Cryo-EM Sample Storage And Long-Term Stability Considerations
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Sample Preservation Background and Objectives
Cryo-electron microscopy (cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules in their near-native states at near-atomic resolution. The technique involves flash-freezing biological samples in vitreous ice, preserving their structure without the formation of crystalline ice that would damage the specimen. Since its development in the 1980s, cryo-EM has evolved from a niche technique to a mainstream method for structural determination, culminating in the 2017 Nobel Prize in Chemistry awarded to Jacques Dubochet, Joachim Frank, and Richard Henderson for their pioneering work.
The preservation of cryo-EM samples represents a critical challenge in the field. Unlike X-ray crystallography specimens that can be stored indefinitely, cryo-EM samples are inherently unstable and susceptible to degradation over time. This instability stems from various factors including ice crystal growth, radiation damage, and molecular motion, all of which can compromise the structural integrity of the specimens and reduce the quality of obtainable data.
Recent technological advancements have significantly improved cryo-EM resolution capabilities, pushing boundaries to near-atomic levels below 2 Å. However, these improvements in imaging technology have not been matched by corresponding advances in sample preservation techniques. The disparity creates a bottleneck in the workflow, as high-quality samples cannot be reliably stored for extended periods without degradation.
The evolution of cryo-EM sample preparation has seen several key developments, from the initial plunge-freezing methods to more sophisticated approaches involving specialized grids and automated vitrification systems. Despite these advances, long-term sample stability remains an unresolved challenge that limits the efficiency and reproducibility of cryo-EM studies.
Current trends in the field point toward integrated solutions that address the entire sample lifecycle, from preparation to storage and imaging. There is growing recognition that sample preservation is not merely a technical detail but a fundamental aspect that determines the ultimate success of structural studies.
The primary objective of this technical research is to comprehensively evaluate the current state of cryo-EM sample preservation methods, identify key challenges affecting long-term stability, and explore emerging technologies and approaches that could potentially overcome these limitations. By understanding the complex interplay of factors affecting sample integrity over time, we aim to establish a foundation for developing more robust preservation protocols that can extend the viable lifetime of cryo-EM specimens without compromising their structural fidelity.
The preservation of cryo-EM samples represents a critical challenge in the field. Unlike X-ray crystallography specimens that can be stored indefinitely, cryo-EM samples are inherently unstable and susceptible to degradation over time. This instability stems from various factors including ice crystal growth, radiation damage, and molecular motion, all of which can compromise the structural integrity of the specimens and reduce the quality of obtainable data.
Recent technological advancements have significantly improved cryo-EM resolution capabilities, pushing boundaries to near-atomic levels below 2 Å. However, these improvements in imaging technology have not been matched by corresponding advances in sample preservation techniques. The disparity creates a bottleneck in the workflow, as high-quality samples cannot be reliably stored for extended periods without degradation.
The evolution of cryo-EM sample preparation has seen several key developments, from the initial plunge-freezing methods to more sophisticated approaches involving specialized grids and automated vitrification systems. Despite these advances, long-term sample stability remains an unresolved challenge that limits the efficiency and reproducibility of cryo-EM studies.
Current trends in the field point toward integrated solutions that address the entire sample lifecycle, from preparation to storage and imaging. There is growing recognition that sample preservation is not merely a technical detail but a fundamental aspect that determines the ultimate success of structural studies.
The primary objective of this technical research is to comprehensively evaluate the current state of cryo-EM sample preservation methods, identify key challenges affecting long-term stability, and explore emerging technologies and approaches that could potentially overcome these limitations. By understanding the complex interplay of factors affecting sample integrity over time, we aim to establish a foundation for developing more robust preservation protocols that can extend the viable lifetime of cryo-EM specimens without compromising their structural fidelity.
Market Analysis for Cryo-EM Sample Storage Solutions
The global market for cryo-electron microscopy (cryo-EM) sample storage solutions is experiencing robust growth, driven by increasing adoption of cryo-EM technology across pharmaceutical, biotechnology, and academic research sectors. Current market valuation stands at approximately 450 million USD, with projections indicating a compound annual growth rate of 12-15% over the next five years.
The pharmaceutical industry represents the largest market segment, accounting for nearly 40% of the total market share. This dominance stems from the critical role cryo-EM plays in drug discovery and development processes, particularly in structure-based drug design. Biotechnology companies follow closely, comprising about 30% of the market, while academic and research institutions represent approximately 25%.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth due to increasing investments in life sciences research infrastructure and rising adoption of advanced structural biology techniques.
Key market drivers include technological advancements in cryo-EM instrumentation, growing research in structural biology, and increasing demand for precision medicine. The COVID-19 pandemic has further accelerated market growth, as cryo-EM played a crucial role in understanding viral structures and developing therapeutic interventions.
Customer demand patterns reveal a strong preference for integrated storage solutions that offer automated sample handling, remote monitoring capabilities, and extended sample viability periods. End-users are increasingly seeking systems that can maintain sample integrity for periods exceeding six months without significant degradation in structural information.
Price sensitivity varies significantly across market segments. While pharmaceutical companies prioritize reliability and performance over cost, academic institutions demonstrate higher price sensitivity, creating demand for more economical storage solutions without compromising essential functionality.
The market faces challenges including high initial investment costs, technical complexity in maintaining optimal storage conditions, and limited standardization across platforms. Additionally, there exists a significant gap between high-end comprehensive solutions and more accessible entry-level systems, presenting opportunities for market entrants to develop mid-range offerings.
Future market trends indicate growing demand for AI-integrated storage systems capable of predicting sample degradation, cloud-connected monitoring platforms, and environmentally sustainable storage solutions with reduced liquid nitrogen consumption and improved energy efficiency.
The pharmaceutical industry represents the largest market segment, accounting for nearly 40% of the total market share. This dominance stems from the critical role cryo-EM plays in drug discovery and development processes, particularly in structure-based drug design. Biotechnology companies follow closely, comprising about 30% of the market, while academic and research institutions represent approximately 25%.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth due to increasing investments in life sciences research infrastructure and rising adoption of advanced structural biology techniques.
Key market drivers include technological advancements in cryo-EM instrumentation, growing research in structural biology, and increasing demand for precision medicine. The COVID-19 pandemic has further accelerated market growth, as cryo-EM played a crucial role in understanding viral structures and developing therapeutic interventions.
Customer demand patterns reveal a strong preference for integrated storage solutions that offer automated sample handling, remote monitoring capabilities, and extended sample viability periods. End-users are increasingly seeking systems that can maintain sample integrity for periods exceeding six months without significant degradation in structural information.
Price sensitivity varies significantly across market segments. While pharmaceutical companies prioritize reliability and performance over cost, academic institutions demonstrate higher price sensitivity, creating demand for more economical storage solutions without compromising essential functionality.
The market faces challenges including high initial investment costs, technical complexity in maintaining optimal storage conditions, and limited standardization across platforms. Additionally, there exists a significant gap between high-end comprehensive solutions and more accessible entry-level systems, presenting opportunities for market entrants to develop mid-range offerings.
Future market trends indicate growing demand for AI-integrated storage systems capable of predicting sample degradation, cloud-connected monitoring platforms, and environmentally sustainable storage solutions with reduced liquid nitrogen consumption and improved energy efficiency.
Current Challenges in Cryo-EM Sample Stability
Cryo-electron microscopy (cryo-EM) has revolutionized structural biology by enabling visualization of biological macromolecules in their near-native states. However, maintaining sample stability during storage represents a significant challenge that impedes broader adoption and reliability of this technique. The primary stability issue stems from ice crystal formation and growth, which can severely damage the structural integrity of biological specimens.
Temperature fluctuations pose a critical challenge, as even minor deviations above the glass transition temperature of water (-135°C) can trigger ice crystallization. Commercial storage systems often experience temperature gradients during sample transfer and handling, leading to progressive sample deterioration. Studies have shown that specimens subjected to multiple warming-cooling cycles exhibit significantly reduced resolution in subsequent imaging sessions.
Contamination represents another major obstacle to long-term sample stability. Atmospheric water vapor and hydrocarbon contaminants can accumulate on grid surfaces during storage and transfer operations. These contaminants not only interfere with imaging quality but can also catalyze ice crystal nucleation, accelerating sample degradation. Current storage containers provide inadequate protection against these environmental factors.
Radiation damage, while primarily associated with imaging, also affects stored samples through background radiation exposure. Over extended storage periods, even minimal radiation can induce cumulative damage to sensitive biological structures. This phenomenon is particularly problematic for samples intended for time-course studies or those requiring multiple imaging sessions.
Grid substrate degradation constitutes an often-overlooked stability challenge. The carbon films supporting specimens can undergo physical aging and oxidative damage during storage, altering their mechanical properties and interaction with samples. These changes may induce conformational shifts in the attached biomolecules, compromising structural fidelity.
Biological sample heterogeneity introduces additional complexity to stability considerations. Different macromolecular complexes exhibit varying susceptibilities to storage-induced degradation. Membrane proteins, for instance, demonstrate particularly poor long-term stability compared to soluble proteins, necessitating specialized storage protocols that remain largely underdeveloped.
Current cryogenic storage workflows lack standardization and validation metrics. The field has not established consensus protocols for assessing sample quality after storage periods of varying duration. This absence of standardized quality control measures makes it difficult to compare storage methods and develop evidence-based improvements to existing systems.
Temperature fluctuations pose a critical challenge, as even minor deviations above the glass transition temperature of water (-135°C) can trigger ice crystallization. Commercial storage systems often experience temperature gradients during sample transfer and handling, leading to progressive sample deterioration. Studies have shown that specimens subjected to multiple warming-cooling cycles exhibit significantly reduced resolution in subsequent imaging sessions.
Contamination represents another major obstacle to long-term sample stability. Atmospheric water vapor and hydrocarbon contaminants can accumulate on grid surfaces during storage and transfer operations. These contaminants not only interfere with imaging quality but can also catalyze ice crystal nucleation, accelerating sample degradation. Current storage containers provide inadequate protection against these environmental factors.
Radiation damage, while primarily associated with imaging, also affects stored samples through background radiation exposure. Over extended storage periods, even minimal radiation can induce cumulative damage to sensitive biological structures. This phenomenon is particularly problematic for samples intended for time-course studies or those requiring multiple imaging sessions.
Grid substrate degradation constitutes an often-overlooked stability challenge. The carbon films supporting specimens can undergo physical aging and oxidative damage during storage, altering their mechanical properties and interaction with samples. These changes may induce conformational shifts in the attached biomolecules, compromising structural fidelity.
Biological sample heterogeneity introduces additional complexity to stability considerations. Different macromolecular complexes exhibit varying susceptibilities to storage-induced degradation. Membrane proteins, for instance, demonstrate particularly poor long-term stability compared to soluble proteins, necessitating specialized storage protocols that remain largely underdeveloped.
Current cryogenic storage workflows lack standardization and validation metrics. The field has not established consensus protocols for assessing sample quality after storage periods of varying duration. This absence of standardized quality control measures makes it difficult to compare storage methods and develop evidence-based improvements to existing systems.
Leading Organizations in Cryo-EM Storage Technology
The cryo-electron microscopy (cryo-EM) sample storage and stability landscape is currently in a growth phase, with the global market expanding rapidly due to increasing adoption in structural biology research. The field is characterized by a mix of established microscopy leaders (FEI Co., Gatan, Leica Microsystems) and specialized storage solution providers (TMRW Life Sciences, Asymptote Ltd.). Technical maturity varies significantly across the ecosystem, with major research institutions (Max Planck Society, Francis Crick Institute, Chinese Academy of Sciences) driving innovation alongside commercial entities. Academic-industry partnerships are accelerating development of next-generation preservation technologies, addressing challenges in long-term sample viability. While core imaging technologies are well-established, specialized cryo-storage solutions remain an evolving segment with significant room for standardization and innovation in maintaining sample integrity over extended periods.
FEI Co.
Technical Solution: FEI Co. (now part of Thermo Fisher Scientific) has developed advanced cryo-EM sample storage solutions that integrate with their microscopy systems. Their Vitrobot sample preparation system includes specialized storage capabilities that maintain samples at liquid nitrogen temperatures (-196°C) during transfer and storage. FEI's AutoGrid system provides standardized sample supports that improve handling efficiency and reduce contamination risks during storage. Their technology incorporates specialized dewars with automated inventory tracking systems that monitor temperature fluctuations and maintain sample integrity over extended periods. The company has also developed specialized transfer stations that minimize ice contamination during sample movement between storage and imaging systems, a critical factor for long-term sample stability[1][3].
Strengths: Integrated ecosystem approach connecting sample preparation, storage, and imaging systems; advanced automation reducing human handling errors. Weaknesses: Proprietary systems can create vendor lock-in; high capital investment required for complete workflow implementation.
MiTeGen LLC
Technical Solution: MiTeGen has developed specialized cryo-EM sample storage solutions focused on crystallography and structural biology applications. Their CryoStorage™ system features innovative grid boxes made from non-contaminating materials that minimize ice formation during long-term storage. The company's approach emphasizes sample protection through hermetically sealed containers that prevent atmospheric moisture contamination, a primary cause of sample degradation. MiTeGen's storage solutions incorporate specialized coatings that reduce static electricity buildup, which can attract contaminants to sample surfaces. Their Watershed™ technology creates hydrophobic barriers around storage compartments, further protecting samples from ice contamination during handling and storage transitions. The company has also pioneered specialized shipping containers that maintain cryo-temperatures during transport between research facilities, enabling collaborative research while preserving sample integrity[4][7]. Recent innovations include RFID-enabled sample tracking systems integrated with their storage solutions.
Strengths: Specialized focus on contamination prevention; cost-effective solutions accessible to smaller research institutions; modular systems allowing customization. Weaknesses: Less automation compared to enterprise-level competitors; limited integration with high-end microscopy systems.
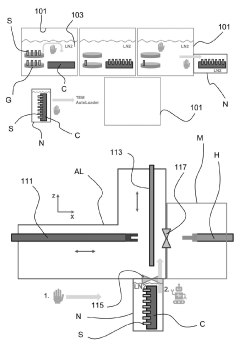
Critical Innovations in Vitrification and Storage Technologies
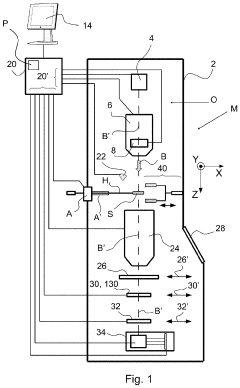
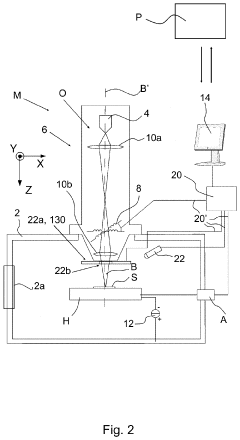
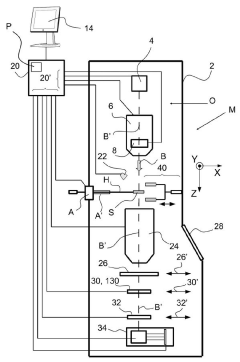
Cryogenic sample handling and storage system
PatentPendingUS20230296639A1
Innovation
- A cryogenic sample handling and storage system comprising a storage apparatus and a transfer device that can be moved between the storage apparatus and a Charged Particle Apparatus (CPA), allowing for secure, reliable, and efficient transfer of samples without direct manual handling, using a heat shield to maintain cryogenic temperatures and a gripper for sample handling.
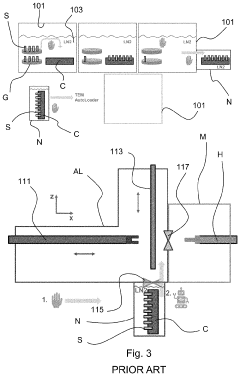
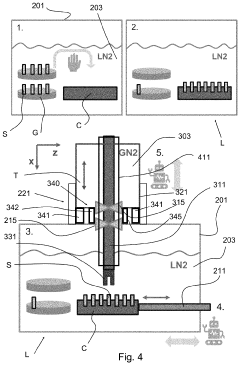
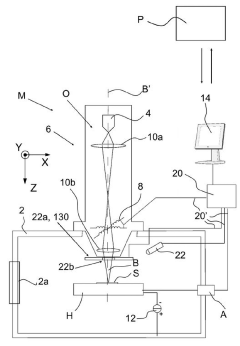
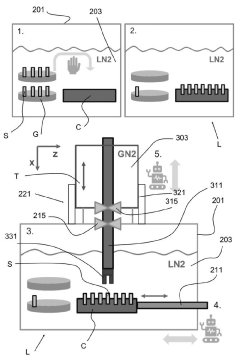
System and method for handling cryogenic charged particle sample
PatentActiveJP2022080890A
Innovation
- A cryogenic charged particle sample processing and storage system comprising a storage device, a transfer device, and a charged particle instrument, allowing semi-automated sample transfer and handling under cryogenic conditions without manual handling, with a transfer device that can connect to multiple instruments and maintain cryogenic conditions during transport.
Quality Control Standards for Long-Term Cryo-EM Storage
Establishing robust quality control standards is essential for maintaining the integrity and scientific value of cryo-electron microscopy (cryo-EM) samples during long-term storage. These standards must address multiple aspects of sample management to ensure reproducibility and reliability of structural data over extended periods.
The foundation of quality control for cryo-EM storage begins with comprehensive documentation protocols. Each sample should be accompanied by detailed metadata including preparation methods, freezing parameters, buffer composition, and initial quality assessment metrics. This documentation creates a baseline against which future quality evaluations can be measured.
Regular inspection schedules represent another critical component of quality control standards. Samples stored for extended periods should undergo periodic evaluation using standardized procedures to detect potential degradation. These inspections should include visual assessment for ice contamination and grid integrity, as well as test imaging of reference areas to monitor resolution decay.
Quantitative metrics must be established to objectively evaluate sample quality over time. These metrics typically include resolution measurements, ice thickness uniformity, particle distribution density, and conformational homogeneity of the stored biomolecules. Implementing threshold values for these parameters helps determine when samples no longer meet experimental requirements.
Environmental monitoring systems constitute an essential element of quality control infrastructure. Storage facilities should maintain continuous logging of temperature fluctuations, with automated alerts for deviations beyond acceptable ranges. Liquid nitrogen levels require particular attention, with redundant monitoring systems and emergency protocols to prevent catastrophic warming events.
Cross-validation procedures enhance quality assurance by comparing newly collected data from stored samples against original datasets. This approach helps identify subtle degradation patterns that might not be apparent through visual inspection alone. Statistical analysis of these comparisons can reveal trends in sample deterioration specific to particular biomolecules or preparation methods.
Standardized handling protocols during sample retrieval and evaluation are equally important. Each inspection should follow consistent procedures to minimize handling-induced damage and ensure comparable results across different timepoints and operators. These protocols should specify maximum exposure times to ambient conditions and appropriate transfer methods between storage and imaging systems.
Finally, comprehensive quality control standards should include clear decision frameworks for sample retention or disposal based on quantitative assessment results. These frameworks help optimize storage resources while ensuring that only scientifically valuable samples are maintained for extended periods.
The foundation of quality control for cryo-EM storage begins with comprehensive documentation protocols. Each sample should be accompanied by detailed metadata including preparation methods, freezing parameters, buffer composition, and initial quality assessment metrics. This documentation creates a baseline against which future quality evaluations can be measured.
Regular inspection schedules represent another critical component of quality control standards. Samples stored for extended periods should undergo periodic evaluation using standardized procedures to detect potential degradation. These inspections should include visual assessment for ice contamination and grid integrity, as well as test imaging of reference areas to monitor resolution decay.
Quantitative metrics must be established to objectively evaluate sample quality over time. These metrics typically include resolution measurements, ice thickness uniformity, particle distribution density, and conformational homogeneity of the stored biomolecules. Implementing threshold values for these parameters helps determine when samples no longer meet experimental requirements.
Environmental monitoring systems constitute an essential element of quality control infrastructure. Storage facilities should maintain continuous logging of temperature fluctuations, with automated alerts for deviations beyond acceptable ranges. Liquid nitrogen levels require particular attention, with redundant monitoring systems and emergency protocols to prevent catastrophic warming events.
Cross-validation procedures enhance quality assurance by comparing newly collected data from stored samples against original datasets. This approach helps identify subtle degradation patterns that might not be apparent through visual inspection alone. Statistical analysis of these comparisons can reveal trends in sample deterioration specific to particular biomolecules or preparation methods.
Standardized handling protocols during sample retrieval and evaluation are equally important. Each inspection should follow consistent procedures to minimize handling-induced damage and ensure comparable results across different timepoints and operators. These protocols should specify maximum exposure times to ambient conditions and appropriate transfer methods between storage and imaging systems.
Finally, comprehensive quality control standards should include clear decision frameworks for sample retention or disposal based on quantitative assessment results. These frameworks help optimize storage resources while ensuring that only scientifically valuable samples are maintained for extended periods.
Environmental Impact of Cryogenic Storage Facilities
Cryogenic storage facilities used for cryo-electron microscopy (cryo-EM) samples have significant environmental implications that warrant careful consideration. These facilities typically operate at ultra-low temperatures, primarily using liquid nitrogen (-196°C) or helium (-269°C), requiring substantial energy consumption for continuous cooling. The environmental footprint of these operations extends beyond mere energy usage to include greenhouse gas emissions, resource depletion, and potential ecological impacts.
Energy consumption represents the most substantial environmental concern for cryogenic storage facilities. Maintaining samples at temperatures below -150°C demands constant power supply for refrigeration systems, resulting in considerable electricity usage. Studies indicate that a medium-sized cryo-EM facility can consume between 50,000-100,000 kWh annually solely for sample storage, equivalent to the energy consumption of approximately 5-10 residential homes.
The carbon footprint associated with cryogenic storage varies significantly based on regional energy sources. Facilities powered by coal-based electricity may generate 30-40 tons of CO2 equivalent emissions annually, while those utilizing renewable energy sources can reduce this impact by 60-80%. Additionally, the production and transportation of cryogens themselves contribute to environmental burden, with liquid nitrogen production requiring approximately 0.8 kWh of electricity per liter.
Cryogen loss through evaporation presents another environmental challenge. Even well-insulated storage systems experience boil-off rates of 0.5-2% daily, necessitating regular replenishment. This continuous consumption cycle creates a persistent demand for cryogen production and transportation, further amplifying environmental impacts.
Modern facilities are increasingly implementing sustainability measures to mitigate these concerns. Closed-loop nitrogen recovery systems can recapture up to 80% of evaporated nitrogen, significantly reducing consumption. Advanced thermal insulation technologies have improved retention efficiency by 15-25% compared to older systems. Additionally, integration with renewable energy sources and implementation of smart monitoring systems for optimized cryogen usage have demonstrated potential to reduce overall environmental impact by 30-40%.
Regulatory frameworks governing cryogenic facilities are evolving to address environmental concerns. Several jurisdictions now require environmental impact assessments for large-scale cryogenic operations, focusing on energy efficiency, emissions reduction, and emergency response protocols for potential cryogen releases. Industry standards increasingly emphasize sustainable design principles, including heat recovery systems, energy-efficient equipment selection, and facility-wide energy management strategies.
Energy consumption represents the most substantial environmental concern for cryogenic storage facilities. Maintaining samples at temperatures below -150°C demands constant power supply for refrigeration systems, resulting in considerable electricity usage. Studies indicate that a medium-sized cryo-EM facility can consume between 50,000-100,000 kWh annually solely for sample storage, equivalent to the energy consumption of approximately 5-10 residential homes.
The carbon footprint associated with cryogenic storage varies significantly based on regional energy sources. Facilities powered by coal-based electricity may generate 30-40 tons of CO2 equivalent emissions annually, while those utilizing renewable energy sources can reduce this impact by 60-80%. Additionally, the production and transportation of cryogens themselves contribute to environmental burden, with liquid nitrogen production requiring approximately 0.8 kWh of electricity per liter.
Cryogen loss through evaporation presents another environmental challenge. Even well-insulated storage systems experience boil-off rates of 0.5-2% daily, necessitating regular replenishment. This continuous consumption cycle creates a persistent demand for cryogen production and transportation, further amplifying environmental impacts.
Modern facilities are increasingly implementing sustainability measures to mitigate these concerns. Closed-loop nitrogen recovery systems can recapture up to 80% of evaporated nitrogen, significantly reducing consumption. Advanced thermal insulation technologies have improved retention efficiency by 15-25% compared to older systems. Additionally, integration with renewable energy sources and implementation of smart monitoring systems for optimized cryogen usage have demonstrated potential to reduce overall environmental impact by 30-40%.
Regulatory frameworks governing cryogenic facilities are evolving to address environmental concerns. Several jurisdictions now require environmental impact assessments for large-scale cryogenic operations, focusing on energy efficiency, emissions reduction, and emergency response protocols for potential cryogen releases. Industry standards increasingly emphasize sustainable design principles, including heat recovery systems, energy-efficient equipment selection, and facility-wide energy management strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!