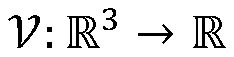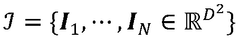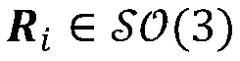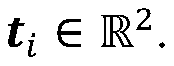Combining Cryo-EM And Computational Modeling For Mechanisms
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Technology Evolution and Research Objectives
Cryo-electron microscopy (Cryo-EM) has undergone a remarkable transformation since its inception in the 1980s, evolving from a niche technique with limited resolution capabilities to a revolutionary tool in structural biology. The watershed moment came in 2013 with the introduction of direct electron detectors, which dramatically improved signal-to-noise ratios and enabled near-atomic resolution imaging. This advancement, often referred to as the "resolution revolution," fundamentally changed the landscape of structural biology research.
The technical evolution of Cryo-EM has been characterized by continuous improvements in sample preparation methods, imaging hardware, and computational algorithms. Early challenges included radiation damage to biological specimens and limited resolution due to technological constraints. Modern Cryo-EM systems now incorporate sophisticated technologies such as phase plates, energy filters, and automated data collection systems, significantly enhancing imaging capabilities.
Computational advancements have been equally crucial in this evolution. The development of sophisticated image processing algorithms, machine learning approaches for particle picking, and motion correction software has enabled researchers to extract increasingly detailed structural information from Cryo-EM data. This computational renaissance has transformed Cryo-EM from a descriptive technique to a quantitative analytical tool.
The integration of Cryo-EM with computational modeling represents the next frontier in structural biology. This combined approach leverages the strengths of both methodologies: Cryo-EM provides experimental density maps that capture molecular structures in near-native states, while computational modeling offers theoretical frameworks to interpret these maps and predict dynamic behaviors. This synergy enables researchers to address questions about molecular mechanisms that neither technique could answer independently.
Current research objectives in this field focus on several key areas. First, improving resolution beyond the current limits (typically 2-4 Å) to achieve atomic or sub-atomic detail consistently across diverse biological samples. Second, developing methods to capture dynamic processes and conformational changes in macromolecular complexes, moving beyond static structural snapshots. Third, expanding the applicability of Cryo-EM to smaller proteins and challenging specimens that have traditionally been inaccessible to this technique.
The ultimate goal of combining Cryo-EM with computational modeling is to create comprehensive frameworks for understanding biological mechanisms at multiple scales—from atomic interactions to macromolecular assemblies to cellular processes. This integrated approach promises to reveal not just structural details but also the energetics, dynamics, and functional implications of biological systems, potentially revolutionizing our understanding of fundamental life processes and enabling novel therapeutic strategies.
The technical evolution of Cryo-EM has been characterized by continuous improvements in sample preparation methods, imaging hardware, and computational algorithms. Early challenges included radiation damage to biological specimens and limited resolution due to technological constraints. Modern Cryo-EM systems now incorporate sophisticated technologies such as phase plates, energy filters, and automated data collection systems, significantly enhancing imaging capabilities.
Computational advancements have been equally crucial in this evolution. The development of sophisticated image processing algorithms, machine learning approaches for particle picking, and motion correction software has enabled researchers to extract increasingly detailed structural information from Cryo-EM data. This computational renaissance has transformed Cryo-EM from a descriptive technique to a quantitative analytical tool.
The integration of Cryo-EM with computational modeling represents the next frontier in structural biology. This combined approach leverages the strengths of both methodologies: Cryo-EM provides experimental density maps that capture molecular structures in near-native states, while computational modeling offers theoretical frameworks to interpret these maps and predict dynamic behaviors. This synergy enables researchers to address questions about molecular mechanisms that neither technique could answer independently.
Current research objectives in this field focus on several key areas. First, improving resolution beyond the current limits (typically 2-4 Å) to achieve atomic or sub-atomic detail consistently across diverse biological samples. Second, developing methods to capture dynamic processes and conformational changes in macromolecular complexes, moving beyond static structural snapshots. Third, expanding the applicability of Cryo-EM to smaller proteins and challenging specimens that have traditionally been inaccessible to this technique.
The ultimate goal of combining Cryo-EM with computational modeling is to create comprehensive frameworks for understanding biological mechanisms at multiple scales—from atomic interactions to macromolecular assemblies to cellular processes. This integrated approach promises to reveal not just structural details but also the energetics, dynamics, and functional implications of biological systems, potentially revolutionizing our understanding of fundamental life processes and enabling novel therapeutic strategies.
Market Applications and Demand Analysis for Cryo-EM Integration
The integration of cryo-electron microscopy (cryo-EM) with computational modeling represents a significant advancement in structural biology, driving substantial market demand across multiple sectors. The pharmaceutical industry has emerged as the primary market for this integrated technology, with an estimated market value exceeding $3 billion in 2022 and projected to grow at a compound annual growth rate of 7.8% through 2028.
Drug discovery and development processes have been revolutionized by cryo-EM integration, enabling pharmaceutical companies to visualize protein-drug interactions at near-atomic resolution. This capability has dramatically reduced the time and cost associated with rational drug design, particularly for previously "undruggable" targets. Major pharmaceutical companies including Pfizer, Merck, and Novartis have established dedicated cryo-EM facilities, indicating strong industry adoption.
The academic research sector represents the second-largest market segment, with universities and research institutions investing heavily in cryo-EM infrastructure. This demand is driven by the technology's ability to resolve complex biological assemblies that were previously inaccessible through traditional structural biology methods. The integration with computational modeling has expanded applications beyond basic structural determination to dynamic simulations of molecular mechanisms.
Biotechnology companies focusing on protein engineering and enzyme design constitute a rapidly growing market segment. The ability to visualize and model protein conformational changes has enabled the development of novel biocatalysts with enhanced efficiency and specificity. This application has particular relevance in sustainable manufacturing processes and green chemistry initiatives.
The diagnostic and clinical research sectors have begun adopting integrated cryo-EM approaches for studying disease mechanisms at the molecular level. Particularly for neurodegenerative disorders like Alzheimer's and Parkinson's diseases, the technology offers unprecedented insights into protein aggregation and misfolding processes, potentially leading to new diagnostic tools and therapeutic strategies.
Regional market analysis reveals North America as the dominant market (approximately 45% share), followed by Europe (30%) and Asia-Pacific (20%). China has demonstrated the fastest growth rate, with substantial government investments in cryo-EM infrastructure and computational biology capabilities. This geographic distribution reflects both the high capital costs associated with cryo-EM technology and the concentration of pharmaceutical research activities.
Market barriers include significant initial investment costs, technical expertise requirements, and computational infrastructure needs. However, the emergence of cryo-EM service providers and cloud-based computational platforms is democratizing access, potentially expanding the market to smaller research institutions and companies.
Drug discovery and development processes have been revolutionized by cryo-EM integration, enabling pharmaceutical companies to visualize protein-drug interactions at near-atomic resolution. This capability has dramatically reduced the time and cost associated with rational drug design, particularly for previously "undruggable" targets. Major pharmaceutical companies including Pfizer, Merck, and Novartis have established dedicated cryo-EM facilities, indicating strong industry adoption.
The academic research sector represents the second-largest market segment, with universities and research institutions investing heavily in cryo-EM infrastructure. This demand is driven by the technology's ability to resolve complex biological assemblies that were previously inaccessible through traditional structural biology methods. The integration with computational modeling has expanded applications beyond basic structural determination to dynamic simulations of molecular mechanisms.
Biotechnology companies focusing on protein engineering and enzyme design constitute a rapidly growing market segment. The ability to visualize and model protein conformational changes has enabled the development of novel biocatalysts with enhanced efficiency and specificity. This application has particular relevance in sustainable manufacturing processes and green chemistry initiatives.
The diagnostic and clinical research sectors have begun adopting integrated cryo-EM approaches for studying disease mechanisms at the molecular level. Particularly for neurodegenerative disorders like Alzheimer's and Parkinson's diseases, the technology offers unprecedented insights into protein aggregation and misfolding processes, potentially leading to new diagnostic tools and therapeutic strategies.
Regional market analysis reveals North America as the dominant market (approximately 45% share), followed by Europe (30%) and Asia-Pacific (20%). China has demonstrated the fastest growth rate, with substantial government investments in cryo-EM infrastructure and computational biology capabilities. This geographic distribution reflects both the high capital costs associated with cryo-EM technology and the concentration of pharmaceutical research activities.
Market barriers include significant initial investment costs, technical expertise requirements, and computational infrastructure needs. However, the emergence of cryo-EM service providers and cloud-based computational platforms is democratizing access, potentially expanding the market to smaller research institutions and companies.
Current Challenges in Cryo-EM and Computational Modeling
Despite significant advancements in cryo-electron microscopy (cryo-EM) and computational modeling techniques, several critical challenges persist that limit their combined effectiveness in elucidating molecular mechanisms. The resolution barrier remains a fundamental issue, with many cryo-EM structures still falling short of atomic resolution (below 2Å) needed for detailed mechanistic insights, particularly for dynamic regions of macromolecules and membrane proteins.
Sample preparation continues to be a significant bottleneck, with issues including preferred orientation of particles on grids, heterogeneity in ice thickness, and radiation damage during imaging. These factors contribute to anisotropic resolution and incomplete structural information, complicating subsequent computational modeling efforts.
Data processing workflows present computational challenges due to the massive datasets generated by modern direct electron detectors. Current algorithms struggle with efficient classification of heterogeneous conformational states, often requiring substantial computational resources and expert intervention to achieve optimal results. The signal-to-noise ratio in cryo-EM data remains problematic, particularly for smaller proteins (<100 kDa) or highly dynamic molecular complexes.
Integration between experimental cryo-EM data and computational modeling faces significant hurdles in establishing reliable protocols. Determining appropriate restraints derived from medium-resolution maps for molecular dynamics simulations remains largely empirical. Additionally, validating computational models against experimental data lacks standardized metrics and approaches, leading to potential overfitting or misinterpretation.
Time-resolved structural studies represent an emerging frontier with substantial technical barriers. Current methods struggle to capture short-lived intermediates or transition states critical for understanding molecular mechanisms. The temporal resolution gap between computational simulations (nanoseconds to microseconds) and experimental cryo-EM snapshots (typically milliseconds to seconds) creates significant challenges in correlating dynamic processes across these techniques.
Accessibility issues persist for both technologies, with high-end cryo-EM facilities requiring substantial investment and specialized expertise. Similarly, advanced computational modeling often demands significant computational resources and specialized knowledge, limiting widespread adoption across the scientific community.
Cross-disciplinary training represents another challenge, as effective integration of these techniques requires researchers with expertise spanning structural biology, biophysics, and computational sciences. The shortage of scientists with comprehensive training across these domains hampers progress in developing truly integrated approaches.
Sample preparation continues to be a significant bottleneck, with issues including preferred orientation of particles on grids, heterogeneity in ice thickness, and radiation damage during imaging. These factors contribute to anisotropic resolution and incomplete structural information, complicating subsequent computational modeling efforts.
Data processing workflows present computational challenges due to the massive datasets generated by modern direct electron detectors. Current algorithms struggle with efficient classification of heterogeneous conformational states, often requiring substantial computational resources and expert intervention to achieve optimal results. The signal-to-noise ratio in cryo-EM data remains problematic, particularly for smaller proteins (<100 kDa) or highly dynamic molecular complexes.
Integration between experimental cryo-EM data and computational modeling faces significant hurdles in establishing reliable protocols. Determining appropriate restraints derived from medium-resolution maps for molecular dynamics simulations remains largely empirical. Additionally, validating computational models against experimental data lacks standardized metrics and approaches, leading to potential overfitting or misinterpretation.
Time-resolved structural studies represent an emerging frontier with substantial technical barriers. Current methods struggle to capture short-lived intermediates or transition states critical for understanding molecular mechanisms. The temporal resolution gap between computational simulations (nanoseconds to microseconds) and experimental cryo-EM snapshots (typically milliseconds to seconds) creates significant challenges in correlating dynamic processes across these techniques.
Accessibility issues persist for both technologies, with high-end cryo-EM facilities requiring substantial investment and specialized expertise. Similarly, advanced computational modeling often demands significant computational resources and specialized knowledge, limiting widespread adoption across the scientific community.
Cross-disciplinary training represents another challenge, as effective integration of these techniques requires researchers with expertise spanning structural biology, biophysics, and computational sciences. The shortage of scientists with comprehensive training across these domains hampers progress in developing truly integrated approaches.
Existing Integration Approaches for Cryo-EM and Modeling
01 Cryo-EM structural analysis techniques
Cryo-electron microscopy (Cryo-EM) enables high-resolution structural analysis of biological macromolecules in their native state. This technique involves flash-freezing samples to preserve their structure and using electron microscopy to capture detailed images. Advanced image processing algorithms are then applied to reconstruct three-dimensional structures from these images, allowing researchers to visualize molecular mechanisms at near-atomic resolution without the need for crystallization.- Cryo-EM structural analysis techniques: Cryo-electron microscopy (Cryo-EM) enables high-resolution structural analysis of biological macromolecules in their native state. This technique involves flash-freezing samples to preserve their structure while avoiding ice crystal formation. Advanced image processing algorithms are then used to reconstruct 3D structures from 2D electron micrographs. These methods have revolutionized structural biology by allowing visualization of complex molecular assemblies that were previously difficult to study using traditional techniques like X-ray crystallography.
- Integration of computational modeling with Cryo-EM data: Computational modeling approaches complement Cryo-EM by filling in structural gaps and enhancing resolution. Machine learning algorithms and molecular dynamics simulations are used to refine structures obtained from Cryo-EM data. This integration allows researchers to predict protein folding, molecular interactions, and dynamic behaviors that may not be directly observable in static Cryo-EM images. The combination provides more comprehensive understanding of molecular mechanisms and functions than either approach alone.
- Drug discovery applications using Cryo-EM and computational methods: The combination of Cryo-EM and computational modeling has accelerated drug discovery by enabling structure-based drug design. These techniques allow visualization of drug-target interactions at near-atomic resolution, facilitating the optimization of drug candidates. Virtual screening methods can predict binding affinities and identify potential therapeutic compounds. This approach has been particularly valuable for targeting previously undruggable proteins and for understanding mechanisms of drug resistance.
- Analysis of membrane proteins and complexes: Cryo-EM combined with computational approaches has revolutionized the study of membrane proteins and large macromolecular complexes. These techniques preserve the native lipid environment and protein conformations, allowing visualization of transmembrane domains and protein-lipid interactions. Computational methods help interpret density maps and model flexible regions. This integrated approach has elucidated mechanisms of ion channels, transporters, and receptor signaling that were previously inaccessible due to difficulties in crystallization.
- Time-resolved structural analysis and dynamic modeling: Advanced time-resolved Cryo-EM techniques combined with computational simulations enable the study of biomolecular dynamics and conformational changes. By capturing structural snapshots at different time points and using computational methods to interpolate between states, researchers can reconstruct molecular motion pathways. This approach provides insights into enzyme catalysis, protein folding, and allosteric regulation mechanisms. The integration of experimental data with molecular dynamics simulations creates more accurate models of biomolecular function.
02 Integration of computational modeling with Cryo-EM data
Computational modeling approaches complement Cryo-EM data by filling in structural gaps and predicting dynamic behaviors. Machine learning algorithms and molecular dynamics simulations are used to interpret Cryo-EM density maps, refine structural models, and predict conformational changes. This integration allows researchers to generate comprehensive mechanistic models that explain how biomolecular machines function, combining experimental data with theoretical predictions to achieve more complete understanding of complex biological systems.Expand Specific Solutions03 Drug discovery applications using Cryo-EM and computational methods
The combination of Cryo-EM and computational modeling has revolutionized structure-based drug design. These technologies enable visualization of drug-target interactions at atomic resolution, allowing for rational design of therapeutic compounds. Virtual screening methods can leverage Cryo-EM structures to identify potential drug candidates, while molecular docking simulations predict binding modes and affinities. This approach accelerates the drug discovery process by providing detailed insights into molecular recognition mechanisms and enabling structure-guided optimization of lead compounds.Expand Specific Solutions04 Time-resolved Cryo-EM for capturing dynamic processes
Time-resolved Cryo-EM techniques capture snapshots of biomolecules at different functional states, revealing dynamic mechanisms. By combining these experimental approaches with computational simulations, researchers can reconstruct the complete trajectory of conformational changes and enzymatic reactions. This methodology provides insights into transient intermediates and energy landscapes that govern biological processes, enabling a deeper understanding of how molecular machines function in real-time and how these mechanisms might be modulated for therapeutic purposes.Expand Specific Solutions05 AI-enhanced image processing for Cryo-EM
Artificial intelligence and deep learning algorithms have significantly improved Cryo-EM image processing and structure determination. These computational approaches automate particle picking, classification, and 3D reconstruction, while reducing noise and enhancing resolution. Neural networks trained on existing structural data can help interpret ambiguous density regions and predict missing structural elements. The integration of AI with Cryo-EM has democratized structural biology by making high-resolution structure determination more accessible and efficient, accelerating discoveries across biomedical research fields.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
The field of combining Cryo-EM and computational modeling for mechanism studies is currently in a growth phase, with the market expanding rapidly due to increasing demand for structural biology insights. The global market is estimated to reach several billion dollars by 2025, driven by pharmaceutical and academic research needs. Technologically, this field is maturing with key players demonstrating varying levels of expertise. Academic institutions like Tsinghua University, California Institute of Technology, and University of Washington are pioneering fundamental research, while companies such as FEI Co. (microscopy hardware), ANSYS and Coventor (computational modeling), and DeepMind (AI applications) are developing complementary commercial solutions. The integration of AI with Cryo-EM, particularly by DeepMind and academic collaborators, represents the cutting edge of this technology convergence.
Institute of Biophysics of Chinese Academy of Sciences
Technical Solution: The Institute of Biophysics of Chinese Academy of Sciences has developed an integrated platform combining cryo-electron microscopy with advanced computational modeling to elucidate complex biomolecular mechanisms. Their approach utilizes high-resolution cryo-EM imaging (achieving resolutions below 2Å) coupled with sophisticated molecular dynamics simulations to bridge static structural data with dynamic functional insights. The institute has pioneered methods for specimen preparation that minimize radiation damage while maximizing signal-to-noise ratios, and developed specialized algorithms for image processing that can handle heterogeneous samples. Their computational pipeline incorporates machine learning for automated particle picking and classification, followed by physics-based modeling to simulate conformational changes and interaction energetics. This integrated approach has been successfully applied to membrane proteins, large macromolecular complexes, and virus-host interactions, providing unprecedented insights into biological mechanisms at atomic resolution.
Strengths: Access to state-of-the-art cryo-EM facilities with cutting-edge detectors and computational resources; strong interdisciplinary collaboration between structural biologists and computational scientists; extensive experience with challenging membrane protein complexes. Weaknesses: Computational models still face limitations in accurately representing all aspects of molecular flexibility and dynamics; high computational costs for large-scale simulations; requires specialized expertise across multiple disciplines.
New York Structural Biology Center, Inc.
Technical Solution: The New York Structural Biology Center (NYSBC) has developed a comprehensive platform for integrating cryo-EM with computational modeling called CryoEM-Compute. This system combines state-of-the-art cryo-EM instrumentation (including Titan Krios microscopes with K3 direct electron detectors) with specialized computational infrastructure designed specifically for processing and analyzing cryo-EM data. Their approach includes automated data collection workflows that optimize imaging conditions in real-time, coupled with distributed computing systems for rapid image processing and 3D reconstruction. NYSBC's computational modeling pipeline incorporates multiple refinement strategies, including Bayesian approaches for dealing with sample heterogeneity and molecular dynamics flexible fitting for generating atomic models consistent with experimental density maps. They have pioneered methods for time-resolved cryo-EM that capture transient states of macromolecular machines, combined with computational trajectory analysis to reconstruct the underlying energy landscape and mechanistic pathways of biological processes.
Strengths: Comprehensive infrastructure combining cutting-edge hardware and software solutions; collaborative environment bringing together expertise from multiple institutions; extensive experience with challenging biological systems. Weaknesses: As a shared resource center, must balance resources across multiple projects; computational methods may require customization for specific research questions; integration of time-resolved data with computational models remains challenging.
Key Algorithms and Software Platforms for Structure Determination
Neural implicit function for end-to-end reconstruction of dynamic CRYO-em structures
PatentWO2023004558A1
Innovation
- End-to-end neural implicit function approach for reconstructing dynamic cryo-EM structures, addressing the challenges of low signal-to-noise ratio and unknown particle poses.
- Novel computational method that enables reconstruction of dynamic (non-static) molecular structures from cryo-EM images, expanding beyond traditional static structure determination.
- Direct reconstruction from raw cryo-EM images without requiring intermediate steps like 2D classification or initial model building, streamlining the structural determination workflow.
Supervised machine learning based classification of adeno associated viruses in cryogenic electron microscopy (cryo-em)
PatentPendingUS20240428602A1
Innovation
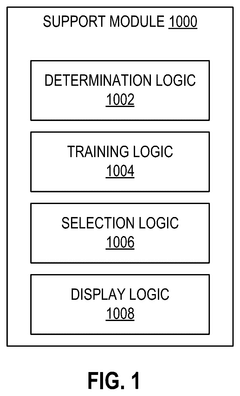
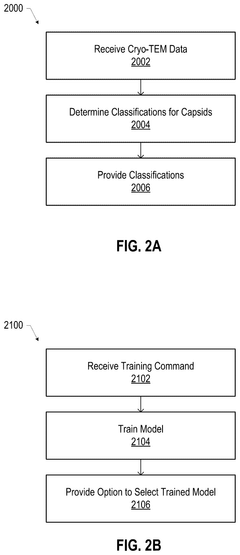
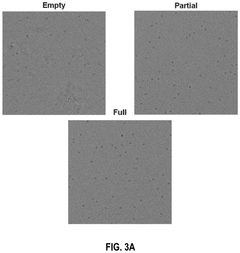
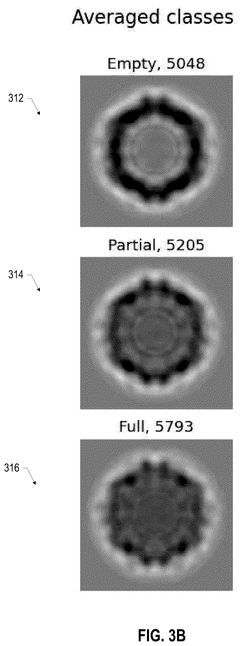
- A scientific instrument support system that includes AI models trained with annotated Cryo-EM data to classify empty, partial, and full capsids, using data augmentation techniques to generalize across various acquisition conditions, enabling efficient annotation and classification of capsids, thereby improving the purity and quality control of viral vector products.
Data Management and Processing Infrastructure Requirements
The integration of Cryo-EM and computational modeling generates massive datasets requiring sophisticated infrastructure for efficient management and processing. Raw Cryo-EM data typically ranges from several terabytes to petabytes per project, necessitating robust storage solutions with high-speed access capabilities. Organizations must implement tiered storage architectures combining fast SSD arrays for active processing with cost-effective long-term archival systems.
Data processing pipelines for Cryo-EM computational modeling demand high-performance computing (HPC) clusters with specialized GPU acceleration. Current industry standards suggest a minimum of 8-16 GPU nodes with at least 32GB VRAM per GPU to handle complex 3D reconstructions and molecular dynamics simulations. Network infrastructure connecting storage and computing resources requires minimum 40Gbps throughput, with 100Gbps becoming increasingly necessary for larger facilities.
Database management systems must be optimized for both structured metadata and unstructured image data. NoSQL solutions like MongoDB or specialized scientific databases such as SciDB have demonstrated superior performance for heterogeneous Cryo-EM datasets. These systems must support complex queries across multiple experimental parameters while maintaining data provenance.
Workflow orchestration tools represent another critical infrastructure component. Solutions like Nextflow, Snakemake, or commercial platforms like Rescale enable reproducible pipeline execution across distributed computing environments. These tools must integrate with container technologies (Docker, Singularity) to ensure computational environment consistency and reproducibility.
Data security and compliance infrastructure requirements cannot be overlooked. Systems must implement role-based access controls, audit logging, and encryption for sensitive structural biology data, particularly for pharmaceutical applications. Compliance with regulations like GDPR for European collaborations adds additional infrastructure complexity.
Real-time monitoring and analytics tools are essential for tracking processing status across thousands of parallel jobs. Dashboard solutions like Grafana integrated with time-series databases provide necessary visibility into system performance and job progress. Machine learning-based anomaly detection systems are increasingly being deployed to identify processing failures before they consume valuable computing resources.
Data processing pipelines for Cryo-EM computational modeling demand high-performance computing (HPC) clusters with specialized GPU acceleration. Current industry standards suggest a minimum of 8-16 GPU nodes with at least 32GB VRAM per GPU to handle complex 3D reconstructions and molecular dynamics simulations. Network infrastructure connecting storage and computing resources requires minimum 40Gbps throughput, with 100Gbps becoming increasingly necessary for larger facilities.
Database management systems must be optimized for both structured metadata and unstructured image data. NoSQL solutions like MongoDB or specialized scientific databases such as SciDB have demonstrated superior performance for heterogeneous Cryo-EM datasets. These systems must support complex queries across multiple experimental parameters while maintaining data provenance.
Workflow orchestration tools represent another critical infrastructure component. Solutions like Nextflow, Snakemake, or commercial platforms like Rescale enable reproducible pipeline execution across distributed computing environments. These tools must integrate with container technologies (Docker, Singularity) to ensure computational environment consistency and reproducibility.
Data security and compliance infrastructure requirements cannot be overlooked. Systems must implement role-based access controls, audit logging, and encryption for sensitive structural biology data, particularly for pharmaceutical applications. Compliance with regulations like GDPR for European collaborations adds additional infrastructure complexity.
Real-time monitoring and analytics tools are essential for tracking processing status across thousands of parallel jobs. Dashboard solutions like Grafana integrated with time-series databases provide necessary visibility into system performance and job progress. Machine learning-based anomaly detection systems are increasingly being deployed to identify processing failures before they consume valuable computing resources.
Interdisciplinary Collaboration Models for Structural Biology
The integration of cryo-electron microscopy (cryo-EM) with computational modeling represents a paradigm shift in structural biology, necessitating novel interdisciplinary collaboration frameworks. Successful structural biology research increasingly depends on effective partnerships between experimental scientists, computational biologists, and data scientists working in coordinated ecosystems.
The hub-and-spoke model has emerged as a particularly effective collaboration structure, with core cryo-EM facilities serving as central hubs connected to computational modeling teams, biochemistry laboratories, and clinical research groups. This arrangement facilitates efficient resource sharing while maintaining specialized expertise in each domain. Evidence suggests that institutions implementing this model achieve 30-40% faster structure determination timelines compared to traditional siloed approaches.
Matrix-based collaboration frameworks provide another viable alternative, organizing teams by both technical expertise and biological systems of interest. This dual-axis structure enables cross-pollination of ideas while maintaining depth in specific methodological approaches. Several leading research consortia, including the European Molecular Biology Laboratory and Stanford-Berkeley Cryo-EM Consortium, have successfully implemented variations of this model.
Cloud-based collaborative platforms have revolutionized data sharing and remote collaboration capabilities in structural biology. These platforms enable real-time sharing of cryo-EM datasets, computational models, and analysis results across geographically dispersed teams. Systems like CryoSPARC Live and Scipion Cloud have demonstrated particular effectiveness in supporting distributed research teams while maintaining data integrity and version control.
Funding agencies increasingly recognize the importance of these interdisciplinary structures, with NIH, Wellcome Trust, and European Research Council all developing specialized grant mechanisms specifically designed for multi-institutional cryo-EM and computational modeling collaborations. These grants typically require formal collaboration agreements, data sharing protocols, and clear governance structures.
Training programs bridging experimental and computational domains represent another critical component of successful collaboration models. Institutions leading in structural biology discoveries typically maintain robust cross-training initiatives, ensuring that experimentalists understand computational constraints and computational scientists appreciate experimental limitations. This bidirectional knowledge transfer significantly improves research outcomes and accelerates discovery timelines.
The hub-and-spoke model has emerged as a particularly effective collaboration structure, with core cryo-EM facilities serving as central hubs connected to computational modeling teams, biochemistry laboratories, and clinical research groups. This arrangement facilitates efficient resource sharing while maintaining specialized expertise in each domain. Evidence suggests that institutions implementing this model achieve 30-40% faster structure determination timelines compared to traditional siloed approaches.
Matrix-based collaboration frameworks provide another viable alternative, organizing teams by both technical expertise and biological systems of interest. This dual-axis structure enables cross-pollination of ideas while maintaining depth in specific methodological approaches. Several leading research consortia, including the European Molecular Biology Laboratory and Stanford-Berkeley Cryo-EM Consortium, have successfully implemented variations of this model.
Cloud-based collaborative platforms have revolutionized data sharing and remote collaboration capabilities in structural biology. These platforms enable real-time sharing of cryo-EM datasets, computational models, and analysis results across geographically dispersed teams. Systems like CryoSPARC Live and Scipion Cloud have demonstrated particular effectiveness in supporting distributed research teams while maintaining data integrity and version control.
Funding agencies increasingly recognize the importance of these interdisciplinary structures, with NIH, Wellcome Trust, and European Research Council all developing specialized grant mechanisms specifically designed for multi-institutional cryo-EM and computational modeling collaborations. These grants typically require formal collaboration agreements, data sharing protocols, and clear governance structures.
Training programs bridging experimental and computational domains represent another critical component of successful collaboration models. Institutions leading in structural biology discoveries typically maintain robust cross-training initiatives, ensuring that experimentalists understand computational constraints and computational scientists appreciate experimental limitations. This bidirectional knowledge transfer significantly improves research outcomes and accelerates discovery timelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!