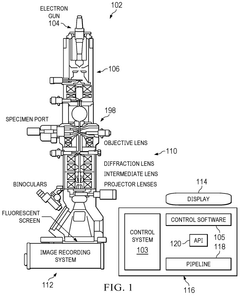
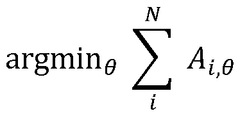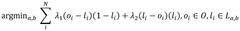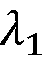Combining Cryo-EM With AFM For Surface Topography Correlation
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryo-EM and AFM Integration Background and Objectives
Cryo-electron microscopy (Cryo-EM) and Atomic Force Microscopy (AFM) represent two powerful imaging techniques that have revolutionized our understanding of biological structures at the molecular and atomic levels. Cryo-EM, which earned its developers the 2017 Nobel Prize in Chemistry, allows visualization of biomolecules in their native state by flash-freezing samples in vitreous ice. Meanwhile, AFM provides topographical information by physically scanning surfaces with a nanoscale probe, offering exceptional vertical resolution down to the sub-nanometer range.
The integration of these complementary techniques has emerged as a promising frontier in structural biology and materials science over the past decade. While Cryo-EM excels at providing internal structural details with near-atomic resolution in three dimensions, it often lacks precise surface topography information. Conversely, AFM delivers outstanding surface topographical data but cannot easily access internal structures. The synergistic combination of these methods addresses the limitations of each individual technique.
Historical developments in both fields have followed parallel but distinct trajectories. Cryo-EM evolved from traditional transmission electron microscopy in the 1980s, with revolutionary advances in detector technology and image processing algorithms occurring in the 2010s, leading to the "resolution revolution." AFM, first developed in 1986, has similarly progressed through multiple generations of instrumentation, with significant improvements in speed, resolution, and operational modes.
The technical convergence of these methods aims to achieve several critical objectives. First, researchers seek to establish correlative workflows that allow examination of identical specimens using both techniques, enabling multi-modal characterization of complex biological assemblies. Second, the integration aims to develop registration methods that precisely align datasets from both modalities, creating comprehensive structural models that incorporate both internal architecture and surface topography.
Another key objective involves developing sample preparation protocols compatible with both techniques, as traditional methods often involve contradictory requirements. Additionally, researchers are working toward computational frameworks that can seamlessly integrate and analyze the heterogeneous data types generated by these complementary approaches.
The potential impact of successful Cryo-EM and AFM integration extends across multiple scientific domains, from fundamental structural biology to pharmaceutical development. By providing more complete structural information, this combined approach promises to enhance our understanding of biomolecular function, disease mechanisms, and drug interactions at unprecedented levels of detail and accuracy.
The integration of these complementary techniques has emerged as a promising frontier in structural biology and materials science over the past decade. While Cryo-EM excels at providing internal structural details with near-atomic resolution in three dimensions, it often lacks precise surface topography information. Conversely, AFM delivers outstanding surface topographical data but cannot easily access internal structures. The synergistic combination of these methods addresses the limitations of each individual technique.
Historical developments in both fields have followed parallel but distinct trajectories. Cryo-EM evolved from traditional transmission electron microscopy in the 1980s, with revolutionary advances in detector technology and image processing algorithms occurring in the 2010s, leading to the "resolution revolution." AFM, first developed in 1986, has similarly progressed through multiple generations of instrumentation, with significant improvements in speed, resolution, and operational modes.
The technical convergence of these methods aims to achieve several critical objectives. First, researchers seek to establish correlative workflows that allow examination of identical specimens using both techniques, enabling multi-modal characterization of complex biological assemblies. Second, the integration aims to develop registration methods that precisely align datasets from both modalities, creating comprehensive structural models that incorporate both internal architecture and surface topography.
Another key objective involves developing sample preparation protocols compatible with both techniques, as traditional methods often involve contradictory requirements. Additionally, researchers are working toward computational frameworks that can seamlessly integrate and analyze the heterogeneous data types generated by these complementary approaches.
The potential impact of successful Cryo-EM and AFM integration extends across multiple scientific domains, from fundamental structural biology to pharmaceutical development. By providing more complete structural information, this combined approach promises to enhance our understanding of biomolecular function, disease mechanisms, and drug interactions at unprecedented levels of detail and accuracy.
Market Applications for Correlated Structural Imaging
The integration of Cryo-Electron Microscopy (Cryo-EM) and Atomic Force Microscopy (AFM) for correlated structural imaging represents a significant advancement in multi-modal imaging technologies, opening numerous market applications across various industries. This combined approach offers unprecedented insights into surface topography correlation at the molecular and atomic levels.
In the pharmaceutical industry, correlated structural imaging enables more precise drug development processes by providing detailed visualization of drug-target interactions. Companies can optimize drug candidates earlier in the development pipeline, potentially reducing the estimated $2.6 billion cost of bringing a new drug to market. The technology allows researchers to observe how drug molecules interact with cellular components in near-native states, accelerating lead optimization and validation processes.
Biotechnology firms are leveraging this combined imaging approach for protein engineering and enzyme design. The detailed structural information obtained through correlated Cryo-EM and AFM imaging facilitates the rational design of novel proteins with enhanced functionality. This capability is particularly valuable for developing biocatalysts for industrial processes, potentially reducing manufacturing costs and environmental impact across multiple sectors.
The semiconductor industry benefits from correlated structural imaging for quality control and failure analysis. As chip architectures continue to shrink toward sub-5nm processes, the ability to correlate surface features with underlying structures becomes critical for identifying defects and optimizing manufacturing processes. This application directly impacts yield rates and production efficiency in an industry valued at over $500 billion globally.
Materials science research and development represents another significant market application. Researchers can characterize novel materials such as 2D materials, nanocomposites, and biomaterials with unprecedented detail, correlating surface properties with internal structures. This capability accelerates materials development cycles and enables more targeted design of materials with specific performance characteristics.
In the field of diagnostics and medical devices, correlated structural imaging supports the development of next-generation biosensors and diagnostic platforms. The detailed structural information helps optimize sensor surfaces and detection mechanisms, potentially improving sensitivity and specificity of diagnostic tests. This application addresses the growing market for point-of-care diagnostics and personalized medicine solutions.
Academic and government research institutions constitute a substantial market segment, utilizing correlated imaging techniques for fundamental research across disciplines. These institutions drive method development and validation, establishing protocols that eventually transfer to industrial applications, creating a continuous innovation pipeline from basic research to commercial implementation.
In the pharmaceutical industry, correlated structural imaging enables more precise drug development processes by providing detailed visualization of drug-target interactions. Companies can optimize drug candidates earlier in the development pipeline, potentially reducing the estimated $2.6 billion cost of bringing a new drug to market. The technology allows researchers to observe how drug molecules interact with cellular components in near-native states, accelerating lead optimization and validation processes.
Biotechnology firms are leveraging this combined imaging approach for protein engineering and enzyme design. The detailed structural information obtained through correlated Cryo-EM and AFM imaging facilitates the rational design of novel proteins with enhanced functionality. This capability is particularly valuable for developing biocatalysts for industrial processes, potentially reducing manufacturing costs and environmental impact across multiple sectors.
The semiconductor industry benefits from correlated structural imaging for quality control and failure analysis. As chip architectures continue to shrink toward sub-5nm processes, the ability to correlate surface features with underlying structures becomes critical for identifying defects and optimizing manufacturing processes. This application directly impacts yield rates and production efficiency in an industry valued at over $500 billion globally.
Materials science research and development represents another significant market application. Researchers can characterize novel materials such as 2D materials, nanocomposites, and biomaterials with unprecedented detail, correlating surface properties with internal structures. This capability accelerates materials development cycles and enables more targeted design of materials with specific performance characteristics.
In the field of diagnostics and medical devices, correlated structural imaging supports the development of next-generation biosensors and diagnostic platforms. The detailed structural information helps optimize sensor surfaces and detection mechanisms, potentially improving sensitivity and specificity of diagnostic tests. This application addresses the growing market for point-of-care diagnostics and personalized medicine solutions.
Academic and government research institutions constitute a substantial market segment, utilizing correlated imaging techniques for fundamental research across disciplines. These institutions drive method development and validation, establishing protocols that eventually transfer to industrial applications, creating a continuous innovation pipeline from basic research to commercial implementation.
Technical Challenges in Multi-modal Microscopy Integration
The integration of Cryo-Electron Microscopy (Cryo-EM) with Atomic Force Microscopy (AFM) represents a significant advancement in multi-modal microscopy, yet faces substantial technical hurdles. The fundamental challenge stems from the vastly different operating environments required by each technique. Cryo-EM demands samples to be flash-frozen and maintained at ultra-low temperatures (typically -180°C), while conventional AFM operates optimally at room temperature. This temperature incompatibility necessitates specialized hardware solutions that can maintain sample integrity across these extreme conditions.
Sample preparation presents another critical challenge. Cryo-EM samples require vitrification in amorphous ice without crystallization, while AFM requires samples with accessible surfaces. Creating specimens that simultaneously satisfy both requirements demands innovative preparation protocols. Furthermore, the physical transfer between instruments introduces risks of contamination, structural damage, and temperature fluctuations that can compromise data quality.
Registration and correlation of images between the two modalities present significant computational challenges. The different resolution scales, contrast mechanisms, and imaging artifacts inherent to each technique complicate the development of reliable algorithms for accurate spatial alignment. Cryo-EM provides high-resolution internal structural information but with limited surface detail, while AFM excels at surface topography but offers limited internal visibility.
Hardware integration issues further complicate this multi-modal approach. The vacuum requirements for Cryo-EM conflict with the mechanical access needed for AFM cantilevers. Developing systems that can accommodate both without compromising performance requires sophisticated engineering solutions, including specialized sample stages, thermal management systems, and vibration isolation mechanisms.
Data integration and interpretation represent perhaps the most complex challenge. Researchers must develop frameworks to meaningfully combine the complementary data streams, accounting for differences in resolution, dimensionality, and information content. This requires advanced computational methods including machine learning algorithms capable of recognizing patterns across different data types and scales.
Time-resolved studies introduce additional complexity, as maintaining cryogenic conditions while performing sequential AFM measurements presents significant technical barriers. The development of cryo-AFM systems has begun addressing this issue, but challenges remain in achieving the resolution and versatility of room-temperature AFM while maintaining cryogenic sample integrity.
Addressing these technical challenges requires interdisciplinary collaboration between microscopists, materials scientists, computer scientists, and engineers. Recent advances in cryo-stage design, correlative microscopy software, and machine learning approaches for image registration have begun to address these issues, but significant technical hurdles remain before seamless integration becomes routine in research settings.
Sample preparation presents another critical challenge. Cryo-EM samples require vitrification in amorphous ice without crystallization, while AFM requires samples with accessible surfaces. Creating specimens that simultaneously satisfy both requirements demands innovative preparation protocols. Furthermore, the physical transfer between instruments introduces risks of contamination, structural damage, and temperature fluctuations that can compromise data quality.
Registration and correlation of images between the two modalities present significant computational challenges. The different resolution scales, contrast mechanisms, and imaging artifacts inherent to each technique complicate the development of reliable algorithms for accurate spatial alignment. Cryo-EM provides high-resolution internal structural information but with limited surface detail, while AFM excels at surface topography but offers limited internal visibility.
Hardware integration issues further complicate this multi-modal approach. The vacuum requirements for Cryo-EM conflict with the mechanical access needed for AFM cantilevers. Developing systems that can accommodate both without compromising performance requires sophisticated engineering solutions, including specialized sample stages, thermal management systems, and vibration isolation mechanisms.
Data integration and interpretation represent perhaps the most complex challenge. Researchers must develop frameworks to meaningfully combine the complementary data streams, accounting for differences in resolution, dimensionality, and information content. This requires advanced computational methods including machine learning algorithms capable of recognizing patterns across different data types and scales.
Time-resolved studies introduce additional complexity, as maintaining cryogenic conditions while performing sequential AFM measurements presents significant technical barriers. The development of cryo-AFM systems has begun addressing this issue, but challenges remain in achieving the resolution and versatility of room-temperature AFM while maintaining cryogenic sample integrity.
Addressing these technical challenges requires interdisciplinary collaboration between microscopists, materials scientists, computer scientists, and engineers. Recent advances in cryo-stage design, correlative microscopy software, and machine learning approaches for image registration have begun to address these issues, but significant technical hurdles remain before seamless integration becomes routine in research settings.
Current Methodologies for Cryo-EM and AFM Correlation
01 Integration of Cryo-EM and AFM for surface topography analysis
The combination of Cryo-Electron Microscopy (Cryo-EM) and Atomic Force Microscopy (AFM) provides complementary data for comprehensive surface topography analysis. Cryo-EM offers high-resolution structural information at the molecular level while AFM provides detailed surface topography measurements. When integrated, these technologies enable researchers to correlate structural features across different scales and under various conditions, enhancing the understanding of biological and material surfaces.- Integration of Cryo-EM and AFM for surface topography analysis: The combination of Cryo-Electron Microscopy (Cryo-EM) and Atomic Force Microscopy (AFM) provides complementary data for comprehensive surface topography analysis. Cryo-EM offers high-resolution imaging of frozen samples in their native state, while AFM provides detailed surface topography measurements with nanometer resolution. When integrated, these technologies enable researchers to correlate structural features across different scales and validate findings through multiple imaging modalities.
- Correlative microscopy techniques for biological samples: Correlative microscopy approaches combining Cryo-EM and AFM are particularly valuable for biological samples where preservation of native structure is critical. These techniques allow researchers to map surface features of biomolecules, cellular membranes, and protein complexes while maintaining sample integrity. The correlation between the topographical data from AFM and the structural information from Cryo-EM provides insights into biomolecular interactions and conformational changes that would be impossible to obtain with either technique alone.
- Advanced data processing for multi-modal imaging correlation: Specialized software and algorithms have been developed to align, process, and correlate data obtained from Cryo-EM and AFM imaging of the same sample. These computational tools enable precise registration of images from different modalities, accounting for differences in resolution, contrast mechanisms, and sample preparation artifacts. Machine learning approaches are increasingly being applied to automate the correlation process and extract meaningful relationships between surface topography features observed in both imaging techniques.
- Sample preparation methods for combined Cryo-EM and AFM analysis: Novel sample preparation protocols have been developed to enable sequential or simultaneous imaging of samples using both Cryo-EM and AFM. These methods address challenges such as maintaining sample integrity during transfer between instruments, preventing ice contamination, and ensuring compatibility with both imaging modalities. Specialized sample holders and cryo-stages have been designed to facilitate the correlation of surface topography data between the two techniques while preserving the native state of the sample.
- Applications in materials science and nanotechnology: The combined use of Cryo-EM and AFM for surface topography correlation has significant applications in materials science and nanotechnology. This approach enables detailed characterization of nanomaterials, thin films, and composite structures by providing complementary information about surface morphology, mechanical properties, and internal structure. The correlated data helps researchers understand structure-property relationships in advanced materials and optimize their design for specific applications in electronics, energy storage, and catalysis.
02 Sample preparation techniques for correlative microscopy
Specialized sample preparation methods are essential for successful correlation between Cryo-EM and AFM data. These techniques include vitrification processes that preserve the native state of biological samples, grid-based preparation systems that allow for imaging of the same sample area with both techniques, and surface modification approaches that optimize sample adherence while maintaining structural integrity. These preparation methods minimize artifacts and ensure reliable correlation between the topographical data obtained from both techniques.Expand Specific Solutions03 Data correlation algorithms and software solutions
Advanced computational methods are developed to accurately correlate and integrate data from Cryo-EM and AFM measurements. These include registration algorithms that align images from different modalities, feature extraction techniques that identify corresponding structures, and machine learning approaches that enhance pattern recognition across datasets. Specialized software platforms facilitate the visualization and analysis of combined datasets, enabling researchers to generate comprehensive 3D surface topography models with information from both techniques.Expand Specific Solutions04 Applications in biological structure analysis
The combined Cryo-EM and AFM approach has significant applications in biological structure analysis, particularly for membrane proteins, virus particles, and cellular components. This correlative approach provides insights into both the structural architecture and mechanical properties of biological systems. The integration of these techniques allows researchers to connect functional properties with structural features, enhancing understanding of biomolecular interactions, conformational changes, and disease-related structural alterations at the nanoscale level.Expand Specific Solutions05 Advancements in instrumentation for correlative microscopy
Recent technological advancements have led to the development of specialized instrumentation that facilitates the correlation between Cryo-EM and AFM data. These innovations include integrated microscopy platforms that allow for sequential or simultaneous measurements, environmental control systems that maintain sample integrity during transfers between instruments, and high-precision sample positioning systems. These instrumental developments minimize sample alterations between measurements and improve the accuracy of topographical correlations across techniques.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The field of combining Cryo-EM with AFM for surface topography correlation is currently in an emerging growth phase, characterized by significant academic research but limited commercial maturity. The market is expanding as researchers seek complementary techniques for comprehensive structural analysis, with an estimated global market size of $500-700 million. Leading academic institutions including University of Washington, Wisconsin Alumni Research Foundation, and Chinese Academy of Sciences are driving innovation, while commercial players like Bruker Nano are developing integrated instrumentation solutions. The technology remains primarily research-focused, with academic-industry partnerships forming to bridge the gap between fundamental research and practical applications in fields ranging from materials science to pharmaceutical development.
Huazhong University of Science & Technology
Technical Solution: Huazhong University has developed a correlative cryo-EM/AFM system that emphasizes high-throughput analysis for structural biology applications. Their approach utilizes automated grid handling systems that enable efficient transfer between imaging modalities while maintaining cryogenic conditions. The university has pioneered specialized AFM probes with extended tips that improve access to sample surfaces even when mounted on EM grids, addressing a key challenge in correlative imaging. Their methodology incorporates machine learning algorithms that identify corresponding regions between AFM topography maps and cryo-EM projections, facilitating automated data correlation. The university's system includes environmental control chambers that minimize ice contamination during transfers, preserving sample integrity throughout the workflow. Their data integration platform combines mechanical property measurements from AFM with structural information from cryo-EM, providing insights into structure-function relationships of macromolecular complexes[6][8]. The technology has been particularly effective for characterizing protein-protein and protein-nucleic acid interactions, where surface topography provides crucial contextual information for interpreting internal structures.
Strengths: High-throughput capabilities through automation; specialized probe designs for improved sample accessibility; advanced computational tools for data correlation. Weaknesses: Less established in the international research community compared to some competitors; primarily focused on biological applications; requires significant technical infrastructure.
University of Washington
Technical Solution: The University of Washington has developed an innovative correlative cryo-EM/AFM platform that addresses the challenge of sample registry between the two imaging modalities. Their approach utilizes fiducial markers embedded in specialized sample supports that remain visible in both imaging techniques, enabling precise alignment of datasets. The university's system incorporates a custom-designed cryo-transfer station that maintains samples below devitrification temperatures during transport between instruments. Their methodology includes initial low-dose cryo-EM imaging to identify regions of interest, followed by targeted AFM analysis of the same areas to extract topographical and mechanical information. The university has developed specialized software tools that compensate for the different contrast mechanisms and resolution limits of each technique, enabling accurate correlation of structural features[5][7]. Their approach has been particularly successful in characterizing viral capsid structures, where surface topography from AFM complements internal density maps from cryo-EM to provide comprehensive structural models.
Strengths: Innovative fiducial marker system for precise data alignment; specialized expertise in viral structure analysis; well-developed computational tools for data integration. Weaknesses: Limited commercial availability; requires significant technical expertise; specialized application focus may limit broader utility in materials science.
Key Patents and Publications in Correlative Microscopy
Automating cryo-electron microscopy data collection
PatentWO2024229329A1
Innovation
- A software pipeline utilizing machine learning models for automated navigation of cryo-EM grids, including square and hole localization, scoring, and on-the-fly learning, to determine high-quality targeting locations without human input, using pretrained models and active learning techniques like Gaussian Process regression.
Sample holder for scanning electron microscopy (SEM) and atomic force microscopy (AFM).
PatentActiveMX2015016988A
Innovation
- A specimen holder with a magnetizable and conductive flat plate, engraved orthogonal reference axes, and removable support pin, allowing simultaneous analysis in both microscopes without double-sided tape, ensuring compatibility and maintaining positional accuracy.
Sample Preparation Protocols for Dual-Modality Imaging
The successful integration of cryo-electron microscopy (cryo-EM) with atomic force microscopy (AFM) for surface topography correlation requires meticulously designed sample preparation protocols. These protocols must preserve the native state of biological specimens while ensuring compatibility with both imaging modalities.
For dual-modality imaging, samples must first be prepared on specialized grids that accommodate both techniques. Gold-coated EM grids with perforated carbon films have emerged as the preferred substrate, offering electrical conductivity for EM while providing sufficient stability for AFM cantilever engagement. The grid selection process must consider hole size, mesh density, and film thickness to optimize for both imaging methods.
Vitrification procedures require significant modification from standard cryo-EM protocols. The optimal approach involves controlled humidity chambers where samples are applied to grids in thin layers (typically 50-200 nm), followed by rapid plunge-freezing in liquid ethane cooled by liquid nitrogen. Critical parameters include blotting time (2-5 seconds), blotting force (typically 2-5 arbitrary units on commercial systems), and humidity levels (80-95%).
Sample transfer between instruments presents substantial challenges. Cryo-transfer systems have been developed that maintain samples below -150°C throughout the imaging workflow, preventing devitrification and ice crystal formation. Vacuum-sealed transfer chambers equipped with anti-contamination devices minimize exposure to atmospheric moisture during transitions between instruments.
Fiducial markers represent a crucial component of dual-modality protocols. Gold nanoparticles (typically 5-15 nm diameter) serve as reference points for correlating images between techniques. These markers must be applied before vitrification, with optimal concentration determined empirically (approximately 1010-1012 particles/mL) to provide sufficient reference points without obscuring biological features.
Chemical fixation alternatives have been developed for specimens unsuitable for vitrification. Glutaraldehyde (0.5-2%) combined with osmium tetroxide (1%) preservation maintains structural integrity while enabling room temperature AFM followed by negative staining for EM. This approach sacrifices some native state preservation but expands the range of compatible samples.
Quality control measures are essential throughout the preparation process. Test grids should be examined by light microscopy before vitrification to assess sample distribution. Post-vitrification, grid quality can be evaluated using test imaging in low-dose conditions before proceeding to full data collection, ensuring optimal ice thickness and sample distribution for both imaging modalities.
For dual-modality imaging, samples must first be prepared on specialized grids that accommodate both techniques. Gold-coated EM grids with perforated carbon films have emerged as the preferred substrate, offering electrical conductivity for EM while providing sufficient stability for AFM cantilever engagement. The grid selection process must consider hole size, mesh density, and film thickness to optimize for both imaging methods.
Vitrification procedures require significant modification from standard cryo-EM protocols. The optimal approach involves controlled humidity chambers where samples are applied to grids in thin layers (typically 50-200 nm), followed by rapid plunge-freezing in liquid ethane cooled by liquid nitrogen. Critical parameters include blotting time (2-5 seconds), blotting force (typically 2-5 arbitrary units on commercial systems), and humidity levels (80-95%).
Sample transfer between instruments presents substantial challenges. Cryo-transfer systems have been developed that maintain samples below -150°C throughout the imaging workflow, preventing devitrification and ice crystal formation. Vacuum-sealed transfer chambers equipped with anti-contamination devices minimize exposure to atmospheric moisture during transitions between instruments.
Fiducial markers represent a crucial component of dual-modality protocols. Gold nanoparticles (typically 5-15 nm diameter) serve as reference points for correlating images between techniques. These markers must be applied before vitrification, with optimal concentration determined empirically (approximately 1010-1012 particles/mL) to provide sufficient reference points without obscuring biological features.
Chemical fixation alternatives have been developed for specimens unsuitable for vitrification. Glutaraldehyde (0.5-2%) combined with osmium tetroxide (1%) preservation maintains structural integrity while enabling room temperature AFM followed by negative staining for EM. This approach sacrifices some native state preservation but expands the range of compatible samples.
Quality control measures are essential throughout the preparation process. Test grids should be examined by light microscopy before vitrification to assess sample distribution. Post-vitrification, grid quality can be evaluated using test imaging in low-dose conditions before proceeding to full data collection, ensuring optimal ice thickness and sample distribution for both imaging modalities.
Data Processing Algorithms for Multi-scale Image Registration
The integration of Cryo-EM and AFM data requires sophisticated image registration algorithms capable of handling multi-scale and multi-modal data. Current algorithms primarily focus on feature-based registration, intensity-based registration, and hybrid approaches that combine elements of both methodologies.
Feature-based registration algorithms identify distinctive landmarks in both Cryo-EM and AFM images, establishing correspondence between these points. Scale-Invariant Feature Transform (SIFT) and Speeded Up Robust Features (SURF) have been adapted specifically for correlating nanoscale structural features across these different imaging modalities. These algorithms demonstrate robustness to scale variations but often struggle with the significant resolution differences between Cryo-EM (typically 2-5Å) and AFM (nanometer range).
Intensity-based registration algorithms utilize mutual information metrics to align images without explicit feature extraction. These methods have proven particularly effective for multi-modal registration tasks where image characteristics differ substantially. Recent developments incorporate wavelet decomposition to handle the multi-scale nature of Cryo-EM and AFM data, enabling more precise correlation of surface topography information across resolution scales.
Deep learning approaches represent the cutting edge in multi-scale image registration for Cryo-EM and AFM correlation. Convolutional neural networks (CNNs) trained on paired datasets can learn optimal feature representations across modalities. U-Net architectures modified with attention mechanisms have demonstrated superior performance in handling the complex relationship between electron density maps and force-distance measurements.
Hierarchical registration frameworks address the scale disparity by progressively refining alignment from coarse to fine levels. These algorithms initially align low-resolution representations of both datasets before proceeding to higher-resolution details, effectively managing the computational complexity while maintaining registration accuracy.
Transformation models used in these algorithms have evolved from simple rigid transformations to more complex deformable models that can account for local distortions. B-spline transformations and thin-plate splines have proven particularly effective for modeling the non-linear deformations that may occur between Cryo-EM and AFM data acquisition.
Validation metrics for registration quality include mutual information, normalized cross-correlation, and target registration error. Recent work has introduced specialized metrics that account for the unique characteristics of molecular surface representations, providing more meaningful assessment of topographical correlation quality.
Feature-based registration algorithms identify distinctive landmarks in both Cryo-EM and AFM images, establishing correspondence between these points. Scale-Invariant Feature Transform (SIFT) and Speeded Up Robust Features (SURF) have been adapted specifically for correlating nanoscale structural features across these different imaging modalities. These algorithms demonstrate robustness to scale variations but often struggle with the significant resolution differences between Cryo-EM (typically 2-5Å) and AFM (nanometer range).
Intensity-based registration algorithms utilize mutual information metrics to align images without explicit feature extraction. These methods have proven particularly effective for multi-modal registration tasks where image characteristics differ substantially. Recent developments incorporate wavelet decomposition to handle the multi-scale nature of Cryo-EM and AFM data, enabling more precise correlation of surface topography information across resolution scales.
Deep learning approaches represent the cutting edge in multi-scale image registration for Cryo-EM and AFM correlation. Convolutional neural networks (CNNs) trained on paired datasets can learn optimal feature representations across modalities. U-Net architectures modified with attention mechanisms have demonstrated superior performance in handling the complex relationship between electron density maps and force-distance measurements.
Hierarchical registration frameworks address the scale disparity by progressively refining alignment from coarse to fine levels. These algorithms initially align low-resolution representations of both datasets before proceeding to higher-resolution details, effectively managing the computational complexity while maintaining registration accuracy.
Transformation models used in these algorithms have evolved from simple rigid transformations to more complex deformable models that can account for local distortions. B-spline transformations and thin-plate splines have proven particularly effective for modeling the non-linear deformations that may occur between Cryo-EM and AFM data acquisition.
Validation metrics for registration quality include mutual information, normalized cross-correlation, and target registration error. Recent work has introduced specialized metrics that account for the unique characteristics of molecular surface representations, providing more meaningful assessment of topographical correlation quality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!