Sample Preparation Automation For High-Throughput Cryo-EM
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM Sample Preparation Evolution and Objectives
Cryo-electron microscopy (cryo-EM) has revolutionized structural biology by enabling the visualization of biological macromolecules at near-atomic resolution. The evolution of sample preparation techniques for cryo-EM represents a critical aspect of this technology's development, transitioning from manual, labor-intensive processes to increasingly automated systems designed to enhance throughput and reproducibility.
The earliest cryo-EM sample preparation methods, pioneered by Jacques Dubochet and colleagues in the 1980s, involved manually blotting thin films of aqueous samples on electron microscopy grids followed by rapid freezing in liquid ethane. This fundamental approach remained largely unchanged for decades, with scientists accepting the inherent variability and low throughput as unavoidable limitations.
By the early 2000s, the first semi-automated vitrification devices emerged, offering improved consistency in blotting times and environmental control. The introduction of the Vitrobot (FEI/Thermo Fisher) and similar instruments represented a significant step forward, reducing operator-dependent variability while maintaining the basic blotting paradigm. However, these systems still required substantial manual intervention and produced relatively few usable grids per day.
The mid-2010s marked a turning point in cryo-EM sample preparation with the "resolution revolution" driving demand for higher throughput methods. Researchers began exploring alternative approaches to traditional blotting, including spray-based methods, inkjet dispensing, and microfluidic devices. These innovations aimed to minimize sample waste, reduce ice thickness variability, and enable processing of multiple samples in parallel.
Recent years have witnessed the emergence of fully automated systems integrating robotic sample handling, environmental control, and quality assessment capabilities. Platforms such as the Chameleon (SPT Labtech), Spotiton (NYSBC), and Vitrojet (Cryosol) represent different technological approaches to achieving true high-throughput sample preparation, with the capacity to prepare dozens of grids with minimal human intervention.
The primary objectives driving cryo-EM sample preparation automation include increasing throughput to match the capabilities of modern electron microscopes, improving reproducibility through standardized protocols, reducing sample consumption (particularly critical for scarce biological specimens), and enabling real-time quality assessment to minimize wasted microscope time on suboptimal samples.
Future development goals focus on creating integrated workflows connecting sample preparation directly to automated data collection systems, implementing machine learning algorithms for optimizing preparation parameters, and developing specialized approaches for challenging sample types such as membrane proteins and macromolecular complexes. The ultimate aim is to transform cryo-EM from a specialized technique requiring significant expertise into a routine high-throughput structural biology method accessible to the broader scientific community.
The earliest cryo-EM sample preparation methods, pioneered by Jacques Dubochet and colleagues in the 1980s, involved manually blotting thin films of aqueous samples on electron microscopy grids followed by rapid freezing in liquid ethane. This fundamental approach remained largely unchanged for decades, with scientists accepting the inherent variability and low throughput as unavoidable limitations.
By the early 2000s, the first semi-automated vitrification devices emerged, offering improved consistency in blotting times and environmental control. The introduction of the Vitrobot (FEI/Thermo Fisher) and similar instruments represented a significant step forward, reducing operator-dependent variability while maintaining the basic blotting paradigm. However, these systems still required substantial manual intervention and produced relatively few usable grids per day.
The mid-2010s marked a turning point in cryo-EM sample preparation with the "resolution revolution" driving demand for higher throughput methods. Researchers began exploring alternative approaches to traditional blotting, including spray-based methods, inkjet dispensing, and microfluidic devices. These innovations aimed to minimize sample waste, reduce ice thickness variability, and enable processing of multiple samples in parallel.
Recent years have witnessed the emergence of fully automated systems integrating robotic sample handling, environmental control, and quality assessment capabilities. Platforms such as the Chameleon (SPT Labtech), Spotiton (NYSBC), and Vitrojet (Cryosol) represent different technological approaches to achieving true high-throughput sample preparation, with the capacity to prepare dozens of grids with minimal human intervention.
The primary objectives driving cryo-EM sample preparation automation include increasing throughput to match the capabilities of modern electron microscopes, improving reproducibility through standardized protocols, reducing sample consumption (particularly critical for scarce biological specimens), and enabling real-time quality assessment to minimize wasted microscope time on suboptimal samples.
Future development goals focus on creating integrated workflows connecting sample preparation directly to automated data collection systems, implementing machine learning algorithms for optimizing preparation parameters, and developing specialized approaches for challenging sample types such as membrane proteins and macromolecular complexes. The ultimate aim is to transform cryo-EM from a specialized technique requiring significant expertise into a routine high-throughput structural biology method accessible to the broader scientific community.
Market Analysis for Automated Cryo-EM Sample Preparation
The global market for automated cryo-electron microscopy (cryo-EM) sample preparation systems is experiencing robust growth, driven by increasing demand for high-resolution structural biology research. Current market valuation stands at approximately 200 million USD, with projections indicating a compound annual growth rate of 15-20% over the next five years. This growth trajectory is primarily fueled by expanding applications in pharmaceutical research, structural biology, and materials science.
The pharmaceutical and biotechnology sectors represent the largest market segments, collectively accounting for over 60% of the total market share. These industries leverage cryo-EM technology for drug discovery and development processes, particularly in structure-based drug design and protein-ligand interaction studies. Academic research institutions constitute another significant market segment, representing approximately 25% of the market, while government laboratories and contract research organizations make up the remaining portion.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate due to increasing investments in life sciences research infrastructure and rising adoption of advanced structural biology techniques.
Key market drivers include the growing need for high-throughput structural analysis in drug discovery, increasing research activities in protein structure determination, and technological advancements that enhance automation capabilities. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of structural biology in understanding viral proteins and developing therapeutic interventions.
Market restraints include the high initial investment required for automated cryo-EM systems, technical challenges in sample preparation standardization, and the need for specialized expertise. The average cost of a fully automated cryo-EM sample preparation system ranges from 500,000 to 1.5 million USD, creating a significant barrier to entry for smaller research institutions.
Customer demand is increasingly focused on systems offering higher throughput, improved reproducibility, and integration capabilities with existing laboratory workflows. End-users are particularly interested in solutions that can reduce sample preparation time from days to hours while maintaining or improving sample quality. There is also growing demand for systems that can handle smaller sample volumes, addressing the challenges associated with limited availability of biological materials.
The competitive landscape features established scientific instrument manufacturers alongside specialized cryo-EM technology providers. Recent market trends indicate a shift toward more integrated solutions that combine sample preparation automation with data acquisition and analysis capabilities, offering end-to-end workflows for structural biology research.
The pharmaceutical and biotechnology sectors represent the largest market segments, collectively accounting for over 60% of the total market share. These industries leverage cryo-EM technology for drug discovery and development processes, particularly in structure-based drug design and protein-ligand interaction studies. Academic research institutions constitute another significant market segment, representing approximately 25% of the market, while government laboratories and contract research organizations make up the remaining portion.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and Japan, is expected to witness the fastest growth rate due to increasing investments in life sciences research infrastructure and rising adoption of advanced structural biology techniques.
Key market drivers include the growing need for high-throughput structural analysis in drug discovery, increasing research activities in protein structure determination, and technological advancements that enhance automation capabilities. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of structural biology in understanding viral proteins and developing therapeutic interventions.
Market restraints include the high initial investment required for automated cryo-EM systems, technical challenges in sample preparation standardization, and the need for specialized expertise. The average cost of a fully automated cryo-EM sample preparation system ranges from 500,000 to 1.5 million USD, creating a significant barrier to entry for smaller research institutions.
Customer demand is increasingly focused on systems offering higher throughput, improved reproducibility, and integration capabilities with existing laboratory workflows. End-users are particularly interested in solutions that can reduce sample preparation time from days to hours while maintaining or improving sample quality. There is also growing demand for systems that can handle smaller sample volumes, addressing the challenges associated with limited availability of biological materials.
The competitive landscape features established scientific instrument manufacturers alongside specialized cryo-EM technology providers. Recent market trends indicate a shift toward more integrated solutions that combine sample preparation automation with data acquisition and analysis capabilities, offering end-to-end workflows for structural biology research.
Current Automation Challenges in Cryo-EM Sample Preparation
Despite significant advancements in cryo-electron microscopy (cryo-EM) technology, sample preparation remains a critical bottleneck in achieving high-throughput workflows. Current automation challenges stem from the inherent complexity and sensitivity of the sample preparation process, which involves multiple delicate steps that must be performed under precise environmental conditions.
The vitrification process, essential for preserving biological samples in their native state, presents significant automation hurdles. This process requires rapid freezing (within milliseconds) to prevent ice crystal formation, which can damage the specimen structure. Current automated systems struggle to consistently achieve optimal freezing rates across different sample types and volumes, resulting in variable ice thickness and quality.
Grid preparation and handling represent another major challenge. The fragile nature of EM grids, typically made of carbon films supported by metal meshes, makes them difficult to manipulate robotically without damage. Automated systems must delicately handle these grids while maintaining precise environmental control to prevent contamination or premature sample degradation.
Sample application uniformity remains problematic for automation systems. The distribution of particles across the grid surface significantly impacts data collection efficiency. Current automated dispensing mechanisms often produce uneven particle distribution, with some areas containing particle aggregates while others remain empty. This inconsistency reduces the usable area for imaging and ultimately limits throughput.
Environmental control during the entire preparation workflow presents substantial technical difficulties. Humidity, temperature, and contamination must be strictly regulated to preserve sample integrity. Existing automation solutions struggle to maintain these conditions consistently throughout all preparation stages, particularly during transfers between different modules or instruments.
Integration challenges exist between sample preparation automation and downstream processes. Many current systems operate as standalone units rather than as components of an end-to-end workflow. This lack of integration creates inefficiencies when transferring samples between preparation, loading, and imaging stages, often requiring manual intervention that introduces variability and reduces throughput.
Quality control and real-time monitoring capabilities remain limited in existing automation solutions. Unlike manual preparation, where experienced operators can make adjustments based on visual feedback, automated systems typically lack sophisticated in-process monitoring to detect and correct preparation issues. This results in the discovery of sample quality problems only during imaging, wasting valuable microscope time and resources.
The vitrification process, essential for preserving biological samples in their native state, presents significant automation hurdles. This process requires rapid freezing (within milliseconds) to prevent ice crystal formation, which can damage the specimen structure. Current automated systems struggle to consistently achieve optimal freezing rates across different sample types and volumes, resulting in variable ice thickness and quality.
Grid preparation and handling represent another major challenge. The fragile nature of EM grids, typically made of carbon films supported by metal meshes, makes them difficult to manipulate robotically without damage. Automated systems must delicately handle these grids while maintaining precise environmental control to prevent contamination or premature sample degradation.
Sample application uniformity remains problematic for automation systems. The distribution of particles across the grid surface significantly impacts data collection efficiency. Current automated dispensing mechanisms often produce uneven particle distribution, with some areas containing particle aggregates while others remain empty. This inconsistency reduces the usable area for imaging and ultimately limits throughput.
Environmental control during the entire preparation workflow presents substantial technical difficulties. Humidity, temperature, and contamination must be strictly regulated to preserve sample integrity. Existing automation solutions struggle to maintain these conditions consistently throughout all preparation stages, particularly during transfers between different modules or instruments.
Integration challenges exist between sample preparation automation and downstream processes. Many current systems operate as standalone units rather than as components of an end-to-end workflow. This lack of integration creates inefficiencies when transferring samples between preparation, loading, and imaging stages, often requiring manual intervention that introduces variability and reduces throughput.
Quality control and real-time monitoring capabilities remain limited in existing automation solutions. Unlike manual preparation, where experienced operators can make adjustments based on visual feedback, automated systems typically lack sophisticated in-process monitoring to detect and correct preparation issues. This results in the discovery of sample quality problems only during imaging, wasting valuable microscope time and resources.
Existing Automation Solutions for High-Throughput Cryo-EM
01 Automated sample handling systems
Automated systems for handling samples can significantly increase throughput in laboratory settings. These systems typically include robotic arms, conveyor belts, or other mechanical components that can move samples between different processing stations without human intervention. By automating the sample handling process, laboratories can process more samples in less time, reduce human error, and maintain consistent processing conditions.- Automated sample handling systems: Automated systems for handling and processing samples can significantly increase throughput in laboratory settings. These systems typically include robotic arms, conveyor belts, and specialized grippers that can manipulate multiple samples simultaneously. By automating the physical movement and positioning of samples, these systems reduce manual intervention, minimize human error, and allow for continuous operation, thereby enhancing overall throughput in sample preparation workflows.
- High-throughput liquid handling technologies: Advanced liquid handling technologies enable rapid and precise dispensing, mixing, and transfer of liquids for sample preparation. These technologies include multi-channel pipetting systems, acoustic liquid handlers, and microfluidic platforms that can process multiple samples in parallel. By optimizing liquid handling operations, these technologies reduce processing time, increase sample throughput, and improve the consistency of sample preparation procedures.
- Integrated workflow management systems: Integrated systems that coordinate and manage the entire sample preparation workflow can significantly enhance throughput. These systems combine hardware automation with sophisticated software that schedules tasks, monitors progress, and optimizes resource utilization. By integrating various steps of the sample preparation process into a cohesive workflow, these systems minimize idle time, reduce bottlenecks, and maximize the efficiency of laboratory operations.
- Miniaturization and parallelization techniques: Miniaturization of sample preparation processes, combined with parallelization techniques, allows for simultaneous processing of multiple samples in reduced volumes. These approaches include microplate technologies, microarray platforms, and lab-on-a-chip devices that can handle hundreds or thousands of samples concurrently. By reducing sample volumes and increasing parallelization, these techniques dramatically improve throughput while conserving valuable reagents and samples.
- Intelligent automation with machine learning: Advanced automation systems incorporating machine learning and artificial intelligence can adaptively optimize sample preparation processes. These intelligent systems can analyze data in real-time, predict optimal processing parameters, and automatically adjust conditions to maximize throughput and quality. By continuously learning from operational data, these systems can identify inefficiencies, suggest improvements, and progressively enhance the speed and reliability of sample preparation workflows.
02 High-throughput liquid handling technologies
Advanced liquid handling technologies enable rapid and precise dispensing, mixing, and transfer of liquid samples. These technologies include multi-channel pipetting systems, microfluidic devices, and automated liquid handlers that can process multiple samples simultaneously. By optimizing liquid handling processes, laboratories can significantly reduce sample preparation time and increase overall throughput while maintaining accuracy and precision.Expand Specific Solutions03 Integrated sample preparation workflows
Integrated workflows combine multiple sample preparation steps into a single automated process. These systems can perform sequential operations such as sample extraction, purification, dilution, and analysis without manual intervention between steps. By integrating these processes, laboratories can eliminate bottlenecks, reduce handling time, and increase the overall efficiency of sample preparation, leading to higher throughput and more consistent results.Expand Specific Solutions04 Parallel processing techniques
Parallel processing techniques allow multiple samples to be prepared simultaneously rather than sequentially. These techniques utilize multi-well plates, batch processing systems, or modular instruments that can operate independently on different samples at the same time. By processing samples in parallel, laboratories can dramatically increase throughput without sacrificing quality or accuracy, making it possible to handle large sample volumes efficiently.Expand Specific Solutions05 Software control and optimization systems
Advanced software systems play a crucial role in optimizing sample preparation automation and throughput. These systems provide intelligent scheduling, real-time monitoring, process optimization, and data management capabilities. By using sophisticated algorithms to coordinate instrument operations, identify bottlenecks, and adjust workflows dynamically, these software solutions can maximize the efficiency of automated sample preparation systems and ensure consistent high-quality results even at increased throughput levels.Expand Specific Solutions
Leading Companies and Research Institutions in Cryo-EM Automation
The field of Sample Preparation Automation for High-Throughput Cryo-EM is currently in its growth phase, with an estimated market size of $300-500 million and expanding at 15-20% annually. The competitive landscape features academic institutions (Oxford University, Tsinghua University, Chinese Academy of Sciences) driving fundamental research, while established microscopy companies (FEI Co., Leica Microsystems) provide commercial hardware solutions. Emerging specialized players like MiTeGen and Engineering Arts are developing innovative automation tools. The technology remains in early-to-mid maturity, with significant advancements in robotics and AI-driven sample handling, though standardization challenges persist. Research institutions are increasingly partnering with industry players to bridge the gap between academic innovation and commercial implementation, accelerating the field's development toward fully automated workflows.
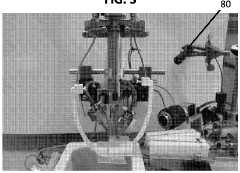
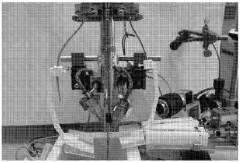
Institute of Biophysics of Chinese Academy of Sciences
Technical Solution: The Institute of Biophysics (IBP) of the Chinese Academy of Sciences has developed an innovative automated cryo-EM sample preparation system called "CryoWriter." This technology employs microfluidic principles to address limitations in traditional blotting methods. The CryoWriter system utilizes a capillary-based approach where samples are precisely deposited onto EM grids in nanoliter volumes through controlled microfluidic channels. Their method eliminates the conventional blotting step, instead using controlled surface tension and capillary forces to create thin liquid films of consistent thickness. The system incorporates a humidity-controlled chamber with precise temperature regulation and automated grid handling mechanisms. A key innovation is their "write-freeze" technology that synchronizes sample deposition with immediate plunge-freezing, minimizing the air-water interface exposure time to milliseconds rather than seconds, which significantly reduces protein denaturation issues common in conventional methods[9]. The IBP system also features integrated image analysis capabilities that provide real-time feedback on ice thickness and sample distribution[10].
Strengths: Minimal sample consumption (nanoliter volumes); reduced protein denaturation at air-water interface; excellent reproducibility between preparations. Weaknesses: Currently optimized primarily for soluble proteins rather than membrane proteins or complexes; requires specialized microfluidic components; limited commercial availability outside research collaborations.
FEI Co.
Technical Solution: FEI Co. (now part of Thermo Fisher Scientific) has developed the Vitrobot™ Mark IV system, a fully automated vitrification device for cryo-EM sample preparation. The system provides controlled humidity and temperature environments while enabling precise blotting parameters to create thin, vitreous ice films. Their technology incorporates automated grid handling with robotic arms that can process multiple grids in sequence, maintaining sample integrity throughout the preparation process. The Vitrobot system features programmable blotting times, forces, and wait times, allowing researchers to optimize conditions for different sample types. Recent advancements include integration with their EPU (Electron Microscopy Software) for streamlined workflow from sample preparation to data acquisition, creating an end-to-end solution for high-throughput cryo-EM studies[1][3]. The system also incorporates ethane/propane mixture capabilities for improved vitrification rates compared to traditional liquid ethane methods.
Strengths: Industry-standard automation with precise environmental control; seamless integration with imaging workflows; high reproducibility between samples. Weaknesses: Limited throughput compared to newer systems; manual grid loading still required; relatively high operational costs for consumables.
Key Technical Innovations in Cryo-EM Sample Preparation
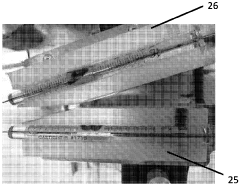
Device and method for cryogenic electron microscopy sample preparation
PatentWO2024080868A1
Innovation
- A cryo-EM sample preparation device with dual applicators and gas outlets for precise and automated deposition and removal of sample materials on a grid, combined with a cooling bath and image capturing apparatus for real-time monitoring, allowing for rapid mixing and vitrification of samples on a millisecond timescale.
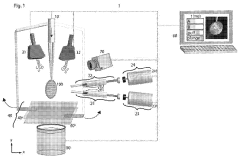
Cryoelectron microscope sample preparation system and preparation method
PatentPendingCN118067469A
Innovation
- A cryo-EM sample preparation system including a spray device, a monitoring device and a detection device is used. Sample droplets are formed through the spray device. The three-dimensional moving device controls the landing of the droplets. The monitoring device monitors and detects the status and distribution of the droplets in real time to improve sample preparation. The process is controllable and reproducible, avoiding sample contamination and achieving high-throughput sample preparation.
Cost-Benefit Analysis of Automated Sample Preparation Systems
The implementation of automated sample preparation systems for high-throughput cryo-electron microscopy (cryo-EM) requires substantial initial investment, making a comprehensive cost-benefit analysis essential for research institutions and pharmaceutical companies considering this technology. The primary capital expenditure includes the automated sample preparation equipment itself, which typically ranges from $500,000 to $2 million depending on the level of automation and throughput capacity. Additional costs include facility modifications, integration with existing workflows, and staff training.
Operating expenses must also be factored into the analysis, including maintenance contracts (approximately 10-15% of equipment cost annually), consumables specific to automated systems, and potential increases in utility costs. However, these costs should be weighed against significant potential benefits in several key areas.
Labor cost reduction represents one of the most quantifiable benefits, with automated systems potentially reducing hands-on time by 70-90% compared to manual preparation methods. For a facility processing 1,000 samples annually, this could translate to savings of 1,500-2,000 labor hours per year. The consistency of sample preparation also improves dramatically, reducing the sample rejection rate from typically 30-40% with manual methods to potentially below 10% with automated systems.
Throughput enhancement constitutes another major benefit, with modern automated systems capable of preparing 50-100 samples per day compared to 5-10 samples with manual methods. This increased capacity can significantly reduce the cost per sample, particularly important for pharmaceutical applications where large sample sets are common.
The quality improvements delivered by automated systems translate directly to financial benefits through higher resolution structures and more reliable results. Studies indicate that consistent sample quality can reduce the number of samples needed for high-resolution structure determination by 30-50%, representing substantial savings in both time and materials.
Return on investment calculations suggest that facilities processing more than 500 samples annually can typically recoup their investment within 3-5 years, with the payback period shortening as sample volumes increase. For pharmaceutical companies, where the value of accelerated drug discovery is particularly high, the ROI timeline may be even shorter when considering the competitive advantages of faster structure determination.
Operating expenses must also be factored into the analysis, including maintenance contracts (approximately 10-15% of equipment cost annually), consumables specific to automated systems, and potential increases in utility costs. However, these costs should be weighed against significant potential benefits in several key areas.
Labor cost reduction represents one of the most quantifiable benefits, with automated systems potentially reducing hands-on time by 70-90% compared to manual preparation methods. For a facility processing 1,000 samples annually, this could translate to savings of 1,500-2,000 labor hours per year. The consistency of sample preparation also improves dramatically, reducing the sample rejection rate from typically 30-40% with manual methods to potentially below 10% with automated systems.
Throughput enhancement constitutes another major benefit, with modern automated systems capable of preparing 50-100 samples per day compared to 5-10 samples with manual methods. This increased capacity can significantly reduce the cost per sample, particularly important for pharmaceutical applications where large sample sets are common.
The quality improvements delivered by automated systems translate directly to financial benefits through higher resolution structures and more reliable results. Studies indicate that consistent sample quality can reduce the number of samples needed for high-resolution structure determination by 30-50%, representing substantial savings in both time and materials.
Return on investment calculations suggest that facilities processing more than 500 samples annually can typically recoup their investment within 3-5 years, with the payback period shortening as sample volumes increase. For pharmaceutical companies, where the value of accelerated drug discovery is particularly high, the ROI timeline may be even shorter when considering the competitive advantages of faster structure determination.
Integration with AI and Machine Learning for Quality Control
The integration of artificial intelligence and machine learning technologies into cryo-electron microscopy (cryo-EM) sample preparation workflows represents a significant advancement in quality control capabilities. These technologies enable real-time analysis of sample quality parameters, dramatically reducing the time between preparation and validation while simultaneously increasing throughput and reliability.
Machine learning algorithms have been developed to automatically assess ice thickness, particle distribution, and contamination levels in cryo-EM grids. These systems can analyze images immediately after grid preparation, providing instant feedback on sample viability before proceeding to expensive microscope time. Deep learning models trained on thousands of previously successful and failed samples can identify subtle patterns invisible to human operators, predicting sample quality with increasing accuracy.
Computer vision systems integrated with automated sample preparation platforms now enable continuous monitoring throughout the vitrification process. These systems can detect anomalies in droplet dispensing, blotting uniformity, and flash-freezing procedures, triggering corrective actions or flagging samples for operator review. The reduction in failed samples translates directly to increased throughput and reduced operational costs.
Recent developments in reinforcement learning have produced adaptive systems capable of optimizing preparation parameters in real-time. These systems analyze outcomes from previous preparation attempts and incrementally adjust variables such as blotting time, humidity levels, and vitrification speed to maximize sample quality for specific protein types. This capability is particularly valuable for challenging samples that resist standardized preparation protocols.
Federated learning approaches are emerging as a powerful tool for quality control improvement across multiple research facilities. By sharing model improvements while maintaining data privacy, institutions can collectively build more robust quality assessment algorithms that account for variations in equipment, protocols, and sample types. This collaborative approach accelerates the development of increasingly sophisticated quality control mechanisms.
Natural language processing techniques are being applied to automatically generate detailed sample preparation reports, correlating preparation parameters with quality outcomes and structural resolution. These systems create searchable databases of preparation conditions that inform future experiments and contribute to the growing knowledge base of optimal sample handling techniques for different biomolecular specimens.
The integration of these AI capabilities with automated sample preparation systems creates a powerful feedback loop that continuously improves both the technology and methodologies for high-throughput cryo-EM. As these systems mature, they promise to remove one of the most significant bottlenecks in structural biology research.
Machine learning algorithms have been developed to automatically assess ice thickness, particle distribution, and contamination levels in cryo-EM grids. These systems can analyze images immediately after grid preparation, providing instant feedback on sample viability before proceeding to expensive microscope time. Deep learning models trained on thousands of previously successful and failed samples can identify subtle patterns invisible to human operators, predicting sample quality with increasing accuracy.
Computer vision systems integrated with automated sample preparation platforms now enable continuous monitoring throughout the vitrification process. These systems can detect anomalies in droplet dispensing, blotting uniformity, and flash-freezing procedures, triggering corrective actions or flagging samples for operator review. The reduction in failed samples translates directly to increased throughput and reduced operational costs.
Recent developments in reinforcement learning have produced adaptive systems capable of optimizing preparation parameters in real-time. These systems analyze outcomes from previous preparation attempts and incrementally adjust variables such as blotting time, humidity levels, and vitrification speed to maximize sample quality for specific protein types. This capability is particularly valuable for challenging samples that resist standardized preparation protocols.
Federated learning approaches are emerging as a powerful tool for quality control improvement across multiple research facilities. By sharing model improvements while maintaining data privacy, institutions can collectively build more robust quality assessment algorithms that account for variations in equipment, protocols, and sample types. This collaborative approach accelerates the development of increasingly sophisticated quality control mechanisms.
Natural language processing techniques are being applied to automatically generate detailed sample preparation reports, correlating preparation parameters with quality outcomes and structural resolution. These systems create searchable databases of preparation conditions that inform future experiments and contribute to the growing knowledge base of optimal sample handling techniques for different biomolecular specimens.
The integration of these AI capabilities with automated sample preparation systems creates a powerful feedback loop that continuously improves both the technology and methodologies for high-throughput cryo-EM. As these systems mature, they promise to remove one of the most significant bottlenecks in structural biology research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!