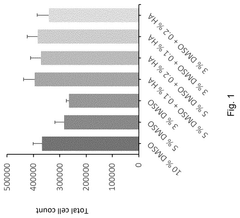
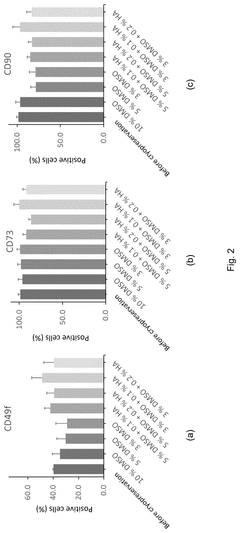
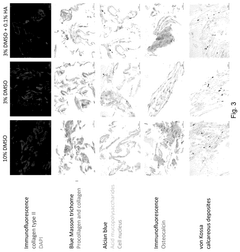
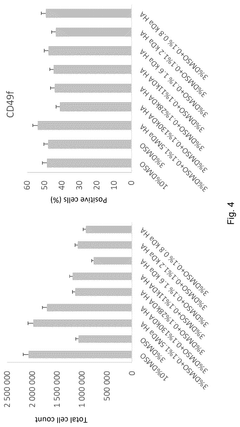
Alternatives to DMSO in cryopreservation: efficacy, toxicity and regulatory perspectives
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryopreservation Background and Objectives
Cryopreservation represents a critical technology in modern biomedical science and clinical applications, enabling the long-term storage of biological materials at ultra-low temperatures. The technique has evolved significantly since its inception in the 1950s when Polge, Smith, and Parkes discovered the cryoprotective properties of glycerol. This breakthrough fundamentally transformed our ability to preserve living cells and tissues, leading to applications across numerous fields including reproductive medicine, stem cell research, organ transplantation, and biodiversity conservation.
Dimethyl sulfoxide (DMSO) emerged in the 1960s as the gold standard cryoprotective agent (CPA) due to its exceptional ability to penetrate cell membranes and prevent intracellular ice crystal formation. For decades, DMSO at concentrations of 5-10% has dominated cryopreservation protocols across various biological specimens, from simple cell suspensions to complex tissues.
However, mounting evidence has revealed significant limitations of DMSO, particularly concerning its cytotoxicity, adverse effects on cellular differentiation pathways, and clinical side effects including nausea, headaches, cardiovascular complications, and in rare cases, anaphylactic reactions. These concerns have intensified with the expansion of cell therapy applications, where directly infused DMSO-preserved products can trigger serious adverse events in patients.
The regulatory landscape surrounding cryopreservation has also evolved substantially. Regulatory bodies including the FDA, EMA, and international standards organizations have implemented increasingly stringent requirements for cryopreservation processes, particularly for clinical applications. These regulations emphasize the need for well-characterized, non-toxic preservation methods that maintain cellular functionality and genetic stability.
The primary objective of this technical research is to comprehensively evaluate alternative cryoprotective agents and strategies that could potentially replace or reduce DMSO usage while maintaining or improving preservation efficacy. We aim to assess these alternatives through multiple critical lenses: cryoprotective efficacy across different cell types and tissues, cytotoxicity profiles and safety considerations, compatibility with existing cryopreservation infrastructure, scalability for industrial applications, and alignment with current and anticipated regulatory frameworks.
Additionally, this research seeks to identify promising technological trajectories in the field, including combination approaches using multiple cryoprotectants, novel delivery systems for CPAs, and advanced vitrification techniques that minimize or eliminate the need for penetrating cryoprotectants altogether. By mapping the current technological landscape and future directions, this report will provide strategic guidance for research investment and product development in next-generation cryopreservation technologies.
Dimethyl sulfoxide (DMSO) emerged in the 1960s as the gold standard cryoprotective agent (CPA) due to its exceptional ability to penetrate cell membranes and prevent intracellular ice crystal formation. For decades, DMSO at concentrations of 5-10% has dominated cryopreservation protocols across various biological specimens, from simple cell suspensions to complex tissues.
However, mounting evidence has revealed significant limitations of DMSO, particularly concerning its cytotoxicity, adverse effects on cellular differentiation pathways, and clinical side effects including nausea, headaches, cardiovascular complications, and in rare cases, anaphylactic reactions. These concerns have intensified with the expansion of cell therapy applications, where directly infused DMSO-preserved products can trigger serious adverse events in patients.
The regulatory landscape surrounding cryopreservation has also evolved substantially. Regulatory bodies including the FDA, EMA, and international standards organizations have implemented increasingly stringent requirements for cryopreservation processes, particularly for clinical applications. These regulations emphasize the need for well-characterized, non-toxic preservation methods that maintain cellular functionality and genetic stability.
The primary objective of this technical research is to comprehensively evaluate alternative cryoprotective agents and strategies that could potentially replace or reduce DMSO usage while maintaining or improving preservation efficacy. We aim to assess these alternatives through multiple critical lenses: cryoprotective efficacy across different cell types and tissues, cytotoxicity profiles and safety considerations, compatibility with existing cryopreservation infrastructure, scalability for industrial applications, and alignment with current and anticipated regulatory frameworks.
Additionally, this research seeks to identify promising technological trajectories in the field, including combination approaches using multiple cryoprotectants, novel delivery systems for CPAs, and advanced vitrification techniques that minimize or eliminate the need for penetrating cryoprotectants altogether. By mapping the current technological landscape and future directions, this report will provide strategic guidance for research investment and product development in next-generation cryopreservation technologies.
Market Analysis of DMSO Alternatives
The global market for cryopreservation media is experiencing significant growth, with the DMSO alternatives segment emerging as a particularly dynamic sector. Current market valuations place the overall cryopreservation media market at approximately 2.1 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 8.7% through 2030. Within this broader market, DMSO alternatives are gaining traction, currently representing about 15% of the total market share with expectations to reach 25-30% by 2028.
The primary market drivers for DMSO alternatives include increasing concerns about DMSO toxicity in clinical applications, growing regulatory scrutiny, and rising demand for higher cell viability rates in cell therapy manufacturing. The cell therapy sector, valued at 14.5 billion USD in 2023, is particularly influential in driving innovation in cryopreservation technologies, as these therapies require effective preservation methods that maintain cell functionality without introducing toxicity.
Geographically, North America dominates the market for DMSO alternatives with approximately 42% market share, followed by Europe (31%) and Asia-Pacific (21%). The Asia-Pacific region, however, is demonstrating the fastest growth rate at 11.3% annually, primarily due to expanding biotechnology sectors in China, Japan, and South Korea.
By application segment, stem cell banking represents the largest market for DMSO alternatives (38%), followed by cell therapy manufacturing (27%), IVF clinics (18%), and research applications (17%). The stem cell banking segment is particularly sensitive to safety concerns, making it a prime target for DMSO alternative adoption.
Key customer segments include pharmaceutical and biotechnology companies, academic and research institutions, cell banks, and fertility clinics. Among these, pharmaceutical companies are the highest-value customers, willing to pay premium prices for solutions that enhance product quality and regulatory compliance.
Price sensitivity varies significantly across market segments. While research institutions remain highly price-sensitive, commercial cell therapy manufacturers demonstrate lower price sensitivity when alternatives can demonstrate superior performance metrics or regulatory advantages. Current pricing for DMSO alternatives ranges from 2-5 times higher than conventional DMSO-based solutions, presenting both a barrier to adoption and an opportunity for cost innovation.
Market penetration of DMSO alternatives remains relatively low at 15-20% of potential applications, indicating substantial room for growth as technologies mature and cost barriers are addressed. The most successful market entrants will likely be those that can demonstrate clear regulatory advantages, reduced toxicity profiles, and comparable or superior cell viability outcomes compared to DMSO-based solutions.
The primary market drivers for DMSO alternatives include increasing concerns about DMSO toxicity in clinical applications, growing regulatory scrutiny, and rising demand for higher cell viability rates in cell therapy manufacturing. The cell therapy sector, valued at 14.5 billion USD in 2023, is particularly influential in driving innovation in cryopreservation technologies, as these therapies require effective preservation methods that maintain cell functionality without introducing toxicity.
Geographically, North America dominates the market for DMSO alternatives with approximately 42% market share, followed by Europe (31%) and Asia-Pacific (21%). The Asia-Pacific region, however, is demonstrating the fastest growth rate at 11.3% annually, primarily due to expanding biotechnology sectors in China, Japan, and South Korea.
By application segment, stem cell banking represents the largest market for DMSO alternatives (38%), followed by cell therapy manufacturing (27%), IVF clinics (18%), and research applications (17%). The stem cell banking segment is particularly sensitive to safety concerns, making it a prime target for DMSO alternative adoption.
Key customer segments include pharmaceutical and biotechnology companies, academic and research institutions, cell banks, and fertility clinics. Among these, pharmaceutical companies are the highest-value customers, willing to pay premium prices for solutions that enhance product quality and regulatory compliance.
Price sensitivity varies significantly across market segments. While research institutions remain highly price-sensitive, commercial cell therapy manufacturers demonstrate lower price sensitivity when alternatives can demonstrate superior performance metrics or regulatory advantages. Current pricing for DMSO alternatives ranges from 2-5 times higher than conventional DMSO-based solutions, presenting both a barrier to adoption and an opportunity for cost innovation.
Market penetration of DMSO alternatives remains relatively low at 15-20% of potential applications, indicating substantial room for growth as technologies mature and cost barriers are addressed. The most successful market entrants will likely be those that can demonstrate clear regulatory advantages, reduced toxicity profiles, and comparable or superior cell viability outcomes compared to DMSO-based solutions.
Current Challenges in Cryoprotectant Development
Despite significant advancements in cryopreservation technology, the field faces several critical challenges that impede broader clinical and commercial applications. The most pressing issue remains the continued reliance on dimethyl sulfoxide (DMSO) as the primary cryoprotective agent (CPA), despite its well-documented cytotoxicity, adverse reactions in patients, and negative effects on cellular functionality post-thaw.
Current alternative CPAs demonstrate insufficient protective efficacy compared to DMSO, particularly for complex biological materials such as stem cells, engineered tissues, and certain primary cell types. Many promising alternatives work effectively only at concentrations that introduce their own toxicity concerns, creating a persistent efficacy-toxicity paradox that researchers struggle to resolve.
The mechanisms of cryoinjury and cryoprotection remain incompletely understood at the molecular level, hampering rational design approaches for next-generation cryoprotectants. This knowledge gap is particularly evident in understanding how different cell types respond to various cryoprotective formulations, leading to empirical rather than systematic development processes.
Scalability presents another significant hurdle, as many laboratory-proven alternatives fail to maintain their effectiveness when scaled to industrial volumes required for biobanking or commercial cell therapy manufacturing. This scale-up challenge is compounded by the need for specialized equipment and protocols that differ from established DMSO-based methods.
Regulatory frameworks worldwide lack standardized approaches for evaluating novel cryoprotectants, creating uncertainty in development pathways. The FDA and EMA have established guidelines for cell-based products but offer limited specific guidance on alternative cryoprotectants, resulting in case-by-case evaluations that extend development timelines and increase costs.
Economic factors further complicate advancement, as developing and validating new cryoprotectants requires substantial investment with uncertain returns. The entrenched position of DMSO in established protocols creates market inertia that new solutions must overcome.
Formulation complexity represents another challenge, as effective alternatives often require precise combinations of multiple compounds working synergistically. These complex formulations introduce additional variables in manufacturing, quality control, and regulatory approval processes.
The field also struggles with reproducibility issues, as cryopreservation outcomes can vary significantly between laboratories using seemingly identical protocols, highlighting the need for more robust standardization in both research and clinical applications.
Current alternative CPAs demonstrate insufficient protective efficacy compared to DMSO, particularly for complex biological materials such as stem cells, engineered tissues, and certain primary cell types. Many promising alternatives work effectively only at concentrations that introduce their own toxicity concerns, creating a persistent efficacy-toxicity paradox that researchers struggle to resolve.
The mechanisms of cryoinjury and cryoprotection remain incompletely understood at the molecular level, hampering rational design approaches for next-generation cryoprotectants. This knowledge gap is particularly evident in understanding how different cell types respond to various cryoprotective formulations, leading to empirical rather than systematic development processes.
Scalability presents another significant hurdle, as many laboratory-proven alternatives fail to maintain their effectiveness when scaled to industrial volumes required for biobanking or commercial cell therapy manufacturing. This scale-up challenge is compounded by the need for specialized equipment and protocols that differ from established DMSO-based methods.
Regulatory frameworks worldwide lack standardized approaches for evaluating novel cryoprotectants, creating uncertainty in development pathways. The FDA and EMA have established guidelines for cell-based products but offer limited specific guidance on alternative cryoprotectants, resulting in case-by-case evaluations that extend development timelines and increase costs.
Economic factors further complicate advancement, as developing and validating new cryoprotectants requires substantial investment with uncertain returns. The entrenched position of DMSO in established protocols creates market inertia that new solutions must overcome.
Formulation complexity represents another challenge, as effective alternatives often require precise combinations of multiple compounds working synergistically. These complex formulations introduce additional variables in manufacturing, quality control, and regulatory approval processes.
The field also struggles with reproducibility issues, as cryopreservation outcomes can vary significantly between laboratories using seemingly identical protocols, highlighting the need for more robust standardization in both research and clinical applications.
Comparative Analysis of Current Cryoprotectant Solutions
01 Natural solvent alternatives to DMSO
Various natural solvents can serve as alternatives to DMSO with potentially lower toxicity profiles. These include plant-derived compounds, essential oils, and other naturally occurring substances that demonstrate similar penetration enhancement capabilities while offering improved safety profiles. These natural alternatives can facilitate the delivery of active ingredients through biological membranes while reducing the risk of adverse effects associated with DMSO.- Natural solvent alternatives to DMSO: Various natural solvents have been identified as potential alternatives to DMSO with lower toxicity profiles. These include plant-derived compounds, essential oils, and other biocompatible solvents that can enhance penetration of active ingredients through biological membranes. These natural alternatives often demonstrate comparable efficacy to DMSO while presenting fewer safety concerns and reduced irritation potential, making them suitable for pharmaceutical and cosmetic applications.
- Synthetic penetration enhancers as DMSO replacements: Novel synthetic compounds have been developed specifically to replace DMSO as penetration enhancers. These include modified polymers, specialized surfactants, and synthetic carrier molecules designed to improve transdermal and cellular delivery of active ingredients. These alternatives offer controlled release properties, targeted delivery capabilities, and reduced cytotoxicity compared to DMSO while maintaining or improving efficacy in various applications.
- Formulation techniques to reduce DMSO concentration: Advanced formulation approaches have been developed to minimize DMSO concentration while maintaining efficacy. These include microemulsion systems, nanoparticle carriers, liposomal formulations, and other delivery technologies that enhance penetration and bioavailability of active ingredients. By combining DMSO with these technologies or partially substituting it, the overall toxicity can be reduced while preserving or enhancing the desired therapeutic effects.
- Comparative toxicity and efficacy assessment methods: Novel methodologies have been developed to evaluate and compare the toxicity and efficacy profiles of DMSO alternatives. These include advanced in vitro cell culture systems, organoid models, computational prediction tools, and specialized analytical techniques that can accurately measure penetration enhancement, cellular toxicity, and biological activity. These assessment methods enable more precise selection of appropriate DMSO alternatives for specific applications based on their safety and performance characteristics.
- Application-specific DMSO alternatives: Specialized alternatives to DMSO have been developed for specific applications such as cryopreservation, drug delivery systems, and diagnostic reagents. These alternatives are tailored to the particular requirements of each application, considering factors such as temperature stability, compatibility with specific active ingredients, and interaction with biological systems. By optimizing the alternative for a specific use case, both efficacy can be maximized and toxicity minimized compared to general-purpose DMSO.
02 Synthetic penetration enhancers as DMSO replacements
Several synthetic compounds have been developed as alternatives to DMSO for enhancing penetration of active ingredients. These include modified polymers, surfactants, and other synthetic molecules designed to improve cellular uptake and distribution of therapeutic agents. These compounds aim to match or exceed DMSO's efficacy in facilitating substance transport across biological barriers while demonstrating reduced cytotoxicity and improved biocompatibility.Expand Specific Solutions03 Formulation techniques to reduce DMSO concentration
Advanced formulation approaches can reduce the required concentration of DMSO in pharmaceutical and research applications. These techniques include microemulsions, liposomes, nanoparticles, and other delivery systems that enhance the efficacy of reduced DMSO concentrations or enable its partial replacement. By optimizing formulation parameters, researchers can maintain therapeutic efficacy while minimizing potential toxicity concerns associated with higher DMSO concentrations.Expand Specific Solutions04 Comparative toxicity and efficacy assessment methods
Various analytical and experimental methods have been developed to evaluate and compare the toxicity and efficacy profiles of DMSO alternatives. These include in vitro cell-based assays, molecular modeling techniques, and advanced analytical methods that can predict or measure penetration enhancement, cellular toxicity, and biological activity. These assessment tools help researchers identify safer alternatives to DMSO while ensuring comparable performance in relevant applications.Expand Specific Solutions05 Application-specific DMSO alternatives
Different applications require specialized alternatives to DMSO based on the specific requirements of the system. For cryopreservation, cell culture, drug delivery, and diagnostic applications, researchers have developed targeted alternatives with optimized properties for each use case. These application-specific alternatives consider factors such as cell type compatibility, temperature stability, interaction with active ingredients, and regulatory considerations to provide safer and more effective options than DMSO in specialized contexts.Expand Specific Solutions
Leading Organizations in Cryopreservation Research
The cryopreservation alternatives to DMSO market is currently in a growth phase, with increasing research focus driven by concerns about DMSO toxicity and regulatory constraints. The global biopreservation market, valued at approximately $3 billion, is expected to expand significantly as cell therapy applications grow. Academic institutions like Peking University Third Hospital, Chinese Academy of Science Institute of Chemistry, and Osaka University are leading fundamental research, while companies such as Cellphire, Life Science Group, and Colossal Biosciences are commercializing novel cryoprotectants. Technical maturity varies across solutions, with natural polymers (developed by Contipro), protein-based alternatives (ActualEyes), and synthetic compounds (Saraya Co.) at different development stages. Regulatory acceptance remains a key challenge, with Japan Science & Technology Agency and similar bodies working to establish standards for these emerging technologies.
Lonza Ltd.
Technical Solution: Lonza has developed a comprehensive portfolio of DMSO alternatives for cryopreservation, focusing on their proprietary CryoStor® and HypoThermosol® formulations. These solutions utilize novel combinations of sugars, polymers, and antioxidants to replace DMSO while maintaining cell viability. Their technology employs specialized buffer systems that mitigate osmotic stress during freezing and thawing processes. Lonza's approach incorporates protein-free formulations that reduce immunogenic responses in clinical applications, particularly important for cell therapies. Their research has demonstrated comparable or superior post-thaw recovery rates (>80%) for various cell types including stem cells and primary cells when compared to traditional DMSO-based methods[1]. The company has also developed specialized protocols for controlled-rate freezing that optimize the performance of their DMSO-alternative solutions.
Strengths: Established regulatory approval pathway with FDA master files supporting their formulations; extensive validation across multiple cell types; reduced toxicity profile compared to DMSO. Weaknesses: Higher cost compared to traditional DMSO methods; may require specialized equipment for optimal results; performance varies depending on specific cell types.
Life Science Group Ltd.
Technical Solution: Life Science Group has developed BioFreeze™, a comprehensive range of DMSO-alternative cryopreservation media specifically designed for different cell types and applications. Their technology utilizes a proprietary blend of sugars, polymers, and amino acids that provide cryoprotection without DMSO's cytotoxicity. The company's approach incorporates specialized osmolytes that maintain cellular osmotic balance during freezing and thawing processes. Their research has demonstrated that their formulations achieve post-thaw viability rates comparable to DMSO (typically 80-85%) while significantly reducing cytotoxic effects and differentiation impacts on sensitive cell types[4]. Life Science Group has also developed specialized protocols for different freezing rates optimized for their DMSO-alternative solutions. Their technology includes novel protein stabilizers that prevent denaturation during the freeze-thaw cycle, particularly important for preserving enzymatic activity in cell therapy products. The company has established comprehensive quality control systems that ensure batch-to-batch consistency of their cryopreservation solutions, critical for clinical applications.
Strengths: Customized formulations for different cell types; reduced cytotoxicity compared to DMSO; compatible with standard freezing equipment; established quality management system supporting GMP applications. Weaknesses: Variable performance across different cell types; requires optimization for each specific application; limited long-term storage data compared to traditional DMSO methods; higher cost for specialized formulations.
Key Innovations in Non-DMSO Cryoprotectants
Cryopreservation formulations
PatentWO2025019671A1
Innovation
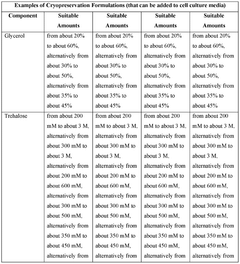
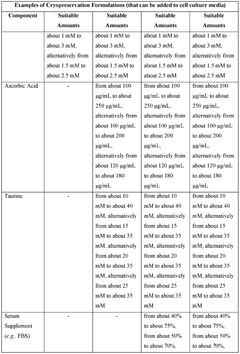
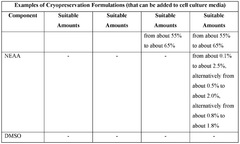
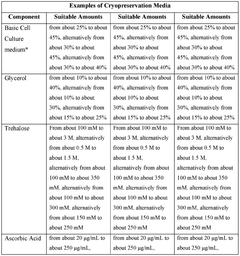
- The development of cryopreservation formulations and media that utilize a combination of cryoprotective agents such as glycerol, trehalose, ascorbic acid, and taurine, without DMSO, to effectively preserve mammalian cells and tissues.
Cryopreservation medium comprising hyaluronic acid, use thereof and method of cryopreservation
PatentPendingUS20250215400A1
Innovation
- A cryopreservation medium comprising native hyaluronic acid with a molecular weight range of 1,000,000 to 2,200,000 g/mol and a concentration of 0.08 to 0.2% (w/v), combined with reduced DMSO concentration (3-5% v/v), is used to preserve stem cells, maintaining their proliferation capacity and pluripotency.
Toxicological Assessment Frameworks
The assessment of toxicological risks associated with cryoprotective agents requires robust frameworks that can systematically evaluate potential hazards. Current toxicological assessment frameworks for cryopreservation agents like DMSO and its alternatives typically follow a tiered approach, beginning with in vitro cytotoxicity screening followed by more complex in vivo studies when warranted.
Standard toxicity testing protocols established by organizations such as the Organization for Economic Cooperation and Development (OECD) and the International Conference on Harmonization (ICH) provide structured guidelines for evaluating acute, sub-chronic, and chronic toxicity. These frameworks have been adapted specifically for cryoprotectants to address their unique exposure scenarios and mechanisms of action.
For alternatives to DMSO, toxicological assessments must consider both direct cellular toxicity and systemic effects. This includes evaluation of membrane integrity, metabolic function, genotoxicity, and immunogenicity. The frameworks typically incorporate dose-response relationships and exposure duration as critical parameters, recognizing that cryoprotectant toxicity often depends on concentration and contact time.
Regulatory bodies including the FDA, EMA, and PMDA have established specific requirements for toxicological data packages needed for cryopreservation agents used in clinical applications. These frameworks emphasize the importance of understanding the toxicokinetics of these compounds, including absorption, distribution, metabolism, and excretion profiles.
Modern toxicological assessment frameworks increasingly incorporate advanced methodologies such as high-throughput screening, toxicogenomics, and computational toxicology to predict potential adverse effects. These approaches are particularly valuable for evaluating novel cryoprotective agents where historical safety data may be limited.
Risk assessment matrices that consider both hazard identification and exposure assessment have been developed specifically for cryopreservation applications. These frameworks help quantify the margin of safety between effective cryoprotective concentrations and toxic thresholds, enabling rational selection of alternatives to DMSO.
The toxicological assessment of cryoprotectants must also consider their potential interactions with biological materials being preserved. This includes evaluating potential chemical modifications to proteins, nucleic acids, and cellular structures that might compromise the functionality of preserved cells or tissues upon thawing.
Standard toxicity testing protocols established by organizations such as the Organization for Economic Cooperation and Development (OECD) and the International Conference on Harmonization (ICH) provide structured guidelines for evaluating acute, sub-chronic, and chronic toxicity. These frameworks have been adapted specifically for cryoprotectants to address their unique exposure scenarios and mechanisms of action.
For alternatives to DMSO, toxicological assessments must consider both direct cellular toxicity and systemic effects. This includes evaluation of membrane integrity, metabolic function, genotoxicity, and immunogenicity. The frameworks typically incorporate dose-response relationships and exposure duration as critical parameters, recognizing that cryoprotectant toxicity often depends on concentration and contact time.
Regulatory bodies including the FDA, EMA, and PMDA have established specific requirements for toxicological data packages needed for cryopreservation agents used in clinical applications. These frameworks emphasize the importance of understanding the toxicokinetics of these compounds, including absorption, distribution, metabolism, and excretion profiles.
Modern toxicological assessment frameworks increasingly incorporate advanced methodologies such as high-throughput screening, toxicogenomics, and computational toxicology to predict potential adverse effects. These approaches are particularly valuable for evaluating novel cryoprotective agents where historical safety data may be limited.
Risk assessment matrices that consider both hazard identification and exposure assessment have been developed specifically for cryopreservation applications. These frameworks help quantify the margin of safety between effective cryoprotective concentrations and toxic thresholds, enabling rational selection of alternatives to DMSO.
The toxicological assessment of cryoprotectants must also consider their potential interactions with biological materials being preserved. This includes evaluating potential chemical modifications to proteins, nucleic acids, and cellular structures that might compromise the functionality of preserved cells or tissues upon thawing.
Regulatory Pathway for Novel Cryoprotectants
The regulatory pathway for novel cryoprotectants represents a complex and multi-faceted process that requires careful navigation through various regulatory frameworks across different jurisdictions. For alternatives to DMSO seeking market approval, understanding these pathways is crucial for successful commercialization and clinical implementation.
In the United States, the FDA categorizes cryoprotectants based on their intended use. Novel cryoprotectants for cell and tissue preservation typically fall under the regulation of the Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on their specific application. The regulatory process generally begins with preclinical testing, followed by an Investigational New Drug (IND) application for clinical trials, and culminates in a Biologics License Application (BLA) or Premarket Approval (PMA).
European regulatory pathways differ significantly, with the European Medicines Agency (EMA) overseeing novel cryoprotectants through the Advanced Therapy Medicinal Products (ATMP) framework when used in cell therapies. The process involves scientific advice consultation, clinical trials authorization, and marketing authorization application. The EMA has established the Committee for Advanced Therapies (CAT) specifically to evaluate these products.
Japan has implemented an expedited approval system through the Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approval for regenerative medicine products after demonstrating safety and probable benefit, which could accelerate the adoption of novel cryoprotectants in certain applications.
Key regulatory considerations for novel cryoprotectants include toxicity profiling, residual presence in final products, impact on cell functionality, and manufacturing consistency. Regulatory bodies increasingly require comprehensive data on the mechanism of action, dose-dependent effects, and potential long-term consequences of exposure to these compounds.
Recent regulatory trends indicate a move toward harmonization of requirements across major markets, with initiatives like the International Council for Harmonisation (ICH) working to standardize testing protocols and safety assessments. This harmonization aims to reduce redundant testing and accelerate global availability of innovative preservation solutions.
For developers of DMSO alternatives, early engagement with regulatory authorities through programs like the FDA's Breakthrough Therapy designation or the EMA's PRIME (Priority Medicines) scheme can provide valuable guidance and potentially expedite the approval process for promising cryoprotectants that address significant unmet needs in biopreservation.
In the United States, the FDA categorizes cryoprotectants based on their intended use. Novel cryoprotectants for cell and tissue preservation typically fall under the regulation of the Center for Biologics Evaluation and Research (CBER) or the Center for Devices and Radiological Health (CDRH), depending on their specific application. The regulatory process generally begins with preclinical testing, followed by an Investigational New Drug (IND) application for clinical trials, and culminates in a Biologics License Application (BLA) or Premarket Approval (PMA).
European regulatory pathways differ significantly, with the European Medicines Agency (EMA) overseeing novel cryoprotectants through the Advanced Therapy Medicinal Products (ATMP) framework when used in cell therapies. The process involves scientific advice consultation, clinical trials authorization, and marketing authorization application. The EMA has established the Committee for Advanced Therapies (CAT) specifically to evaluate these products.
Japan has implemented an expedited approval system through the Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approval for regenerative medicine products after demonstrating safety and probable benefit, which could accelerate the adoption of novel cryoprotectants in certain applications.
Key regulatory considerations for novel cryoprotectants include toxicity profiling, residual presence in final products, impact on cell functionality, and manufacturing consistency. Regulatory bodies increasingly require comprehensive data on the mechanism of action, dose-dependent effects, and potential long-term consequences of exposure to these compounds.
Recent regulatory trends indicate a move toward harmonization of requirements across major markets, with initiatives like the International Council for Harmonisation (ICH) working to standardize testing protocols and safety assessments. This harmonization aims to reduce redundant testing and accelerate global availability of innovative preservation solutions.
For developers of DMSO alternatives, early engagement with regulatory authorities through programs like the FDA's Breakthrough Therapy designation or the EMA's PRIME (Priority Medicines) scheme can provide valuable guidance and potentially expedite the approval process for promising cryoprotectants that address significant unmet needs in biopreservation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!