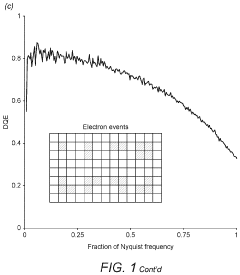
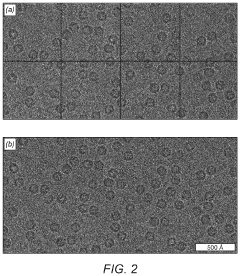
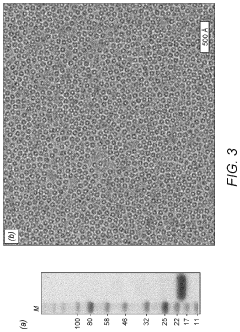
Cryo-EM Detector Calibration And Dose Fractionation Methods
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Detector Technology Evolution and Objectives
Cryo-electron microscopy (cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules at near-atomic resolution. The evolution of cryo-EM detector technology represents one of the most significant advancements in this field over the past two decades, transforming what was once referred to as "blobology" into a high-resolution structural determination technique.
The journey of cryo-EM detectors began with photographic film, which offered high detective quantum efficiency (DQE) but required laborious development and digitization processes. The transition to charge-coupled devices (CCDs) in the early 2000s brought automation but suffered from indirect detection limitations, as electrons were first converted to photons via a scintillator, resulting in signal spread and resolution loss.
A paradigm shift occurred around 2012-2013 with the introduction of direct electron detectors (DEDs), particularly complementary metal-oxide-semiconductor (CMOS) based detectors. These revolutionary devices directly detect electrons without intermediate conversion, dramatically improving DQE and enabling single-electron counting capabilities. This advancement, coupled with faster readout rates, made dose fractionation possible—a technique where the total electron dose is divided into multiple frames that can be aligned to mitigate beam-induced motion.
The technical objectives in cryo-EM detector development have consistently focused on several key parameters: improving DQE across spatial frequencies, increasing readout speed, enhancing radiation hardness, expanding detector area, and reducing pixel size. These improvements aim to maximize signal-to-noise ratio while minimizing radiation damage to specimens.
Current state-of-the-art detectors like the Gatan K3, Thermo Fisher Falcon 4, and Direct Electron DE-64 represent the culmination of these efforts, offering counting modes with DQE values approaching theoretical limits at low spatial frequencies. These detectors enable sophisticated dose fractionation strategies that have become essential for high-resolution structure determination.
Looking forward, the field is moving toward even faster frame rates, larger detector areas, and improved radiation hardness. Emerging technologies such as event-driven readout architectures promise to further revolutionize data collection by recording individual electron events with precise timing information, potentially enabling new experimental approaches.
Calibration methods have evolved in parallel with detector technology, from basic gain normalization to sophisticated algorithms that account for coincidence loss, electron counting artifacts, and detector-specific characteristics. These calibration procedures are critical for extracting maximum information from the collected data and ensuring accurate structure determination.
The journey of cryo-EM detectors began with photographic film, which offered high detective quantum efficiency (DQE) but required laborious development and digitization processes. The transition to charge-coupled devices (CCDs) in the early 2000s brought automation but suffered from indirect detection limitations, as electrons were first converted to photons via a scintillator, resulting in signal spread and resolution loss.
A paradigm shift occurred around 2012-2013 with the introduction of direct electron detectors (DEDs), particularly complementary metal-oxide-semiconductor (CMOS) based detectors. These revolutionary devices directly detect electrons without intermediate conversion, dramatically improving DQE and enabling single-electron counting capabilities. This advancement, coupled with faster readout rates, made dose fractionation possible—a technique where the total electron dose is divided into multiple frames that can be aligned to mitigate beam-induced motion.
The technical objectives in cryo-EM detector development have consistently focused on several key parameters: improving DQE across spatial frequencies, increasing readout speed, enhancing radiation hardness, expanding detector area, and reducing pixel size. These improvements aim to maximize signal-to-noise ratio while minimizing radiation damage to specimens.
Current state-of-the-art detectors like the Gatan K3, Thermo Fisher Falcon 4, and Direct Electron DE-64 represent the culmination of these efforts, offering counting modes with DQE values approaching theoretical limits at low spatial frequencies. These detectors enable sophisticated dose fractionation strategies that have become essential for high-resolution structure determination.
Looking forward, the field is moving toward even faster frame rates, larger detector areas, and improved radiation hardness. Emerging technologies such as event-driven readout architectures promise to further revolutionize data collection by recording individual electron events with precise timing information, potentially enabling new experimental approaches.
Calibration methods have evolved in parallel with detector technology, from basic gain normalization to sophisticated algorithms that account for coincidence loss, electron counting artifacts, and detector-specific characteristics. These calibration procedures are critical for extracting maximum information from the collected data and ensuring accurate structure determination.
Market Analysis for Advanced Cryo-EM Detection Systems
The global market for advanced Cryo-EM detection systems is experiencing robust growth, driven primarily by increasing investments in structural biology research and pharmaceutical drug discovery. The market size was valued at approximately $650 million in 2022 and is projected to reach $1.2 billion by 2028, representing a compound annual growth rate of 10.7% during the forecast period.
North America currently dominates the market with a share of 42%, followed by Europe at 31% and Asia-Pacific at 21%. The remaining 6% is distributed across other regions. This regional distribution reflects the concentration of research institutions and pharmaceutical companies with advanced research capabilities in these areas.
Key market segments include direct electron detectors, which account for 65% of the market, and hybrid detectors at 35%. The direct electron detector segment is growing faster due to superior performance characteristics, particularly in dose fractionation applications where radiation damage mitigation is critical.
The primary end-users driving market demand are academic and research institutions (48%), pharmaceutical and biotechnology companies (37%), and contract research organizations (15%). The pharmaceutical sector is showing the fastest growth rate as companies increasingly utilize Cryo-EM for drug discovery and development processes.
Market dynamics are heavily influenced by technological advancements in detector sensitivity, frame rates, and data processing capabilities. Customers are increasingly demanding systems that offer improved detective quantum efficiency (DQE), higher frame rates for better temporal resolution, and integrated data processing solutions that streamline the workflow from image acquisition to structure determination.
Price sensitivity varies significantly across market segments. While academic institutions are more price-sensitive and often rely on grants for purchases, pharmaceutical companies prioritize performance and integration capabilities over cost considerations. The average selling price for advanced detector systems ranges from $400,000 to $1.2 million, depending on specifications and capabilities.
Emerging markets in Asia, particularly China and India, are showing the highest growth potential, with annual growth rates exceeding 15%. This is attributed to increasing government investments in life sciences research infrastructure and the expansion of pharmaceutical R&D activities in these regions.
Customer buying behavior is characterized by long sales cycles (typically 6-18 months), extensive evaluation processes, and significant emphasis on technical support and training services. Successful market penetration requires not only superior technology but also comprehensive customer support infrastructure and educational resources.
North America currently dominates the market with a share of 42%, followed by Europe at 31% and Asia-Pacific at 21%. The remaining 6% is distributed across other regions. This regional distribution reflects the concentration of research institutions and pharmaceutical companies with advanced research capabilities in these areas.
Key market segments include direct electron detectors, which account for 65% of the market, and hybrid detectors at 35%. The direct electron detector segment is growing faster due to superior performance characteristics, particularly in dose fractionation applications where radiation damage mitigation is critical.
The primary end-users driving market demand are academic and research institutions (48%), pharmaceutical and biotechnology companies (37%), and contract research organizations (15%). The pharmaceutical sector is showing the fastest growth rate as companies increasingly utilize Cryo-EM for drug discovery and development processes.
Market dynamics are heavily influenced by technological advancements in detector sensitivity, frame rates, and data processing capabilities. Customers are increasingly demanding systems that offer improved detective quantum efficiency (DQE), higher frame rates for better temporal resolution, and integrated data processing solutions that streamline the workflow from image acquisition to structure determination.
Price sensitivity varies significantly across market segments. While academic institutions are more price-sensitive and often rely on grants for purchases, pharmaceutical companies prioritize performance and integration capabilities over cost considerations. The average selling price for advanced detector systems ranges from $400,000 to $1.2 million, depending on specifications and capabilities.
Emerging markets in Asia, particularly China and India, are showing the highest growth potential, with annual growth rates exceeding 15%. This is attributed to increasing government investments in life sciences research infrastructure and the expansion of pharmaceutical R&D activities in these regions.
Customer buying behavior is characterized by long sales cycles (typically 6-18 months), extensive evaluation processes, and significant emphasis on technical support and training services. Successful market penetration requires not only superior technology but also comprehensive customer support infrastructure and educational resources.
Current Challenges in Detector Calibration Technologies
Despite significant advancements in cryo-electron microscopy (cryo-EM) detector technology, several critical challenges persist in calibration methodologies that impede optimal performance and data quality. The primary challenge lies in the accurate characterization of detector gain variations across the sensor area. These non-uniform response patterns require sophisticated calibration procedures that must account for both spatial and temporal variations, particularly as detectors age or experience radiation damage.
Dose-dependent detector behavior presents another significant obstacle. Current calibration methods struggle to maintain accuracy across the wide dynamic range of electron doses used in cryo-EM experiments. At low doses, noise characteristics dominate, while at higher doses, detector saturation and non-linearity become problematic. This dose-dependent response necessitates complex calibration models that few facilities have fully implemented.
Temperature stability during calibration represents a persistent technical hurdle. Modern direct electron detectors are typically operated at sub-zero temperatures to minimize noise, but temperature fluctuations during operation can invalidate calibration parameters. Current systems lack robust real-time temperature compensation mechanisms, resulting in calibration drift during extended data collection sessions.
Radiation damage to detector components progressively alters calibration parameters over time. The cumulative electron dose received by detectors gradually changes their response characteristics, requiring frequent recalibration. However, the industry lacks standardized protocols for determining optimal recalibration intervals or for tracking detector degradation quantitatively.
The absence of universal calibration standards across different detector models and manufacturers creates significant interoperability challenges. Each detector type requires unique calibration procedures, making cross-platform data comparison difficult and limiting the transferability of processing workflows between facilities using different hardware.
For dose fractionation applications, current calibration methods inadequately address frame-dependent detector behavior. The detector response can vary between initial and subsequent frames in a fractionation series, yet most calibration approaches treat all frames equivalently. This oversimplification leads to suboptimal alignment and averaging of dose-fractionated data.
Finally, computational limitations affect real-time calibration correction. The processing overhead required for sophisticated calibration models can introduce latency in data acquisition workflows, forcing compromises between calibration accuracy and acquisition speed. Next-generation detectors with higher frame rates and larger pixel counts will further exacerbate this computational bottleneck.
Dose-dependent detector behavior presents another significant obstacle. Current calibration methods struggle to maintain accuracy across the wide dynamic range of electron doses used in cryo-EM experiments. At low doses, noise characteristics dominate, while at higher doses, detector saturation and non-linearity become problematic. This dose-dependent response necessitates complex calibration models that few facilities have fully implemented.
Temperature stability during calibration represents a persistent technical hurdle. Modern direct electron detectors are typically operated at sub-zero temperatures to minimize noise, but temperature fluctuations during operation can invalidate calibration parameters. Current systems lack robust real-time temperature compensation mechanisms, resulting in calibration drift during extended data collection sessions.
Radiation damage to detector components progressively alters calibration parameters over time. The cumulative electron dose received by detectors gradually changes their response characteristics, requiring frequent recalibration. However, the industry lacks standardized protocols for determining optimal recalibration intervals or for tracking detector degradation quantitatively.
The absence of universal calibration standards across different detector models and manufacturers creates significant interoperability challenges. Each detector type requires unique calibration procedures, making cross-platform data comparison difficult and limiting the transferability of processing workflows between facilities using different hardware.
For dose fractionation applications, current calibration methods inadequately address frame-dependent detector behavior. The detector response can vary between initial and subsequent frames in a fractionation series, yet most calibration approaches treat all frames equivalently. This oversimplification leads to suboptimal alignment and averaging of dose-fractionated data.
Finally, computational limitations affect real-time calibration correction. The processing overhead required for sophisticated calibration models can introduce latency in data acquisition workflows, forcing compromises between calibration accuracy and acquisition speed. Next-generation detectors with higher frame rates and larger pixel counts will further exacerbate this computational bottleneck.
Established Calibration and Dose Fractionation Techniques
01 Detector calibration techniques for cryo-EM
Various calibration techniques are employed to ensure accurate performance of detectors in cryo-electron microscopy. These include gain normalization, dark current correction, and defect pixel mapping. Calibration procedures help minimize systematic errors and improve the quality of collected data. Advanced algorithms can automatically detect and correct for detector-specific artifacts, ensuring optimal image quality for structural analysis.- Detector calibration techniques for cryo-EM: Various calibration techniques are employed to ensure accurate performance of detectors in cryo-electron microscopy. These include gain normalization, dark current correction, and defect pixel mapping. Calibration procedures help minimize systematic errors and improve the quality of collected data. Advanced algorithms can automatically detect and correct for detector artifacts, ensuring reliable imaging results even under challenging experimental conditions.
- Dose fractionation strategies in cryo-EM imaging: Dose fractionation involves dividing the total electron dose into multiple frames during data acquisition. This approach allows for motion correction and reduces radiation damage effects on the specimen. By collecting a series of frames with lower individual doses, researchers can better preserve high-resolution information while still achieving adequate signal-to-noise ratios. Various algorithms have been developed to optimize the fractionation scheme based on sample characteristics and research objectives.
- Motion correction and image processing in cryo-EM: Motion correction algorithms compensate for specimen movement during imaging, which is critical for high-resolution structure determination. These methods align individual frames from dose-fractionated data to produce sharper composite images. Advanced processing techniques can account for beam-induced motion, stage drift, and local deformations within the sample. Integration with machine learning approaches has further improved the accuracy and efficiency of motion correction procedures.
- Direct electron detector technology for cryo-EM: Direct electron detectors have revolutionized cryo-EM by offering superior detection efficiency and reduced noise compared to traditional detectors. These devices directly sense electrons without intermediate conversion steps, resulting in improved detective quantum efficiency. They feature fast readout capabilities that enable dose fractionation and can operate in various modes including counting and integration. Ongoing developments focus on enhancing sensitivity, reducing noise, and increasing frame rates.
- Radiation damage mitigation in cryo-EM: Strategies to mitigate radiation damage are essential for preserving specimen integrity during cryo-EM imaging. These include optimizing electron dose distribution, employing low-dose imaging techniques, and implementing specialized sample preparation methods. Software solutions can selectively weight different frames based on their estimated radiation damage levels. Cryogenic temperatures help reduce secondary damage effects, while advanced processing algorithms can extract maximum information from radiation-sensitive samples.
02 Dose fractionation strategies in cryo-EM imaging
Dose fractionation involves dividing the total electron dose into multiple frames during data acquisition. This approach allows for motion correction and reduces the impact of beam-induced damage on biological specimens. By collecting image data as a series of frames with lower individual doses, researchers can optimize the signal-to-noise ratio while minimizing radiation damage. Advanced fractionation protocols can be tailored to specific sample types and imaging conditions.Expand Specific Solutions03 Direct electron detector technology for cryo-EM
Direct electron detectors represent a significant advancement in cryo-EM technology, offering superior detection efficiency and reduced noise compared to traditional detectors. These detectors directly sense electrons without intermediate conversion steps, resulting in improved detective quantum efficiency. They enable high-speed readout necessary for dose fractionation and provide enhanced sensitivity at lower electron doses, which is crucial for imaging radiation-sensitive biological specimens.Expand Specific Solutions04 Motion correction algorithms for cryo-EM data processing
Motion correction algorithms compensate for specimen movement during image acquisition, which is critical when using dose fractionation techniques. These algorithms align individual frames from fractionated exposures to produce a composite image with reduced blurring. Advanced computational methods can account for both global and local motion patterns, improving the resolution of the final reconstructions. Real-time motion correction implementations allow for immediate feedback during data collection.Expand Specific Solutions05 Radiation damage mitigation in cryo-EM
Strategies to mitigate radiation damage are essential in cryo-EM to preserve the structural integrity of biological specimens. These include optimizing electron dose distribution, employing energy filters, and implementing specialized imaging protocols. By carefully controlling the accumulated electron dose and its distribution across the specimen, researchers can extract maximum structural information before significant damage occurs. Computational methods can also selectively weight data from early frames that contain high-resolution information before radiation damage becomes severe.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Cryo-EM
Cryo-EM detector calibration and dose fractionation methods are advancing through a competitive landscape characterized by early commercial maturity and growing market adoption. The global market is expanding rapidly as research institutions and pharmaceutical companies increasingly utilize cryo-EM for structural biology applications. Key technology leaders include established scientific instrument manufacturers like JEOL Ltd. and FEI Co. (now part of Thermo Fisher), who provide comprehensive electron microscopy solutions. Academic institutions such as Oxford University, University of California, and Wisconsin Alumni Research Foundation are driving innovation through fundamental research. Specialized companies like Direct Electron LP and MiTeGen LLC are developing niche detector technologies, while pharmaceutical entities including BioNTech SE, Sanofi, and Merck are investing in applications for drug discovery and development.
Oxford University Innovation Ltd.
Technical Solution: Oxford University Innovation has developed a comprehensive Cryo-EM detector calibration and dose fractionation system based on research from Oxford University's structural biology departments. Their approach implements a physics-based detector modeling framework that accounts for the complex interactions between electrons and detector materials. The calibration protocol incorporates a reference-based methodology using standardized test specimens to characterize detector response across varying electron energies and dose rates[8]. Oxford's dose fractionation technology employs an adaptive frame acquisition strategy that dynamically adjusts exposure parameters based on real-time analysis of specimen behavior and radiation sensitivity. Their "radiation-aware" algorithm incorporates predictive modeling of structural degradation to optimize frame weighting during image reconstruction. The system also features sophisticated motion correction capabilities that track and compensate for both beam-induced and mechanical specimen movements with sub-pixel accuracy. Oxford's solution includes comprehensive validation tools that provide quantitative metrics of detector performance and calibration quality[9].
Strengths: Scientifically rigorous approach based on fundamental understanding of electron-specimen interactions; highly adaptable algorithms that can be optimized for diverse specimen types. Weaknesses: Less commercialized than solutions from established instrument manufacturers; may require more technical expertise for implementation and operation.
JEOL Ltd.
Technical Solution: JEOL has developed advanced Cryo-EM detector calibration systems that integrate direct electron detection technology with sophisticated dose fractionation methods. Their solution employs a multi-frame acquisition approach where the total electron dose is distributed across numerous short exposure frames, allowing for precise motion correction and optimal signal-to-noise ratio preservation. JEOL's calibration protocol includes automated gain normalization that accounts for pixel-to-pixel sensitivity variations and corrects for detector non-uniformities. Their dose fractionation algorithm dynamically adjusts exposure times based on specimen characteristics, implementing a "dose-symmetric" acquisition scheme that places equal radiation damage across the tilt series[1]. The system also incorporates real-time drift monitoring with sub-pixel accuracy, enabling frame alignment before final image reconstruction to minimize motion-induced blurring effects.
Strengths: Superior motion correction algorithms that effectively mitigate beam-induced specimen movement; integrated workflow from calibration to image processing that streamlines the entire Cryo-EM pipeline. Weaknesses: Higher computational requirements for processing the large volumes of fractionated data; system complexity requires specialized training for optimal operation.
Critical Patents and Innovations in Detector Technology
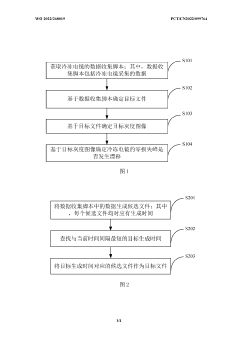
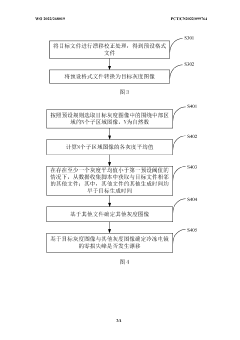
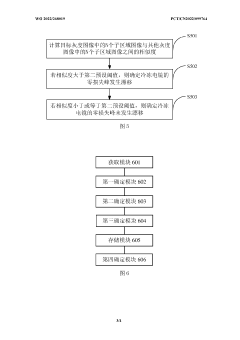
Detection method and apparatus for zero-loss peak drift of cryogenic electron microscopy
PatentWO2022268019A1
Innovation
- By obtaining the data collection script of the cryo-electron microscope, the target file and target grayscale image are determined, and whether the zero-loss peak drifts is automatically detected. If it drifts, a centering operation is performed to improve data collection efficiency and image quality.
System and method for electron cryomicroscopy
PatentPendingUS20230135352A1
Innovation
- An electron cryomicroscopy system with a field-emission gun generating an 80 keV to 120 keV electron beam, an objective lens with selected chromatic aberration for high resolution, a cryostage for specimen cooling, and a direct electron detector with pixels capable of detecting incident electrons, optimizing the system for reduced radiation damage and cost while enhancing information content.
Regulatory Standards for Electron Microscopy Equipment
Regulatory standards for electron microscopy equipment, particularly those related to cryo-electron microscopy (cryo-EM) detector calibration and dose fractionation methods, have evolved significantly in recent years. These standards are crucial for ensuring the reliability, reproducibility, and safety of research outcomes in structural biology and materials science.
The International Electrotechnical Commission (IEC) has established comprehensive guidelines (IEC 61010) specifically addressing safety requirements for electron microscopes, including those used in cryo-EM applications. These standards mandate specific calibration protocols for detectors to ensure accurate measurement of electron doses and proper implementation of dose fractionation techniques.
In the United States, the Food and Drug Administration (FDA) has implemented regulations for cryo-EM equipment used in pharmaceutical research and development. These regulations require validation of detector calibration methods and dose fractionation protocols when the resulting structural data will be used for drug development submissions. The FDA's 21 CFR Part 11 compliance requirements extend to the software systems controlling detector operation and dose fractionation.
The European Union, through its CE marking requirements, enforces the EN 61326 standard for electromagnetic compatibility of laboratory equipment, including electron microscopes and their detector systems. Additionally, the EU's Medical Device Regulation (MDR) applies when cryo-EM systems are used for diagnostic purposes, imposing stricter calibration and validation requirements.
ISO/IEC 17025 accreditation standards have become increasingly important for facilities operating cryo-EM equipment, requiring documented calibration procedures for detectors and validation of dose fractionation methods. These standards emphasize traceability to international measurement standards and uncertainty quantification in detector response.
Japan's Ministry of Health, Labor and Welfare has established specific guidelines for cryo-EM equipment used in pharmaceutical research, with particular emphasis on detector calibration accuracy and dose control methodologies. These guidelines align with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) standards.
Emerging regulatory frameworks in China through the National Medical Products Administration (NMPA) are increasingly focusing on standardization of cryo-EM detector technologies, with specific requirements for calibration frequency and documentation of dose fractionation parameters.
Professional organizations such as the Microscopy Society of America and the European Microscopy Society have developed best practice guidelines that, while not legally binding, often inform regulatory decisions and are widely adopted as industry standards for detector calibration and dose management in cryo-EM applications.
The International Electrotechnical Commission (IEC) has established comprehensive guidelines (IEC 61010) specifically addressing safety requirements for electron microscopes, including those used in cryo-EM applications. These standards mandate specific calibration protocols for detectors to ensure accurate measurement of electron doses and proper implementation of dose fractionation techniques.
In the United States, the Food and Drug Administration (FDA) has implemented regulations for cryo-EM equipment used in pharmaceutical research and development. These regulations require validation of detector calibration methods and dose fractionation protocols when the resulting structural data will be used for drug development submissions. The FDA's 21 CFR Part 11 compliance requirements extend to the software systems controlling detector operation and dose fractionation.
The European Union, through its CE marking requirements, enforces the EN 61326 standard for electromagnetic compatibility of laboratory equipment, including electron microscopes and their detector systems. Additionally, the EU's Medical Device Regulation (MDR) applies when cryo-EM systems are used for diagnostic purposes, imposing stricter calibration and validation requirements.
ISO/IEC 17025 accreditation standards have become increasingly important for facilities operating cryo-EM equipment, requiring documented calibration procedures for detectors and validation of dose fractionation methods. These standards emphasize traceability to international measurement standards and uncertainty quantification in detector response.
Japan's Ministry of Health, Labor and Welfare has established specific guidelines for cryo-EM equipment used in pharmaceutical research, with particular emphasis on detector calibration accuracy and dose control methodologies. These guidelines align with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) standards.
Emerging regulatory frameworks in China through the National Medical Products Administration (NMPA) are increasingly focusing on standardization of cryo-EM detector technologies, with specific requirements for calibration frequency and documentation of dose fractionation parameters.
Professional organizations such as the Microscopy Society of America and the European Microscopy Society have developed best practice guidelines that, while not legally binding, often inform regulatory decisions and are widely adopted as industry standards for detector calibration and dose management in cryo-EM applications.
Data Management Solutions for Large-Scale Cryo-EM Datasets
The exponential growth of cryo-electron microscopy (cryo-EM) datasets presents significant challenges for data management infrastructure. Modern cryo-EM experiments routinely generate terabytes of data per session, necessitating robust solutions for storage, processing, and long-term archiving.
Cloud-based storage systems have emerged as a viable solution for managing large-scale cryo-EM datasets. These platforms offer scalable storage capacity with built-in redundancy and geographical distribution to ensure data integrity. Major providers like AWS, Google Cloud, and Microsoft Azure have developed specialized scientific data management services that accommodate the unique requirements of cryo-EM data, including support for metadata tagging and version control.
On-premises solutions remain essential for many research institutions due to data security concerns and the need for high-speed access during processing. Hybrid approaches combining local high-performance storage for active projects with cloud archiving for completed work represent the current best practice for many facilities.
Database management systems specifically designed for scientific imaging data have evolved to handle the complex metadata associated with cryo-EM experiments. These systems track detector calibration parameters, dose fractionation schemes, and processing histories, enabling researchers to maintain complete provenance records for their structural determinations.
Automated data pipelines have become increasingly important for efficient workflow management. These pipelines integrate detector calibration data, dose fractionation frames, and processing parameters to streamline the path from raw data acquisition to final structure determination. Real-time monitoring systems allow researchers to assess data quality during collection, optimizing microscope time utilization.
Data compression techniques specifically optimized for electron microscopy images have been developed to reduce storage requirements without compromising scientific integrity. Lossy compression methods that preserve critical structural information while significantly reducing file sizes are gaining acceptance in the field, particularly for long-term archiving of processed datasets.
Standardized data formats and metadata schemas, such as those proposed by the Electron Microscopy Public Image Archive (EMPIAR), facilitate data sharing and reanalysis. These standards incorporate detector calibration parameters and dose fractionation information, ensuring that datasets remain interpretable and reusable by the broader scientific community.
Cloud-based storage systems have emerged as a viable solution for managing large-scale cryo-EM datasets. These platforms offer scalable storage capacity with built-in redundancy and geographical distribution to ensure data integrity. Major providers like AWS, Google Cloud, and Microsoft Azure have developed specialized scientific data management services that accommodate the unique requirements of cryo-EM data, including support for metadata tagging and version control.
On-premises solutions remain essential for many research institutions due to data security concerns and the need for high-speed access during processing. Hybrid approaches combining local high-performance storage for active projects with cloud archiving for completed work represent the current best practice for many facilities.
Database management systems specifically designed for scientific imaging data have evolved to handle the complex metadata associated with cryo-EM experiments. These systems track detector calibration parameters, dose fractionation schemes, and processing histories, enabling researchers to maintain complete provenance records for their structural determinations.
Automated data pipelines have become increasingly important for efficient workflow management. These pipelines integrate detector calibration data, dose fractionation frames, and processing parameters to streamline the path from raw data acquisition to final structure determination. Real-time monitoring systems allow researchers to assess data quality during collection, optimizing microscope time utilization.
Data compression techniques specifically optimized for electron microscopy images have been developed to reduce storage requirements without compromising scientific integrity. Lossy compression methods that preserve critical structural information while significantly reducing file sizes are gaining acceptance in the field, particularly for long-term archiving of processed datasets.
Standardized data formats and metadata schemas, such as those proposed by the Electron Microscopy Public Image Archive (EMPIAR), facilitate data sharing and reanalysis. These standards incorporate detector calibration parameters and dose fractionation information, ensuring that datasets remain interpretable and reusable by the broader scientific community.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!