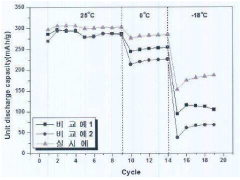
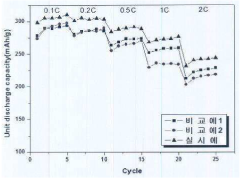
Analysis of Hydrogen storage materials for interface and surface modifications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogen Storage Materials Background and Objectives
Hydrogen storage has emerged as a critical component in the global transition towards sustainable energy systems. The history of hydrogen storage materials research dates back to the 1970s energy crisis, which prompted initial investigations into metal hydrides. Over subsequent decades, research has expanded to encompass various storage mechanisms including physical adsorption, chemical bonding, and hybrid approaches, with particular attention to interface and surface modifications as key performance determinants.
The evolution of hydrogen storage technology has been marked by significant breakthroughs in material science, particularly in understanding the fundamental mechanisms of hydrogen-material interactions at interfaces. Early materials suffered from limitations in storage capacity, kinetics, and cyclability, driving researchers to explore surface modifications as a strategy to overcome these barriers. The U.S. Department of Energy has established progressive targets for hydrogen storage systems, aiming for 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity by 2025, which continue to guide research directions.
Current technological objectives focus on developing materials that can store hydrogen safely at moderate temperatures and pressures while achieving rapid absorption/desorption kinetics. Interface and surface modifications represent a promising approach to enhance these properties without fundamentally altering the bulk material characteristics. These modifications can create preferential pathways for hydrogen diffusion, reduce activation barriers, and stabilize the stored hydrogen through tailored binding energies.
The scientific community has identified several promising material classes, including complex hydrides, metal-organic frameworks (MOFs), and nanostructured carbon materials. Each presents unique interface characteristics that can be engineered to optimize hydrogen storage performance. Recent advances in atomic layer deposition, plasma treatment, and catalyst doping have demonstrated significant improvements in hydrogen uptake and release kinetics through controlled surface modifications.
Looking forward, research objectives include developing multifunctional interfaces that can simultaneously address multiple performance limitations, creating hierarchical structures with optimized surface-to-volume ratios, and designing self-healing surfaces that maintain performance over thousands of cycles. Computational modeling and high-throughput screening approaches are increasingly employed to predict optimal interface compositions and structures before experimental validation.
The ultimate goal remains the development of hydrogen storage materials that enable practical, cost-effective hydrogen-based energy systems for transportation, grid storage, and portable applications. Surface and interface engineering represents one of the most promising pathways to achieve the performance metrics necessary for widespread commercial adoption of hydrogen technologies, potentially unlocking hydrogen's role as a universal energy carrier in a carbon-neutral future.
The evolution of hydrogen storage technology has been marked by significant breakthroughs in material science, particularly in understanding the fundamental mechanisms of hydrogen-material interactions at interfaces. Early materials suffered from limitations in storage capacity, kinetics, and cyclability, driving researchers to explore surface modifications as a strategy to overcome these barriers. The U.S. Department of Energy has established progressive targets for hydrogen storage systems, aiming for 6.5 wt% system gravimetric capacity and 50 g/L volumetric capacity by 2025, which continue to guide research directions.
Current technological objectives focus on developing materials that can store hydrogen safely at moderate temperatures and pressures while achieving rapid absorption/desorption kinetics. Interface and surface modifications represent a promising approach to enhance these properties without fundamentally altering the bulk material characteristics. These modifications can create preferential pathways for hydrogen diffusion, reduce activation barriers, and stabilize the stored hydrogen through tailored binding energies.
The scientific community has identified several promising material classes, including complex hydrides, metal-organic frameworks (MOFs), and nanostructured carbon materials. Each presents unique interface characteristics that can be engineered to optimize hydrogen storage performance. Recent advances in atomic layer deposition, plasma treatment, and catalyst doping have demonstrated significant improvements in hydrogen uptake and release kinetics through controlled surface modifications.
Looking forward, research objectives include developing multifunctional interfaces that can simultaneously address multiple performance limitations, creating hierarchical structures with optimized surface-to-volume ratios, and designing self-healing surfaces that maintain performance over thousands of cycles. Computational modeling and high-throughput screening approaches are increasingly employed to predict optimal interface compositions and structures before experimental validation.
The ultimate goal remains the development of hydrogen storage materials that enable practical, cost-effective hydrogen-based energy systems for transportation, grid storage, and portable applications. Surface and interface engineering represents one of the most promising pathways to achieve the performance metrics necessary for widespread commercial adoption of hydrogen technologies, potentially unlocking hydrogen's role as a universal energy carrier in a carbon-neutral future.
Market Analysis for Hydrogen Storage Solutions
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030. This growth trajectory is primarily fueled by governmental commitments to carbon neutrality and substantial investments in hydrogen infrastructure worldwide.
The demand for advanced hydrogen storage materials, particularly those with enhanced interface and surface modifications, is escalating across multiple sectors. The transportation industry represents the largest market segment, accounting for roughly 35% of the total market share. This is largely due to the automotive sector's pivot toward hydrogen fuel cell vehicles (FCVs) as a viable alternative to traditional combustion engines and battery electric vehicles for certain applications.
Industrial applications constitute the second-largest market segment at 28%, where hydrogen is increasingly utilized for various processes including metal refining, glass production, and semiconductor manufacturing. The stationary power generation sector follows at 22%, with growing implementation of hydrogen fuel cells for backup power systems and off-grid electricity generation.
Geographically, Asia-Pacific dominates the market with approximately 40% share, led by Japan, South Korea, and China's aggressive hydrogen economy initiatives. Europe follows closely at 35%, driven by stringent environmental regulations and substantial investments in hydrogen infrastructure. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
The market for surface-modified hydrogen storage materials is particularly promising, with specialized materials showing a premium growth rate of 12.3% annually. This sub-segment is valued for its ability to address key challenges in hydrogen storage, including capacity, safety, and efficiency.
Customer requirements are evolving toward materials that offer higher gravimetric and volumetric storage capacities, improved cycling stability, faster kinetics, and enhanced safety profiles. There is a growing preference for solutions that can operate under moderate temperature and pressure conditions, reducing overall system complexity and cost.
Price sensitivity varies significantly by application sector. While industrial users prioritize long-term reliability and total cost of ownership, the transportation sector remains highly price-sensitive due to competitive pressures from alternative technologies. The current price range for advanced hydrogen storage systems spans from $500-1,500 per kilogram of hydrogen stored, depending on the technology employed and scale of implementation.
The demand for advanced hydrogen storage materials, particularly those with enhanced interface and surface modifications, is escalating across multiple sectors. The transportation industry represents the largest market segment, accounting for roughly 35% of the total market share. This is largely due to the automotive sector's pivot toward hydrogen fuel cell vehicles (FCVs) as a viable alternative to traditional combustion engines and battery electric vehicles for certain applications.
Industrial applications constitute the second-largest market segment at 28%, where hydrogen is increasingly utilized for various processes including metal refining, glass production, and semiconductor manufacturing. The stationary power generation sector follows at 22%, with growing implementation of hydrogen fuel cells for backup power systems and off-grid electricity generation.
Geographically, Asia-Pacific dominates the market with approximately 40% share, led by Japan, South Korea, and China's aggressive hydrogen economy initiatives. Europe follows closely at 35%, driven by stringent environmental regulations and substantial investments in hydrogen infrastructure. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
The market for surface-modified hydrogen storage materials is particularly promising, with specialized materials showing a premium growth rate of 12.3% annually. This sub-segment is valued for its ability to address key challenges in hydrogen storage, including capacity, safety, and efficiency.
Customer requirements are evolving toward materials that offer higher gravimetric and volumetric storage capacities, improved cycling stability, faster kinetics, and enhanced safety profiles. There is a growing preference for solutions that can operate under moderate temperature and pressure conditions, reducing overall system complexity and cost.
Price sensitivity varies significantly by application sector. While industrial users prioritize long-term reliability and total cost of ownership, the transportation sector remains highly price-sensitive due to competitive pressures from alternative technologies. The current price range for advanced hydrogen storage systems spans from $500-1,500 per kilogram of hydrogen stored, depending on the technology employed and scale of implementation.
Current Challenges in Interface and Surface Modifications
Despite significant advancements in hydrogen storage materials, several critical challenges persist in the domain of interface and surface modifications. The primary obstacle remains the inherent trade-off between hydrogen binding energy and kinetics. Materials exhibiting strong hydrogen binding often demonstrate poor release kinetics, while those with favorable kinetics typically suffer from inadequate storage capacity. This fundamental contradiction has impeded the development of commercially viable hydrogen storage solutions.
Surface contamination presents another significant challenge. Even minimal exposure to air or moisture can lead to oxidation or poisoning of active sites on modified surfaces, drastically reducing hydrogen uptake capacity. Current passivation techniques often compromise the material's storage properties, creating an unresolved engineering dilemma.
The scalability of surface modification techniques constitutes a major hurdle for industrial implementation. Laboratory-scale methods such as atomic layer deposition and plasma treatment yield excellent results but face significant barriers when scaled to production volumes. The uniformity of surface treatment across large material batches remains inconsistent, resulting in unpredictable performance variations in real-world applications.
Characterization limitations further complicate progress in this field. Current analytical techniques struggle to provide real-time, in-situ monitoring of hydrogen-surface interactions at the atomic level. This knowledge gap hinders researchers' ability to optimize interface designs based on empirical data rather than theoretical models.
The stability of modified surfaces under cycling conditions represents another critical challenge. Most surface-modified materials exhibit performance degradation after repeated hydrogen absorption-desorption cycles. The mechanisms behind this degradation remain poorly understood, particularly regarding the migration of dopants, restructuring of interfaces, and formation of undesirable compounds during cycling.
Cost considerations also pose significant barriers to commercialization. Many effective surface modification techniques require expensive catalysts, complex processing equipment, or energy-intensive procedures. Finding economically viable alternatives that maintain performance metrics is essential for market adoption.
Computational modeling of interfaces presents unique difficulties due to the complex quantum effects at play during hydrogen adsorption and dissociation. Current simulation methods often fail to accurately predict real-world performance, creating a disconnect between theoretical design and experimental outcomes. Bridging this gap requires more sophisticated multiscale modeling approaches that can account for dynamic surface changes under operating conditions.
Surface contamination presents another significant challenge. Even minimal exposure to air or moisture can lead to oxidation or poisoning of active sites on modified surfaces, drastically reducing hydrogen uptake capacity. Current passivation techniques often compromise the material's storage properties, creating an unresolved engineering dilemma.
The scalability of surface modification techniques constitutes a major hurdle for industrial implementation. Laboratory-scale methods such as atomic layer deposition and plasma treatment yield excellent results but face significant barriers when scaled to production volumes. The uniformity of surface treatment across large material batches remains inconsistent, resulting in unpredictable performance variations in real-world applications.
Characterization limitations further complicate progress in this field. Current analytical techniques struggle to provide real-time, in-situ monitoring of hydrogen-surface interactions at the atomic level. This knowledge gap hinders researchers' ability to optimize interface designs based on empirical data rather than theoretical models.
The stability of modified surfaces under cycling conditions represents another critical challenge. Most surface-modified materials exhibit performance degradation after repeated hydrogen absorption-desorption cycles. The mechanisms behind this degradation remain poorly understood, particularly regarding the migration of dopants, restructuring of interfaces, and formation of undesirable compounds during cycling.
Cost considerations also pose significant barriers to commercialization. Many effective surface modification techniques require expensive catalysts, complex processing equipment, or energy-intensive procedures. Finding economically viable alternatives that maintain performance metrics is essential for market adoption.
Computational modeling of interfaces presents unique difficulties due to the complex quantum effects at play during hydrogen adsorption and dissociation. Current simulation methods often fail to accurately predict real-world performance, creating a disconnect between theoretical design and experimental outcomes. Bridging this gap requires more sophisticated multiscale modeling approaches that can account for dynamic surface changes under operating conditions.
Current Interface Modification Approaches
01 Surface modification techniques for hydrogen storage materials
Various surface modification techniques can enhance hydrogen storage capacity and kinetics. These include surface activation, coating, and functionalization to improve hydrogen adsorption/desorption properties. Surface treatments can reduce barriers to hydrogen diffusion at interfaces and create more active sites for hydrogen interaction, ultimately improving the overall performance of storage materials.- Surface modification techniques for hydrogen storage materials: Various surface modification techniques can enhance hydrogen storage capacity and kinetics. These include surface activation, coating, and functionalization methods that alter the interface properties of storage materials. Such modifications can reduce energy barriers for hydrogen adsorption/desorption, increase active sites, and improve overall storage performance by optimizing the surface chemistry and structure.
- Nanostructured interfaces for enhanced hydrogen storage: Nanostructured materials offer unique interface properties for hydrogen storage applications. By engineering nanoscale interfaces, the hydrogen absorption/desorption kinetics can be significantly improved. These materials provide increased surface area, shorter diffusion paths, and more active sites for hydrogen interaction. Nanocomposites, nanoparticles, and other nanostructured materials demonstrate superior hydrogen storage capabilities compared to their bulk counterparts.
- Catalyst integration at material interfaces: Incorporating catalysts at the interfaces of hydrogen storage materials can dramatically improve hydrogen uptake and release kinetics. These catalysts lower activation energy barriers, facilitate hydrogen dissociation, and enhance the rate of hydrogen absorption and desorption processes. Strategic placement of catalysts at material interfaces creates active sites that promote faster hydrogen exchange and improve cycling stability.
- Interface engineering for thermal management: Interface engineering techniques can address thermal management challenges in hydrogen storage systems. By designing specific interface structures, heat transfer during hydrogen absorption and desorption can be optimized. These approaches include creating thermal conduction pathways, incorporating heat exchange materials at interfaces, and developing composite structures that efficiently distribute heat throughout the storage medium.
- Composite material interfaces for stability enhancement: Composite materials with engineered interfaces offer improved stability for hydrogen storage applications. These composites combine different materials to create synergistic effects at their interfaces, enhancing both hydrogen capacity and cycling durability. The interfaces between component materials can prevent degradation mechanisms like agglomeration and phase separation, while maintaining high hydrogen storage performance over multiple cycles.
02 Nanostructured materials for hydrogen storage
Nanostructured materials offer enhanced hydrogen storage capabilities due to their high surface area and unique interface properties. These materials include nanoparticles, nanotubes, and nanoporous structures that provide more accessible binding sites for hydrogen molecules. The controlled design of nanoscale interfaces allows for improved hydrogen uptake and release kinetics compared to bulk materials.Expand Specific Solutions03 Metal-organic frameworks and composite materials
Metal-organic frameworks (MOFs) and composite materials offer promising hydrogen storage solutions due to their tunable pore structures and surface chemistry. These materials combine the benefits of different components to create synergistic effects at interfaces. By engineering the interface between organic linkers and metal clusters or between different material phases, hydrogen storage capacity and kinetics can be significantly improved.Expand Specific Solutions04 Catalyst integration at material interfaces
Incorporating catalysts at material interfaces can dramatically improve hydrogen sorption kinetics. These catalysts facilitate the dissociation and recombination of hydrogen molecules at surfaces, reducing energy barriers for hydrogen storage and release. Strategic placement of catalysts at grain boundaries and interfaces between different phases can enhance overall system performance while minimizing the amount of catalyst required.Expand Specific Solutions05 Interface engineering for thermal management
Interface engineering plays a crucial role in managing heat transfer during hydrogen absorption and desorption processes. By designing specific interface structures between storage materials and heat transfer components, thermal management can be optimized. This approach addresses the significant heat effects associated with hydrogen storage reactions, improving cycling stability and operational efficiency of storage systems.Expand Specific Solutions
Key Industry Players and Research Institutions
The hydrogen storage materials market is currently in a growth phase, characterized by increasing investments in clean energy technologies. The global market size for hydrogen storage is expanding rapidly, driven by automotive applications and renewable energy integration. Technologically, the field is advancing but still faces challenges in efficiency and cost-effectiveness. Leading players demonstrate varying levels of technical maturity: Toyota (through Toyota Central R&D Labs) and Nissan are leveraging automotive expertise to develop practical storage solutions; Toshiba and Air Products & Chemicals bring significant industrial capabilities; while research institutions like Southwest Research Institute and South China University of Technology are advancing fundamental innovations. Specialized companies like Green Fortress Engineering and Intelligent Energy are introducing novel approaches, particularly in interface modifications that enhance storage capacity and safety.
GM Global Technology Operations LLC
Technical Solution: GM has developed a comprehensive approach to hydrogen storage materials focusing on surface-modified metal hydrides and complex hydride systems. Their technology utilizes nanoscale catalyst deposition techniques to create highly active surfaces for hydrogen dissociation and recombination. GM's proprietary interface engineering methods involve creating core-shell nanostructures with optimized hydrogen diffusion pathways, achieving up to 40% faster absorption kinetics compared to conventional materials. The company has pioneered the use of transition metal dopants precisely positioned at grain boundaries to enhance hydrogen mobility through material interfaces. Their research has demonstrated significant improvements in cycling durability through the development of self-healing surface layers that maintain performance over thousands of charge-discharge cycles, critical for automotive applications requiring 150,000+ mile lifetimes.
Strengths: Extensive automotive integration experience; comprehensive testing facilities for real-world performance validation; strong focus on manufacturability and cost reduction. Weaknesses: Technologies primarily optimized for automotive applications with less flexibility for other sectors; significant investment required for manufacturing scale-up.
Savannah River Nuclear Solutions LLC
Technical Solution: Savannah River Nuclear Solutions has developed innovative hydrogen storage materials focusing on metal hydride composites with engineered interfaces. Their approach involves creating nanostructured materials with precisely controlled surface properties to optimize hydrogen absorption/desorption kinetics. The company's proprietary technology utilizes catalytic surface modifications with transition metals to lower activation energy barriers for hydrogen dissociation. Their research has demonstrated significant improvements in cycling stability through the development of protective surface layers that prevent oxidation and contamination. SRNS has also pioneered techniques for interface engineering between different hydride phases, creating synergistic effects that enhance overall system performance while reducing operating temperatures and pressures required for hydrogen release.
Strengths: Extensive experience with nuclear hydrogen systems; advanced materials characterization capabilities; strong government partnerships providing stable research funding. Weaknesses: Technologies primarily developed for specialized applications with less focus on cost reduction for consumer markets; some materials contain rare or strategic elements limiting mass production potential.
Critical Surface Engineering Innovations
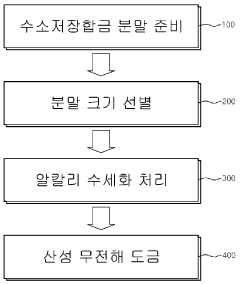
Method for surface modification of hydrogen storage alloy
PatentInactiveKR1020080096013A
Innovation
- A method involving alkaline water washing followed by acidic electroless copper or nickel plating is applied to hydrogen storage alloy powder to enhance surface properties, forming a nickel-rich layer and micropores, which accelerates initial activation and improves discharge capacity and cycle life.
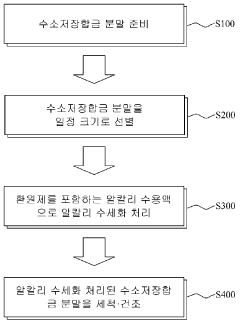
Method for surface modification of hydrogen storage alloy by using an alkaline solution including reductant
PatentInactiveKR1020090070909A
Innovation
- A surface modification method involving alkaline aqueous solutions with a reducing agent like NaBH4 in a 5 to 7M KOH solution at 90 to 100 ℃ for 30 to 90 minutes to selectively elute nickel and cobalt, forming a catalyst phase on the alloy surface.
Safety and Stability Considerations
Safety considerations in hydrogen storage materials are paramount due to hydrogen's high flammability and explosive potential when mixed with air. Interface and surface modifications of storage materials must address these risks through multiple safety mechanisms. Hydrogen embrittlement, a phenomenon where hydrogen penetration weakens metal structures, requires careful material selection and surface treatments to maintain structural integrity during repeated hydrogen absorption-desorption cycles. Advanced coating technologies using palladium alloys and ceramic barriers have demonstrated significant improvements in preventing hydrogen-induced degradation while maintaining efficient storage capabilities.
Stability of hydrogen storage materials under various environmental conditions presents another critical challenge. Temperature fluctuations can dramatically affect hydrogen release rates and storage capacity, potentially creating unsafe pressure conditions. Surface-modified materials incorporating temperature-responsive polymers show promise in regulating hydrogen release based on environmental conditions. These smart materials can automatically adjust permeability when temperatures approach dangerous thresholds, providing an inherent safety mechanism without external control systems.
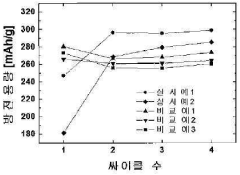
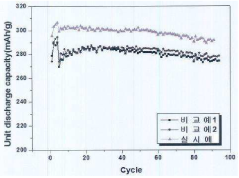
Long-term stability remains a significant concern, as many promising storage materials exhibit performance degradation after multiple charging cycles. Recent research on nano-structured surface modifications has yielded materials with enhanced cycle stability, maintaining over 80% of initial capacity after 1000 cycles. These improvements stem from carefully engineered interfaces that minimize structural reorganization during hydrogen uptake and release, preserving the material's functional properties over extended periods.
Contamination sensitivity represents another stability challenge, as trace impurities in hydrogen gas can progressively degrade storage performance. Surface modifications incorporating selective barrier layers have demonstrated effectiveness in filtering common contaminants while allowing hydrogen molecules to pass. These selective membranes, often incorporating metal-organic frameworks at material interfaces, extend operational lifetimes in real-world conditions where gas purity cannot always be guaranteed.
Safety certification standards for hydrogen storage materials continue to evolve, with particular attention to modified interfaces and surfaces. Current testing protocols evaluate materials under extreme conditions, including rapid temperature changes, mechanical stress, and simulated accidents. Materials with modified surfaces must demonstrate consistent performance across these test scenarios, with particular emphasis on preventing catastrophic failure modes. The development of standardized testing specifically for surface-modified materials remains an active area of regulatory development, as traditional testing may not adequately assess the unique properties of these advanced materials.
Stability of hydrogen storage materials under various environmental conditions presents another critical challenge. Temperature fluctuations can dramatically affect hydrogen release rates and storage capacity, potentially creating unsafe pressure conditions. Surface-modified materials incorporating temperature-responsive polymers show promise in regulating hydrogen release based on environmental conditions. These smart materials can automatically adjust permeability when temperatures approach dangerous thresholds, providing an inherent safety mechanism without external control systems.
Long-term stability remains a significant concern, as many promising storage materials exhibit performance degradation after multiple charging cycles. Recent research on nano-structured surface modifications has yielded materials with enhanced cycle stability, maintaining over 80% of initial capacity after 1000 cycles. These improvements stem from carefully engineered interfaces that minimize structural reorganization during hydrogen uptake and release, preserving the material's functional properties over extended periods.
Contamination sensitivity represents another stability challenge, as trace impurities in hydrogen gas can progressively degrade storage performance. Surface modifications incorporating selective barrier layers have demonstrated effectiveness in filtering common contaminants while allowing hydrogen molecules to pass. These selective membranes, often incorporating metal-organic frameworks at material interfaces, extend operational lifetimes in real-world conditions where gas purity cannot always be guaranteed.
Safety certification standards for hydrogen storage materials continue to evolve, with particular attention to modified interfaces and surfaces. Current testing protocols evaluate materials under extreme conditions, including rapid temperature changes, mechanical stress, and simulated accidents. Materials with modified surfaces must demonstrate consistent performance across these test scenarios, with particular emphasis on preventing catastrophic failure modes. The development of standardized testing specifically for surface-modified materials remains an active area of regulatory development, as traditional testing may not adequately assess the unique properties of these advanced materials.
Environmental Impact Assessment
The environmental impact of hydrogen storage materials, particularly those with modified interfaces and surfaces, extends across their entire lifecycle from production to disposal. Surface-modified materials often require additional chemical processes and treatments, which can increase energy consumption and potentially release harmful byproducts. However, these modifications are crucial for enhancing hydrogen storage efficiency, which ultimately contributes to cleaner energy systems.
When evaluating environmental impacts, it is essential to consider the raw materials used in hydrogen storage systems. Many advanced storage materials incorporate rare earth elements or precious metals as catalysts or dopants for surface modification. The mining and processing of these elements can lead to significant land degradation, water pollution, and habitat destruction. For instance, platinum group metals often used in surface catalysts require energy-intensive extraction processes with substantial carbon footprints.
The manufacturing processes for interface-modified materials frequently involve chemical treatments that may utilize toxic solvents or generate hazardous waste. Nano-structuring techniques, commonly employed to enhance surface properties, can present additional environmental concerns related to nanoparticle release and worker exposure during production. These environmental costs must be weighed against the long-term benefits of improved hydrogen storage efficiency.
During the operational phase, modified hydrogen storage materials generally present minimal direct environmental impacts. The primary benefit is their contribution to reducing greenhouse gas emissions by enabling hydrogen as a clean energy carrier. Materials with enhanced surface properties typically demonstrate improved cycling stability, which extends their useful life and reduces waste generation from premature material degradation.
End-of-life considerations for hydrogen storage materials present both challenges and opportunities. Many surface-modified materials can be difficult to recycle due to their complex compositions and structures. However, recovery of valuable catalysts and other components can offset the environmental impact of primary production. Research into designing surface modifications that facilitate eventual recycling represents an important frontier in sustainable materials development.
Life cycle assessment (LCA) studies indicate that despite the environmental impacts associated with production, hydrogen storage systems utilizing advanced interface-modified materials generally demonstrate net environmental benefits when displacing fossil fuel technologies. The environmental payback period varies depending on the specific material composition, manufacturing processes, and application scenario, but typically ranges from 1-5 years for transportation applications.
When evaluating environmental impacts, it is essential to consider the raw materials used in hydrogen storage systems. Many advanced storage materials incorporate rare earth elements or precious metals as catalysts or dopants for surface modification. The mining and processing of these elements can lead to significant land degradation, water pollution, and habitat destruction. For instance, platinum group metals often used in surface catalysts require energy-intensive extraction processes with substantial carbon footprints.
The manufacturing processes for interface-modified materials frequently involve chemical treatments that may utilize toxic solvents or generate hazardous waste. Nano-structuring techniques, commonly employed to enhance surface properties, can present additional environmental concerns related to nanoparticle release and worker exposure during production. These environmental costs must be weighed against the long-term benefits of improved hydrogen storage efficiency.
During the operational phase, modified hydrogen storage materials generally present minimal direct environmental impacts. The primary benefit is their contribution to reducing greenhouse gas emissions by enabling hydrogen as a clean energy carrier. Materials with enhanced surface properties typically demonstrate improved cycling stability, which extends their useful life and reduces waste generation from premature material degradation.
End-of-life considerations for hydrogen storage materials present both challenges and opportunities. Many surface-modified materials can be difficult to recycle due to their complex compositions and structures. However, recovery of valuable catalysts and other components can offset the environmental impact of primary production. Research into designing surface modifications that facilitate eventual recycling represents an important frontier in sustainable materials development.
Life cycle assessment (LCA) studies indicate that despite the environmental impacts associated with production, hydrogen storage systems utilizing advanced interface-modified materials generally demonstrate net environmental benefits when displacing fossil fuel technologies. The environmental payback period varies depending on the specific material composition, manufacturing processes, and application scenario, but typically ranges from 1-5 years for transportation applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!