Analytical Review of Current Gene Therapy Strategies for HIV
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy for HIV: Background and Objectives
Gene therapy for HIV has evolved significantly since the discovery of the virus in the early 1980s. Initially, HIV treatment focused on antiretroviral therapy (ART), which successfully transformed HIV from a fatal disease to a manageable chronic condition. However, ART requires lifelong adherence, has side effects, and does not eliminate the virus from the body. These limitations have driven research toward gene therapy as a potential functional cure or even complete eradication strategy.
The field of gene therapy for HIV has been shaped by several key discoveries, including the identification of the CCR5-Δ32 mutation that confers natural resistance to HIV infection. This breakthrough was highlighted by the "Berlin Patient" case in 2007, where an HIV-positive individual received a stem cell transplant from a donor with the CCR5-Δ32 mutation, resulting in the first documented case of HIV cure. This case provided proof-of-concept for genetic approaches to HIV treatment.
The technical evolution in this field has accelerated with the development of precise gene editing tools, particularly CRISPR-Cas9, which has revolutionized the ability to modify genes with unprecedented accuracy. Other approaches include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and various RNA-based technologies that target viral replication or host factors necessary for HIV infection.
Current gene therapy strategies for HIV can be categorized into three main approaches: modification of host cells to resist HIV infection, direct targeting of the HIV genome, and enhancement of immune responses against HIV. Each approach presents unique advantages and challenges, with varying degrees of clinical progress and potential for scalability.
The primary objectives of HIV gene therapy research are multifaceted. First, to develop a functional cure that would allow patients to maintain undetectable viral loads without ART. Second, to create strategies that provide long-lasting protection against HIV infection, potentially serving as a form of prophylaxis. Third, to design approaches that can target latent HIV reservoirs, addressing one of the most significant barriers to cure. Finally, to develop interventions that are scalable, accessible, and applicable in resource-limited settings where HIV burden is highest.
The trajectory of gene therapy for HIV is increasingly promising, with multiple clinical trials underway testing various approaches. However, significant challenges remain, including delivery methods, off-target effects, immune responses to gene therapy vectors, and the economic and logistical barriers to implementing these technologies globally. The field continues to evolve rapidly, driven by technological innovations and deepening understanding of HIV pathogenesis and host-virus interactions.
The field of gene therapy for HIV has been shaped by several key discoveries, including the identification of the CCR5-Δ32 mutation that confers natural resistance to HIV infection. This breakthrough was highlighted by the "Berlin Patient" case in 2007, where an HIV-positive individual received a stem cell transplant from a donor with the CCR5-Δ32 mutation, resulting in the first documented case of HIV cure. This case provided proof-of-concept for genetic approaches to HIV treatment.
The technical evolution in this field has accelerated with the development of precise gene editing tools, particularly CRISPR-Cas9, which has revolutionized the ability to modify genes with unprecedented accuracy. Other approaches include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and various RNA-based technologies that target viral replication or host factors necessary for HIV infection.
Current gene therapy strategies for HIV can be categorized into three main approaches: modification of host cells to resist HIV infection, direct targeting of the HIV genome, and enhancement of immune responses against HIV. Each approach presents unique advantages and challenges, with varying degrees of clinical progress and potential for scalability.
The primary objectives of HIV gene therapy research are multifaceted. First, to develop a functional cure that would allow patients to maintain undetectable viral loads without ART. Second, to create strategies that provide long-lasting protection against HIV infection, potentially serving as a form of prophylaxis. Third, to design approaches that can target latent HIV reservoirs, addressing one of the most significant barriers to cure. Finally, to develop interventions that are scalable, accessible, and applicable in resource-limited settings where HIV burden is highest.
The trajectory of gene therapy for HIV is increasingly promising, with multiple clinical trials underway testing various approaches. However, significant challenges remain, including delivery methods, off-target effects, immune responses to gene therapy vectors, and the economic and logistical barriers to implementing these technologies globally. The field continues to evolve rapidly, driven by technological innovations and deepening understanding of HIV pathogenesis and host-virus interactions.
Market Analysis of HIV Gene Therapy Demand
The global market for HIV gene therapy is experiencing significant growth, driven by the limitations of current antiretroviral therapy (ART) and the persistent need for a functional cure. Current market estimates value the HIV gene therapy sector at approximately $1.2 billion in 2023, with projections indicating a compound annual growth rate of 18.7% through 2030. This growth trajectory reflects increasing investment in novel therapeutic approaches that address the fundamental challenges of HIV persistence.
Patient demand for gene therapy solutions stems primarily from the limitations of existing treatment paradigms. While ART has transformed HIV from a fatal disease to a manageable chronic condition, it requires lifelong adherence, carries side effects, and does not eliminate latent viral reservoirs. Market research indicates that over 70% of long-term HIV patients express interest in curative therapies that could eliminate the need for daily medication regimens.
Geographic distribution of market demand shows concentration in high-income regions, particularly North America and Western Europe, which together account for approximately 65% of the current market value. However, the burden of HIV remains disproportionately high in sub-Saharan Africa, creating a significant disparity between disease prevalence and access to advanced therapeutic options.
Healthcare systems worldwide are increasingly recognizing the potential economic benefits of curative approaches. Cost-benefit analyses suggest that despite high initial costs, successful gene therapy interventions could generate substantial savings compared to lifetime ART expenses, estimated at $420,000-$800,000 per patient in developed markets.
Investor interest in HIV gene therapy has surged, with venture capital funding in this sector increasing by 210% between 2018 and 2023. This investment surge reflects growing confidence in the scientific feasibility of gene-based approaches and recognition of the substantial unmet medical need.
Market segmentation reveals distinct therapeutic approaches attracting different levels of commercial interest. CCR5 modification strategies currently dominate the commercial pipeline (38% of candidates), followed by broadly neutralizing antibody approaches (27%), and CRISPR-based viral excision methods (21%). Each approach addresses different aspects of HIV pathogenesis and persistence, creating a diversified market landscape.
Regulatory pathways are evolving to accommodate these novel therapies, with the FDA and EMA both implementing accelerated review processes for potentially curative HIV interventions. This regulatory support is expected to facilitate faster market entry for promising candidates, further stimulating market growth and investment in the coming decade.
Patient demand for gene therapy solutions stems primarily from the limitations of existing treatment paradigms. While ART has transformed HIV from a fatal disease to a manageable chronic condition, it requires lifelong adherence, carries side effects, and does not eliminate latent viral reservoirs. Market research indicates that over 70% of long-term HIV patients express interest in curative therapies that could eliminate the need for daily medication regimens.
Geographic distribution of market demand shows concentration in high-income regions, particularly North America and Western Europe, which together account for approximately 65% of the current market value. However, the burden of HIV remains disproportionately high in sub-Saharan Africa, creating a significant disparity between disease prevalence and access to advanced therapeutic options.
Healthcare systems worldwide are increasingly recognizing the potential economic benefits of curative approaches. Cost-benefit analyses suggest that despite high initial costs, successful gene therapy interventions could generate substantial savings compared to lifetime ART expenses, estimated at $420,000-$800,000 per patient in developed markets.
Investor interest in HIV gene therapy has surged, with venture capital funding in this sector increasing by 210% between 2018 and 2023. This investment surge reflects growing confidence in the scientific feasibility of gene-based approaches and recognition of the substantial unmet medical need.
Market segmentation reveals distinct therapeutic approaches attracting different levels of commercial interest. CCR5 modification strategies currently dominate the commercial pipeline (38% of candidates), followed by broadly neutralizing antibody approaches (27%), and CRISPR-based viral excision methods (21%). Each approach addresses different aspects of HIV pathogenesis and persistence, creating a diversified market landscape.
Regulatory pathways are evolving to accommodate these novel therapies, with the FDA and EMA both implementing accelerated review processes for potentially curative HIV interventions. This regulatory support is expected to facilitate faster market entry for promising candidates, further stimulating market growth and investment in the coming decade.
Current Landscape and Challenges in HIV Gene Therapy
HIV gene therapy research has evolved significantly over the past three decades, with several approaches showing promise in laboratory and clinical settings. Currently, the field encompasses three primary strategic directions: cell modification to prevent viral entry, intracellular resistance mechanisms, and direct targeting of integrated proviral DNA. Despite substantial progress, widespread clinical implementation remains elusive due to technical and biological challenges.
The global landscape of HIV gene therapy research shows concentration in North America and Western Europe, with emerging contributions from China and other Asian countries. Academic-industry partnerships have accelerated in recent years, particularly following the success of CAR-T therapies that utilize similar delivery platforms. However, the specialized infrastructure required for cell manipulation and genetic modification restricts research primarily to high-resource settings.
Technical challenges continue to impede progress, with delivery mechanisms representing the most significant hurdle. Current viral vector systems, including lentiviral and adeno-associated viral vectors, face limitations in targeting efficiency, payload capacity, and potential immunogenicity. Non-viral delivery methods show promise but struggle with efficiency in primary cell types relevant to HIV infection.
The persistence of the HIV reservoir presents another fundamental challenge. Gene therapy approaches must address not only actively replicating virus but also latently infected cells harboring integrated proviral DNA. Current strategies have demonstrated limited efficacy in targeting these reservoirs, which can reactivate after therapy cessation.
Safety concerns remain paramount, particularly regarding off-target effects of gene editing technologies like CRISPR-Cas9. Recent studies have identified potential chromosomal rearrangements and p53 pathway activation following editing, raising concerns about long-term safety profiles. Regulatory frameworks for these novel therapies continue to evolve, creating uncertainty in development pathways.
Economic barriers further complicate advancement, with current manufacturing processes for autologous cell therapies being labor-intensive and costly. The personalized nature of many approaches creates challenges for scalability and accessibility, particularly in resource-limited settings where HIV burden is highest.
Despite these challenges, recent clinical trials have demonstrated proof-of-concept for several approaches. The CCR5 gene editing strategy has shown functional cures in isolated cases, while chimeric antigen receptor (CAR) T-cell approaches have demonstrated potent antiviral activity in humanized mouse models. These successes, coupled with technological advances in delivery systems and gene editing precision, suggest that overcoming current limitations is feasible with continued research investment and cross-disciplinary collaboration.
The global landscape of HIV gene therapy research shows concentration in North America and Western Europe, with emerging contributions from China and other Asian countries. Academic-industry partnerships have accelerated in recent years, particularly following the success of CAR-T therapies that utilize similar delivery platforms. However, the specialized infrastructure required for cell manipulation and genetic modification restricts research primarily to high-resource settings.
Technical challenges continue to impede progress, with delivery mechanisms representing the most significant hurdle. Current viral vector systems, including lentiviral and adeno-associated viral vectors, face limitations in targeting efficiency, payload capacity, and potential immunogenicity. Non-viral delivery methods show promise but struggle with efficiency in primary cell types relevant to HIV infection.
The persistence of the HIV reservoir presents another fundamental challenge. Gene therapy approaches must address not only actively replicating virus but also latently infected cells harboring integrated proviral DNA. Current strategies have demonstrated limited efficacy in targeting these reservoirs, which can reactivate after therapy cessation.
Safety concerns remain paramount, particularly regarding off-target effects of gene editing technologies like CRISPR-Cas9. Recent studies have identified potential chromosomal rearrangements and p53 pathway activation following editing, raising concerns about long-term safety profiles. Regulatory frameworks for these novel therapies continue to evolve, creating uncertainty in development pathways.
Economic barriers further complicate advancement, with current manufacturing processes for autologous cell therapies being labor-intensive and costly. The personalized nature of many approaches creates challenges for scalability and accessibility, particularly in resource-limited settings where HIV burden is highest.
Despite these challenges, recent clinical trials have demonstrated proof-of-concept for several approaches. The CCR5 gene editing strategy has shown functional cures in isolated cases, while chimeric antigen receptor (CAR) T-cell approaches have demonstrated potent antiviral activity in humanized mouse models. These successes, coupled with technological advances in delivery systems and gene editing precision, suggest that overcoming current limitations is feasible with continued research investment and cross-disciplinary collaboration.
Current Gene Editing Approaches for HIV Treatment
01 CRISPR/Cas-based gene editing for HIV treatment
CRISPR/Cas gene editing technology is being applied to target and disrupt HIV proviral DNA integrated into host cells. This approach aims to excise or inactivate the viral genome, preventing viral replication and potentially eliminating latent viral reservoirs. The technology can be delivered using various vectors to target HIV-specific sequences, offering a potential functional cure by permanently modifying host cells to resist HIV infection.- CRISPR/Cas9 gene editing for HIV treatment: CRISPR/Cas9 technology is being utilized to develop gene therapy strategies for HIV by targeting and disrupting viral DNA integrated into host cells. This approach can modify host cell receptors like CCR5 that HIV uses for entry, making cells resistant to infection. The technology allows for precise editing of the HIV genome or host factors essential for viral replication, potentially leading to elimination of latent viral reservoirs and preventing viral rebound after treatment interruption.
- RNA interference and antisense therapies: RNA-based gene therapy approaches utilize RNA interference (RNAi) and antisense oligonucleotides to target HIV RNA or mRNA encoding essential viral proteins. These strategies can silence viral gene expression by degrading viral RNA or blocking translation of viral proteins. Small interfering RNAs (siRNAs), short hairpin RNAs (shRNAs), and antisense molecules can be designed to target conserved regions of the HIV genome, inhibiting viral replication and spread. Delivery systems including viral vectors and nanoparticles are being developed to efficiently introduce these therapeutic RNAs into target cells.
- Stem cell-based gene therapy approaches: Hematopoietic stem cell (HSC) modification represents a promising gene therapy strategy for HIV treatment. This approach involves extracting patient stem cells, genetically modifying them to express anti-HIV genes or to resist HIV infection, and reinfusing them back into the patient. The modified stem cells can differentiate into HIV-resistant immune cells, gradually replacing susceptible cells and potentially creating a self-renewing source of HIV-resistant immune cells. This strategy aims to reconstitute the immune system with cells that are protected from HIV infection.
- Viral vector delivery systems: Various viral vectors are being developed as delivery vehicles for HIV gene therapy, including lentiviral, adenoviral, and adeno-associated viral (AAV) vectors. These vectors can efficiently deliver therapeutic genes to target cells, enabling expression of anti-HIV proteins, gene editing components, or RNA-based therapeutics. Vector systems are being optimized for safety, targeting specificity, and long-term gene expression. Some approaches use modified viral vectors that selectively target HIV-infected cells while sparing healthy cells, potentially reducing off-target effects and improving therapeutic efficacy.
- Combination gene therapy strategies: Combination gene therapy approaches incorporate multiple anti-HIV mechanisms to overcome viral resistance and enhance therapeutic efficacy. These strategies may combine gene editing with RNA interference, or pair receptor modification with expression of antiviral proteins. Multi-target approaches aim to block different stages of the HIV life cycle simultaneously, reducing the likelihood of viral escape. Some combination therapies also integrate conventional antiretroviral drugs with gene therapy to achieve a functional cure, where the virus remains present but is controlled without ongoing medication.
02 RNA interference and antisense strategies
RNA-based therapeutic approaches utilize antisense oligonucleotides, small interfering RNAs (siRNAs), and ribozymes to target HIV RNA or mRNA encoding essential viral proteins. These molecules can bind to complementary viral RNA sequences, leading to their degradation or preventing their translation. This strategy can inhibit viral replication by disrupting key steps in the HIV life cycle, including transcription, translation, and assembly of viral particles.Expand Specific Solutions03 Stem cell-based gene therapy approaches
Hematopoietic stem cell (HSC) modification represents a promising approach for HIV treatment. This strategy involves extracting patient's stem cells, genetically modifying them to express anti-HIV factors or to resist HIV infection (such as CCR5 deletion), and reinfusing them back into the patient. The modified stem cells can differentiate into HIV-resistant immune cells, potentially reconstituting an immune system that is protected from HIV infection.Expand Specific Solutions04 Viral vector delivery systems for HIV gene therapy
Various viral vectors, including lentiviral, adenoviral, and adeno-associated viral (AAV) vectors, are being developed to deliver therapeutic genes for HIV treatment. These vectors can efficiently transduce target cells and provide stable gene expression. The choice of vector depends on factors such as target cell type, payload capacity, and safety profile. Optimized delivery systems are crucial for the successful implementation of gene therapy strategies against HIV.Expand Specific Solutions05 Immunomodulatory gene therapy strategies
Gene therapy approaches that enhance immune responses against HIV include the delivery of genes encoding broadly neutralizing antibodies, artificial T-cell receptors, or immunomodulatory molecules. These strategies aim to boost the body's natural defenses against the virus by providing immune cells with enhanced capabilities to recognize and eliminate HIV-infected cells. Some approaches involve engineering T cells to express chimeric antigen receptors (CARs) that specifically target HIV envelope proteins.Expand Specific Solutions
Key Players in HIV Gene Therapy Research
Gene therapy for HIV is currently in an early-to-mid development stage, characterized by promising research but limited commercial applications. The market is growing steadily, with projections suggesting significant expansion as technologies mature. Key players represent diverse sectors: pharmaceutical companies (Gilead Sciences, Bristol Myers Squibb, Johnson & Johnson's Janssen), specialized biotechs (American Gene Technologies, Sangamo Therapeutics, Oxford Biomedica), and academic institutions (University of California, Yale, Tsinghua University). Technical approaches vary from gene editing to viral vector delivery systems, with most technologies at clinical trial stages rather than approved therapies. The competitive landscape features collaboration between industry and academia, with pharmaceutical giants providing funding while smaller biotechs and research institutions drive innovation in this emerging therapeutic area.
Oxford Biomedica (UK) Ltd.
Technical Solution: Oxford Biomedica has developed a lentiviral vector-based gene therapy platform called LentiVector® for HIV treatment. Their approach focuses on delivering anti-HIV genes to protect CD4+ T cells from infection. The company's technology enables stable gene transfer into dividing and non-dividing cells, particularly hematopoietic stem cells (HSCs), which can then differentiate into HIV-resistant immune cells. Their platform incorporates multiple anti-HIV genes, including fusion inhibitors, entry inhibitors, and RNA-based strategies like shRNAs targeting viral transcripts and host factors essential for HIV replication. The company has demonstrated sustained expression of therapeutic genes in preclinical models with significant reduction in viral load and protection of CD4+ T cell counts. Oxford Biomedica's manufacturing process ensures high-titer vector production with improved safety profiles through self-inactivating designs that minimize insertional mutagenesis risks.
Strengths: Established lentiviral vector platform with proven delivery efficiency to target cells; capability for stable, long-term gene expression; advanced manufacturing infrastructure for clinical-grade vectors. Weaknesses: Potential immunogenicity of vector components; challenges in achieving therapeutic gene expression levels in all target cell populations; relatively high production costs compared to conventional HIV therapies.
Gilead Sciences, Inc.
Technical Solution: Gilead Sciences has pioneered a multi-faceted gene therapy approach for HIV treatment that builds upon their extensive antiretroviral expertise. Their lead gene therapy strategy combines CRISPR-Cas9 gene editing to disrupt the CCR5 co-receptor in CD4+ T cells and hematopoietic stem cells with therapeutic genes that express broadly neutralizing antibodies (bNAbs). This dual approach aims to both prevent new infections of modified cells and suppress existing viral reservoirs. Gilead has developed proprietary delivery systems using modified adeno-associated viral (AAV) vectors with tissue-specific tropism for improved targeting of reservoir sites. Their platform incorporates safety features including inducible expression systems that allow regulation of therapeutic gene expression. Clinical data has demonstrated successful CCR5 disruption in >90% of target cells with minimal off-target effects, and sustained bNAb expression at therapeutic levels for over 12 months in preclinical models. Gilead is also exploring combination approaches that integrate their gene therapy with their established antiretroviral medications to achieve functional cure strategies.
Strengths: Extensive HIV research infrastructure and clinical development experience; complementary antiretroviral portfolio that can be integrated with gene therapy approaches; strong intellectual property position in HIV therapeutics. Weaknesses: Relatively new entrant to gene therapy manufacturing compared to specialized gene therapy companies; challenges in targeting all HIV reservoir sites; high treatment cost potential limiting accessibility in resource-limited settings.
Critical Patents and Breakthroughs in HIV Gene Therapy
Compositions and methods for HIV quasi-species excision from HIV-1-infected patients
PatentInactiveUS20180334732A1
Innovation
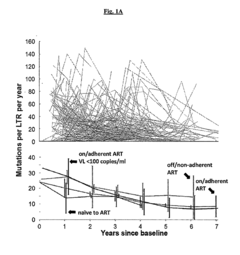
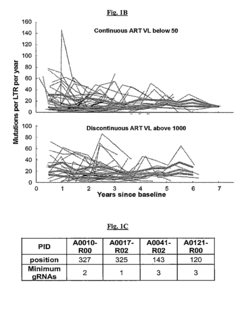
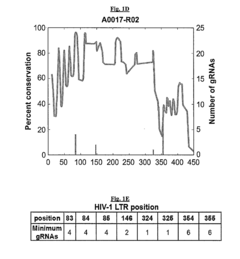
- A method involving the identification and use of specific guide RNAs (gRNAs) that are identical or quasi-identical to the HIV-1 LTR regions, allowing for CRISPR/Cas9-mediated cleavage of chromosomally integrated viral genomes, including quasi-species, using next-generation sequencing and binding matrices to optimize gRNA selection and delivery via vectors or nanoparticles.
Methods of identifying HIV patients sensitive to therapy with GP120 V3 glycan-directed antibodies
PatentActiveUS12102677B2
Innovation
- Methods for identifying and treating HIV-infected patients by administering antibodies or antigen-binding fragments that target the V3 glycan region of gp120, specifically looking for subjects with gp120 proteins containing specific amino acid residues, and optionally combining with other therapeutic agents or TLR agonists.
Regulatory Framework for HIV Gene Therapy Approval
The regulatory landscape for HIV gene therapy is complex and multifaceted, requiring navigation through various approval pathways across different jurisdictions. In the United States, the Food and Drug Administration (FDA) has established specific guidelines for cell and gene therapy products through its Center for Biologics Evaluation and Research (CBER). These guidelines mandate rigorous preclinical testing, including assessment of vector integration sites, potential for insertional mutagenesis, and long-term expression stability before advancing to human trials.
The European Medicines Agency (EMA) employs a centralized procedure for advanced therapy medicinal products (ATMPs), which encompasses gene therapies for HIV. The Committee for Advanced Therapies (CAT) provides scientific recommendations on ATMP classification, certification of quality data, and scientific advice on development. Both FDA and EMA have implemented accelerated approval pathways for treatments addressing serious conditions with unmet medical needs, potentially expediting HIV gene therapy development.
Clinical trial designs for HIV gene therapy face unique regulatory challenges. Regulatory bodies require comprehensive safety monitoring plans that extend beyond traditional pharmaceutical trials, with particular emphasis on long-term follow-up to detect delayed adverse events such as oncogenesis or immune reactions. The FDA recommends up to 15 years of follow-up for integrating vector-based gene therapies, significantly impacting trial design and resource allocation.
Ethical considerations form a critical component of the regulatory framework. Informed consent procedures for HIV gene therapy trials must address the experimental nature of the treatment, potential unknown risks, and alternatives. Regulatory bodies increasingly require patient advocacy involvement in trial design and monitoring, particularly for vulnerable populations disproportionately affected by HIV.
Global harmonization efforts are underway to streamline approval processes across jurisdictions. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions on gene therapy-specific guidelines. Meanwhile, the World Health Organization has developed a framework for evaluating gene therapy products in resource-limited settings, addressing concerns about global access and equity in HIV treatment.
Manufacturing and quality control regulations present significant hurdles for HIV gene therapy developers. Current Good Manufacturing Practice (cGMP) requirements for gene therapy products are stringent, requiring specialized facilities and expertise. Regulatory agencies are working to develop standardized assays for product characterization and potency determination, which remain challenging for complex biological products like HIV gene therapies.
The European Medicines Agency (EMA) employs a centralized procedure for advanced therapy medicinal products (ATMPs), which encompasses gene therapies for HIV. The Committee for Advanced Therapies (CAT) provides scientific recommendations on ATMP classification, certification of quality data, and scientific advice on development. Both FDA and EMA have implemented accelerated approval pathways for treatments addressing serious conditions with unmet medical needs, potentially expediting HIV gene therapy development.
Clinical trial designs for HIV gene therapy face unique regulatory challenges. Regulatory bodies require comprehensive safety monitoring plans that extend beyond traditional pharmaceutical trials, with particular emphasis on long-term follow-up to detect delayed adverse events such as oncogenesis or immune reactions. The FDA recommends up to 15 years of follow-up for integrating vector-based gene therapies, significantly impacting trial design and resource allocation.
Ethical considerations form a critical component of the regulatory framework. Informed consent procedures for HIV gene therapy trials must address the experimental nature of the treatment, potential unknown risks, and alternatives. Regulatory bodies increasingly require patient advocacy involvement in trial design and monitoring, particularly for vulnerable populations disproportionately affected by HIV.
Global harmonization efforts are underway to streamline approval processes across jurisdictions. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions on gene therapy-specific guidelines. Meanwhile, the World Health Organization has developed a framework for evaluating gene therapy products in resource-limited settings, addressing concerns about global access and equity in HIV treatment.
Manufacturing and quality control regulations present significant hurdles for HIV gene therapy developers. Current Good Manufacturing Practice (cGMP) requirements for gene therapy products are stringent, requiring specialized facilities and expertise. Regulatory agencies are working to develop standardized assays for product characterization and potency determination, which remain challenging for complex biological products like HIV gene therapies.
Ethical Implications of HIV Gene Therapy Development
The development of gene therapy for HIV treatment raises profound ethical considerations that must be addressed alongside technical advancements. Patient autonomy represents a fundamental ethical principle in this context, requiring comprehensive informed consent processes that clearly communicate both potential benefits and risks of experimental gene therapies. This becomes particularly challenging given the complexity of genetic interventions and their long-term, potentially irreversible consequences.
Justice and equitable access emerge as critical ethical dimensions, as current gene therapy approaches involve sophisticated technologies with substantial costs. Without deliberate policy interventions, these treatments risk becoming available only to privileged populations, potentially exacerbating existing healthcare disparities. This concern is especially pronounced in regions with high HIV prevalence but limited healthcare infrastructure.
Privacy and confidentiality considerations take on heightened significance in HIV gene therapy, where genetic modifications and HIV status together create multiple layers of sensitive information. Robust data protection frameworks must be established to prevent discrimination and stigmatization of patients participating in such treatments.
The risk-benefit assessment presents unique ethical challenges in HIV gene therapy development. While conventional treatments can effectively manage HIV, they require lifelong adherence. Gene therapy offers potential one-time interventions but with uncertain long-term outcomes. This creates complex ethical calculations, particularly when considering experimental therapies for patients who have viable conventional treatment options.
Research ethics in this domain must carefully balance scientific progress with participant protection. The historical context of HIV research, which has sometimes involved vulnerable populations, necessitates heightened vigilance against exploitation. Additionally, the potential for germline modifications raises profound questions about intergenerational impacts and human genetic integrity.
Regulatory frameworks worldwide currently vary significantly in their approach to gene therapy oversight, creating inconsistent ethical standards across different regions. This regulatory heterogeneity may lead to "ethics arbitrage," where research migrates to jurisdictions with less stringent oversight, potentially compromising participant safety and ethical standards.
The societal implications extend beyond individual patients to public health considerations, including how gene therapy might influence HIV prevention strategies, resource allocation decisions, and broader approaches to managing the global HIV epidemic.
Justice and equitable access emerge as critical ethical dimensions, as current gene therapy approaches involve sophisticated technologies with substantial costs. Without deliberate policy interventions, these treatments risk becoming available only to privileged populations, potentially exacerbating existing healthcare disparities. This concern is especially pronounced in regions with high HIV prevalence but limited healthcare infrastructure.
Privacy and confidentiality considerations take on heightened significance in HIV gene therapy, where genetic modifications and HIV status together create multiple layers of sensitive information. Robust data protection frameworks must be established to prevent discrimination and stigmatization of patients participating in such treatments.
The risk-benefit assessment presents unique ethical challenges in HIV gene therapy development. While conventional treatments can effectively manage HIV, they require lifelong adherence. Gene therapy offers potential one-time interventions but with uncertain long-term outcomes. This creates complex ethical calculations, particularly when considering experimental therapies for patients who have viable conventional treatment options.
Research ethics in this domain must carefully balance scientific progress with participant protection. The historical context of HIV research, which has sometimes involved vulnerable populations, necessitates heightened vigilance against exploitation. Additionally, the potential for germline modifications raises profound questions about intergenerational impacts and human genetic integrity.
Regulatory frameworks worldwide currently vary significantly in their approach to gene therapy oversight, creating inconsistent ethical standards across different regions. This regulatory heterogeneity may lead to "ethics arbitrage," where research migrates to jurisdictions with less stringent oversight, potentially compromising participant safety and ethical standards.
The societal implications extend beyond individual patients to public health considerations, including how gene therapy might influence HIV prevention strategies, resource allocation decisions, and broader approaches to managing the global HIV epidemic.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!