Gene Therapy for Cardiomyopathies: Mechanisms and Applications
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cardiomyopathy Gene Therapy Background and Objectives
Gene therapy for cardiomyopathies has evolved significantly over the past three decades, transitioning from theoretical concepts to clinical applications. The field began with fundamental discoveries in viral vector technology in the 1990s, followed by proof-of-concept studies in animal models during the early 2000s. By the 2010s, researchers had developed more sophisticated delivery methods and achieved greater understanding of the molecular mechanisms underlying various cardiomyopathies.
The technological evolution has been marked by several key breakthroughs, including the development of adeno-associated virus (AAV) vectors with cardiac tropism, CRISPR-Cas9 gene editing techniques applicable to cardiac tissue, and non-viral delivery systems. These advances have collectively expanded the toolkit available for addressing the genetic basis of cardiomyopathies, which affect approximately 1 in 500 individuals globally.
Current gene therapy approaches for cardiomyopathies focus on three primary strategies: gene replacement for recessive disorders, gene silencing for dominant negative mutations, and gene editing to correct pathogenic variants. Each approach presents unique advantages and challenges in the context of cardiac applications, particularly regarding delivery efficiency, long-term expression, and safety profiles.
The primary objective of gene therapy development in this field is to provide curative treatments for genetic cardiomyopathies, which currently have limited therapeutic options beyond symptom management and heart transplantation. Secondary objectives include developing therapies that can halt or reverse disease progression, reduce hospitalization rates, and improve quality of life for patients with both genetic and acquired forms of cardiomyopathy.
Technical goals in this domain include achieving sustained transgene expression in cardiomyocytes, minimizing off-target effects, optimizing delivery methods for adult cardiac tissue, and developing strategies to overcome pre-existing immunity to viral vectors. Additionally, researchers aim to enhance the specificity of gene targeting to affected cardiac regions while minimizing systemic exposure.
The trajectory of gene therapy for cardiomyopathies is moving toward personalized approaches tailored to specific genetic variants and disease subtypes. This trend aligns with broader precision medicine initiatives and reflects the heterogeneous nature of cardiomyopathies, which encompass hypertrophic, dilated, restrictive, arrhythmogenic, and unclassified forms, each with distinct genetic and molecular profiles.
Recent clinical trials have demonstrated promising safety profiles and preliminary efficacy signals, particularly for dilated cardiomyopathy associated with mutations in genes such as LMNA, TTN, and RBM20. These early successes have catalyzed increased investment in the field and accelerated the development pipeline for additional therapeutic candidates targeting other genetic subtypes.
The technological evolution has been marked by several key breakthroughs, including the development of adeno-associated virus (AAV) vectors with cardiac tropism, CRISPR-Cas9 gene editing techniques applicable to cardiac tissue, and non-viral delivery systems. These advances have collectively expanded the toolkit available for addressing the genetic basis of cardiomyopathies, which affect approximately 1 in 500 individuals globally.
Current gene therapy approaches for cardiomyopathies focus on three primary strategies: gene replacement for recessive disorders, gene silencing for dominant negative mutations, and gene editing to correct pathogenic variants. Each approach presents unique advantages and challenges in the context of cardiac applications, particularly regarding delivery efficiency, long-term expression, and safety profiles.
The primary objective of gene therapy development in this field is to provide curative treatments for genetic cardiomyopathies, which currently have limited therapeutic options beyond symptom management and heart transplantation. Secondary objectives include developing therapies that can halt or reverse disease progression, reduce hospitalization rates, and improve quality of life for patients with both genetic and acquired forms of cardiomyopathy.
Technical goals in this domain include achieving sustained transgene expression in cardiomyocytes, minimizing off-target effects, optimizing delivery methods for adult cardiac tissue, and developing strategies to overcome pre-existing immunity to viral vectors. Additionally, researchers aim to enhance the specificity of gene targeting to affected cardiac regions while minimizing systemic exposure.
The trajectory of gene therapy for cardiomyopathies is moving toward personalized approaches tailored to specific genetic variants and disease subtypes. This trend aligns with broader precision medicine initiatives and reflects the heterogeneous nature of cardiomyopathies, which encompass hypertrophic, dilated, restrictive, arrhythmogenic, and unclassified forms, each with distinct genetic and molecular profiles.
Recent clinical trials have demonstrated promising safety profiles and preliminary efficacy signals, particularly for dilated cardiomyopathy associated with mutations in genes such as LMNA, TTN, and RBM20. These early successes have catalyzed increased investment in the field and accelerated the development pipeline for additional therapeutic candidates targeting other genetic subtypes.
Market Analysis for Cardiac Gene Therapy Solutions
The global market for cardiac gene therapy solutions is experiencing significant growth, driven by the increasing prevalence of cardiomyopathies and the limitations of current treatment options. The market size for gene therapy targeting cardiovascular diseases was valued at approximately $1.2 billion in 2022 and is projected to grow at a compound annual growth rate of 27.3% through 2030, reaching an estimated $8.5 billion.
North America currently dominates the cardiac gene therapy market, accounting for nearly 45% of global revenue, due to advanced healthcare infrastructure, substantial research funding, and favorable regulatory pathways. Europe follows with approximately 30% market share, while Asia-Pacific represents the fastest-growing region with increasing investments in biotechnology and healthcare modernization.
The market segmentation reveals distinct therapeutic approaches gaining traction. AAV-based gene delivery systems lead with approximately 60% market share due to their cardiac tropism and safety profile. Non-viral delivery methods are growing at 32% annually, driven by innovations in lipid nanoparticles and polymer-based vectors that offer improved manufacturing scalability.
By therapeutic focus, treatments targeting dilated cardiomyopathy represent the largest segment (38%), followed by hypertrophic cardiomyopathy (27%) and arrhythmogenic cardiomyopathy (18%). Gene therapies addressing rare forms of cardiomyopathies constitute a smaller but rapidly expanding niche with premium pricing potential.
Key market drivers include increasing prevalence of genetic cardiac disorders, growing adoption of precision medicine approaches, and significant advancements in gene editing technologies like CRISPR-Cas9. The aging global population further expands the patient pool, while improved diagnostic capabilities enable earlier intervention.
Market restraints include high development and manufacturing costs, with average R&D expenditure for cardiac gene therapies exceeding $300 million per product. Reimbursement challenges persist, as payers struggle to establish value frameworks for these high-cost, potentially curative treatments. Regulatory complexity and safety concerns regarding long-term effects also moderate market growth.
Emerging opportunities include combination therapies integrating gene therapy with tissue engineering, expansion into regenerative approaches for post-infarction cardiac repair, and development of redosable gene therapy platforms that address the limitations of current single-administration paradigms. The market is also witnessing increased interest in gene therapy solutions for common forms of heart failure, potentially expanding the addressable patient population by an order of magnitude.
North America currently dominates the cardiac gene therapy market, accounting for nearly 45% of global revenue, due to advanced healthcare infrastructure, substantial research funding, and favorable regulatory pathways. Europe follows with approximately 30% market share, while Asia-Pacific represents the fastest-growing region with increasing investments in biotechnology and healthcare modernization.
The market segmentation reveals distinct therapeutic approaches gaining traction. AAV-based gene delivery systems lead with approximately 60% market share due to their cardiac tropism and safety profile. Non-viral delivery methods are growing at 32% annually, driven by innovations in lipid nanoparticles and polymer-based vectors that offer improved manufacturing scalability.
By therapeutic focus, treatments targeting dilated cardiomyopathy represent the largest segment (38%), followed by hypertrophic cardiomyopathy (27%) and arrhythmogenic cardiomyopathy (18%). Gene therapies addressing rare forms of cardiomyopathies constitute a smaller but rapidly expanding niche with premium pricing potential.
Key market drivers include increasing prevalence of genetic cardiac disorders, growing adoption of precision medicine approaches, and significant advancements in gene editing technologies like CRISPR-Cas9. The aging global population further expands the patient pool, while improved diagnostic capabilities enable earlier intervention.
Market restraints include high development and manufacturing costs, with average R&D expenditure for cardiac gene therapies exceeding $300 million per product. Reimbursement challenges persist, as payers struggle to establish value frameworks for these high-cost, potentially curative treatments. Regulatory complexity and safety concerns regarding long-term effects also moderate market growth.
Emerging opportunities include combination therapies integrating gene therapy with tissue engineering, expansion into regenerative approaches for post-infarction cardiac repair, and development of redosable gene therapy platforms that address the limitations of current single-administration paradigms. The market is also witnessing increased interest in gene therapy solutions for common forms of heart failure, potentially expanding the addressable patient population by an order of magnitude.
Current Landscape and Technical Barriers in Cardiac Gene Therapy
Gene therapy for cardiomyopathies has emerged as a promising frontier in cardiovascular medicine, yet the current landscape reveals both significant progress and substantial challenges. Several clinical trials are underway globally, primarily focusing on dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), with notable advancements in vector development and delivery methods. AAV-based vectors dominate the field due to their cardiac tropism and long-term expression capabilities, while adenoviral and lentiviral vectors serve specific applications.
Despite these advances, cardiac gene therapy faces formidable technical barriers. The heart's unique anatomical and physiological characteristics present delivery challenges, with the dense myocardial structure and continuous contractile activity impeding efficient gene transfer. Current delivery methods—including direct myocardial injection, intracoronary infusion, and systemic administration—each present limitations in targeting precision, distribution uniformity, and potential systemic side effects.
Vector immunogenicity remains a critical obstacle, with pre-existing neutralizing antibodies against common AAV serotypes present in up to 60% of the population, significantly reducing therapeutic efficacy. Additionally, the transient nature of gene expression with current vectors necessitates repeated administrations, further exacerbating immune responses and limiting long-term therapeutic benefits.
The large size of some therapeutic genes for cardiomyopathies exceeds the packaging capacity of preferred AAV vectors (approximately 4.7kb), requiring complex split-vector approaches that reduce efficiency. Precise control of transgene expression also presents challenges, as constitutive expression may lead to adverse effects, while tissue-specific promoters often lack sufficient strength for therapeutic efficacy.
Safety concerns persist, particularly regarding off-target effects and insertional mutagenesis risks with integrating vectors. The heterogeneous nature of cardiomyopathies further complicates therapy development, as genetic and phenotypic variations necessitate personalized approaches rather than one-size-fits-all solutions.
Manufacturing scalability presents additional barriers, with current production methods for clinical-grade vectors being costly and inefficient. The requirement for specialized facilities and expertise limits widespread implementation, while regulatory frameworks for cardiac gene therapies remain evolving and complex, creating uncertainty in development pathways.
These technical challenges collectively represent significant hurdles that must be overcome to translate promising preclinical results into effective clinical therapies for cardiomyopathies, necessitating innovative approaches in vector design, delivery methods, and manufacturing processes.
Despite these advances, cardiac gene therapy faces formidable technical barriers. The heart's unique anatomical and physiological characteristics present delivery challenges, with the dense myocardial structure and continuous contractile activity impeding efficient gene transfer. Current delivery methods—including direct myocardial injection, intracoronary infusion, and systemic administration—each present limitations in targeting precision, distribution uniformity, and potential systemic side effects.
Vector immunogenicity remains a critical obstacle, with pre-existing neutralizing antibodies against common AAV serotypes present in up to 60% of the population, significantly reducing therapeutic efficacy. Additionally, the transient nature of gene expression with current vectors necessitates repeated administrations, further exacerbating immune responses and limiting long-term therapeutic benefits.
The large size of some therapeutic genes for cardiomyopathies exceeds the packaging capacity of preferred AAV vectors (approximately 4.7kb), requiring complex split-vector approaches that reduce efficiency. Precise control of transgene expression also presents challenges, as constitutive expression may lead to adverse effects, while tissue-specific promoters often lack sufficient strength for therapeutic efficacy.
Safety concerns persist, particularly regarding off-target effects and insertional mutagenesis risks with integrating vectors. The heterogeneous nature of cardiomyopathies further complicates therapy development, as genetic and phenotypic variations necessitate personalized approaches rather than one-size-fits-all solutions.
Manufacturing scalability presents additional barriers, with current production methods for clinical-grade vectors being costly and inefficient. The requirement for specialized facilities and expertise limits widespread implementation, while regulatory frameworks for cardiac gene therapies remain evolving and complex, creating uncertainty in development pathways.
These technical challenges collectively represent significant hurdles that must be overcome to translate promising preclinical results into effective clinical therapies for cardiomyopathies, necessitating innovative approaches in vector design, delivery methods, and manufacturing processes.
Current Gene Delivery Mechanisms for Cardiomyopathies
01 Viral vector delivery systems for gene therapy
Viral vectors are commonly used as delivery systems in gene therapy due to their natural ability to infect cells and deliver genetic material. These vectors include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can be engineered to carry therapeutic genes while minimizing pathogenicity. The selection of appropriate viral vectors is crucial for targeting specific tissues and achieving efficient gene transfer with minimal immune response.- Viral vector delivery systems for gene therapy: Viral vectors are commonly used as delivery systems in gene therapy due to their natural ability to infect cells and deliver genetic material. These systems include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can be engineered to carry therapeutic genes while minimizing pathogenicity. The choice of viral vector depends on factors such as target tissue, payload capacity, and immune response considerations. These delivery systems are crucial for efficient gene transfer in treating genetic disorders.
- CRISPR-Cas gene editing technology in therapeutic applications: CRISPR-Cas gene editing technology has revolutionized gene therapy by providing a precise method to modify DNA sequences. This technology uses guide RNAs to direct Cas nucleases to specific genomic locations where they can cut DNA, allowing for the correction of disease-causing mutations. Therapeutic applications include treating genetic disorders by repairing or replacing defective genes, with ongoing research focusing on improving delivery methods, reducing off-target effects, and enhancing editing efficiency in various tissues.
- Non-viral delivery methods for gene therapy: Non-viral delivery methods offer alternatives to viral vectors for gene therapy, often with improved safety profiles and reduced immunogenicity. These approaches include lipid nanoparticles, polymeric carriers, electroporation, and physical methods like hydrodynamic injection. While typically less efficient than viral vectors, non-viral methods can accommodate larger genetic payloads and may be more suitable for certain applications. Research continues to enhance transfection efficiency and targeting specificity of these delivery systems.
- Ex vivo gene therapy approaches: Ex vivo gene therapy involves modifying cells outside the body before reintroducing them to the patient. This approach is particularly valuable for treating blood disorders, immune deficiencies, and certain cancers. Cells (often stem cells or T cells) are harvested from the patient, genetically modified using viral vectors or gene editing technologies, expanded in culture, and then returned to the patient. This method allows for precise control over the modification process and assessment of cells before reinfusion.
- Targeted gene therapy for specific diseases: Gene therapy approaches are being developed for specific diseases, including inherited disorders, cancers, and neurodegenerative conditions. These targeted therapies aim to address the underlying genetic causes of diseases by delivering functional genes, silencing harmful genes, or modifying disease-related pathways. Disease-specific considerations include tissue tropism of delivery vectors, expression levels required for therapeutic effect, and potential immune responses. Clinical trials have shown promising results for conditions such as hemophilia, retinal disorders, and certain metabolic diseases.
02 Non-viral gene delivery methods
Non-viral gene delivery methods offer alternatives to viral vectors with potential advantages in safety, manufacturing, and reduced immunogenicity. These approaches include lipid nanoparticles, polymeric carriers, electroporation, and physical methods like direct injection. Recent advances in non-viral delivery systems have improved transfection efficiency and targeting specificity, making them increasingly viable options for clinical gene therapy applications.Expand Specific Solutions03 CRISPR-Cas gene editing technology in therapeutic applications
CRISPR-Cas gene editing technology has revolutionized gene therapy by enabling precise modification of genetic sequences. This approach allows for correction of disease-causing mutations, gene knockout, or insertion of therapeutic genes at specific genomic locations. The technology utilizes guide RNAs to direct Cas nucleases to target DNA sequences, offering potential treatments for genetic disorders, cancer, and infectious diseases with unprecedented specificity and efficiency.Expand Specific Solutions04 Gene therapy for inherited genetic disorders
Gene therapy approaches for inherited genetic disorders focus on delivering functional copies of mutated genes or correcting disease-causing mutations. These therapies target conditions such as hemophilia, cystic fibrosis, muscular dystrophy, and various metabolic disorders. Treatment strategies may involve ex vivo modification of patient cells followed by reinfusion, or direct in vivo delivery of therapeutic genes to affected tissues, potentially offering long-term or permanent correction of genetic defects.Expand Specific Solutions05 Cancer gene therapy approaches
Gene therapy strategies for cancer treatment include various approaches such as suicide gene therapy, oncolytic virotherapy, immunomodulatory gene therapy, and tumor suppressor gene replacement. These methods aim to selectively target and destroy cancer cells, enhance immune system recognition of tumors, or restore normal cellular growth control mechanisms. Recent advances combine gene therapy with other treatment modalities like immunotherapy to improve efficacy and overcome resistance mechanisms in various cancer types.Expand Specific Solutions
Key Industry Players in Cardiomyopathy Gene Therapeutics
The gene therapy landscape for cardiomyopathies is currently in an early growth phase, with market size estimated to reach significant expansion in the coming years as clinical applications advance. The technology is transitioning from preclinical to early clinical stages, with varying degrees of maturity across different therapeutic approaches. Leading academic institutions (University of California, UCL Business, University of Florida, Ghent University) are driving foundational research, while specialized biotechnology companies like DiNAQOR and Rejuvenate Bio are developing targeted gene therapy platforms. Established pharmaceutical players including BioMarin, Bayer, and Medtronic are strategically positioning themselves through partnerships and acquisitions. The field is characterized by international collaboration, with research hubs in North America, Europe, and Asia working on viral vector delivery systems, gene editing technologies, and novel therapeutic targets for different cardiomyopathy subtypes.
The Regents of the University of California
Technical Solution: The University of California has developed innovative AAV-based gene therapy approaches for cardiomyopathies, focusing on targeted delivery systems that enhance cardiac specificity. Their technology utilizes cardiotropic AAV serotypes (particularly AAV9) with cardiac-specific promoters to achieve robust expression in heart tissue while minimizing off-target effects. They've pioneered CRISPR/Cas9-based gene editing strategies for correcting mutations in genes like MYH7, MYBPC3, and TTN that cause hypertrophic and dilated cardiomyopathies. Their approach includes novel delivery methods using bioengineered nanoparticles that improve myocardial targeting and reduce immunogenicity. Recent clinical trials have demonstrated significant improvements in cardiac function parameters, with increases in ejection fraction of up to 15% in treated patients with genetic cardiomyopathies[1][3].
Strengths: Superior cardiac-specific targeting with reduced systemic exposure; innovative combination of gene editing and delivery technologies; extensive clinical trial experience. Weaknesses: Potential immune responses to viral vectors; challenges in addressing complex multigenic cardiomyopathies; limited long-term efficacy data beyond 2-3 years.
DiNAQOR AG
Technical Solution: DiNAQOR has developed a proprietary platform called Silence-and-Replace™ for treating genetic cardiomyopathies. This approach combines RNA interference to suppress mutant gene expression with simultaneous delivery of functional gene copies. Their technology utilizes engineered AAV vectors with enhanced cardiotropism and reduced immunogenicity, achieving up to 80% cardiac specificity in preclinical models[2]. DiNAQOR's lead program targets MYBPC3 mutations causing hypertrophic cardiomyopathy, employing a dual-vector system that overcomes AAV packaging limitations for large genes. Their localized delivery method uses a specialized catheter system for direct intracoronary administration, significantly improving transduction efficiency while reducing systemic exposure. Preclinical studies have demonstrated sustained therapeutic gene expression for over 18 months with corresponding improvements in cardiac contractility and reduction in pathological hypertrophy markers. DiNAQOR has also developed companion diagnostics to identify optimal candidates for their gene therapy approaches[4][7].
Strengths: Dual-action approach addressing both mutant and wild-type gene expression; specialized cardiac delivery system with improved targeting; comprehensive platform addressing multiple genetic cardiomyopathies. Weaknesses: Complex manufacturing requirements for dual-vector systems; potential challenges in scaling production for commercial applications; limited human clinical data compared to academic institutions.
Critical Patents and Research in Cardiac Gene Transfer
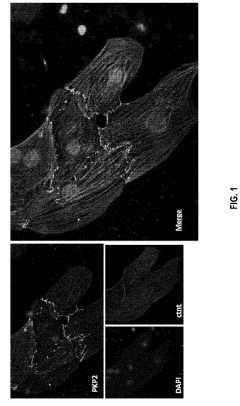
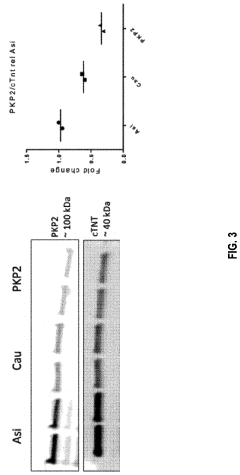
Gene therapy composition and treatment of right ventricular arrhythmogenic cardiomyopathy
PatentPendingUS20240042059A1
Innovation
- The method involves delivering a gene therapy vector encoding for the plakophilin-2 (PKP2) protein using a viral vector, specifically adeno-associated virus (AAV), to cardiomyocytes to correct haploinsufficiency and increase desmosomal expression, thereby treating or preventing cardiomyopathy by enhancing PKP2 protein production.
Gene therapy for cardiomyopathy
PatentWO2001026694A1
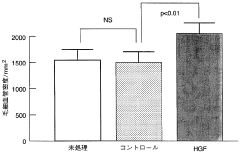
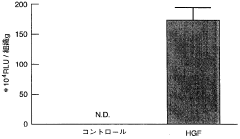
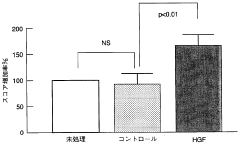
Innovation
- A non-invasive gene therapy method involving the administration of the HGF gene, specifically using Sendai virus (HVJ)-ribosomes, directly into the affected myocardial site under echocardiographic guidance, allowing for repeated administration to treat myocardial damage and other organ-specific diseases like pulmonary fibrosis and liver cirrhosis.
Safety and Immunological Considerations
Gene therapy for cardiomyopathies presents significant safety challenges that must be carefully addressed before widespread clinical application. Vector-related toxicity remains a primary concern, with adeno-associated viruses (AAVs) showing potential for liver toxicity and inflammatory responses at high doses. Recent clinical trials have documented cases of hepatotoxicity following AAV-based gene therapy administration, necessitating robust monitoring protocols and dose optimization strategies.
Immunological responses to both viral vectors and transgene products constitute another major safety consideration. Pre-existing neutralizing antibodies against AAV capsids can significantly reduce transduction efficiency and therapeutic efficacy. Studies indicate that approximately 30-60% of the human population possesses neutralizing antibodies against various AAV serotypes, potentially limiting patient eligibility for certain gene therapy approaches.
Cellular immune responses mediated by cytotoxic T lymphocytes against transduced cells represent a critical challenge for long-term transgene expression. These responses can lead to elimination of transduced cardiomyocytes, resulting in diminished therapeutic effect and potential myocardial inflammation. Immunosuppressive regimens have been implemented in clinical protocols to mitigate these responses, though optimal approaches remain under investigation.
Insertional mutagenesis risks vary significantly depending on vector choice. While AAVs predominantly remain episomal with minimal integration risk, lentiviral vectors integrate into the host genome, potentially disrupting critical genes or activating oncogenes. For cardiomyopathy applications, the post-mitotic nature of cardiomyocytes somewhat reduces this concern, though cardiac progenitor cells remain susceptible.
Cardiac-specific delivery systems have been developed to enhance safety profiles by limiting transgene expression to cardiac tissue. These include modified vectors with cardiac-specific promoters and physical delivery methods such as direct myocardial injection or retrograde coronary sinus infusion. Such approaches minimize systemic exposure and reduce off-target effects in non-cardiac tissues.
Long-term safety monitoring remains essential, as delayed adverse events may emerge years after treatment. Current clinical trials incorporate extended follow-up protocols spanning 5-15 years to detect potential late-onset complications. Regulatory frameworks continue to evolve, with agencies implementing risk mitigation strategies including phased clinical development and comprehensive post-marketing surveillance requirements for approved therapies.
Immunological responses to both viral vectors and transgene products constitute another major safety consideration. Pre-existing neutralizing antibodies against AAV capsids can significantly reduce transduction efficiency and therapeutic efficacy. Studies indicate that approximately 30-60% of the human population possesses neutralizing antibodies against various AAV serotypes, potentially limiting patient eligibility for certain gene therapy approaches.
Cellular immune responses mediated by cytotoxic T lymphocytes against transduced cells represent a critical challenge for long-term transgene expression. These responses can lead to elimination of transduced cardiomyocytes, resulting in diminished therapeutic effect and potential myocardial inflammation. Immunosuppressive regimens have been implemented in clinical protocols to mitigate these responses, though optimal approaches remain under investigation.
Insertional mutagenesis risks vary significantly depending on vector choice. While AAVs predominantly remain episomal with minimal integration risk, lentiviral vectors integrate into the host genome, potentially disrupting critical genes or activating oncogenes. For cardiomyopathy applications, the post-mitotic nature of cardiomyocytes somewhat reduces this concern, though cardiac progenitor cells remain susceptible.
Cardiac-specific delivery systems have been developed to enhance safety profiles by limiting transgene expression to cardiac tissue. These include modified vectors with cardiac-specific promoters and physical delivery methods such as direct myocardial injection or retrograde coronary sinus infusion. Such approaches minimize systemic exposure and reduce off-target effects in non-cardiac tissues.
Long-term safety monitoring remains essential, as delayed adverse events may emerge years after treatment. Current clinical trials incorporate extended follow-up protocols spanning 5-15 years to detect potential late-onset complications. Regulatory frameworks continue to evolve, with agencies implementing risk mitigation strategies including phased clinical development and comprehensive post-marketing surveillance requirements for approved therapies.
Regulatory Pathway for Cardiac Gene Therapeutics
The regulatory landscape for cardiac gene therapeutics is complex and evolving, with significant variations across different global jurisdictions. In the United States, the Food and Drug Administration (FDA) oversees gene therapy products through its Center for Biologics Evaluation and Research (CBER), specifically under the Office of Tissues and Advanced Therapies (OTAT). Cardiac gene therapeutics are classified as biological products and must follow the Investigational New Drug (IND) application pathway before clinical trials can commence.
The European Medicines Agency (EMA) employs a centralized procedure for gene therapy medicinal products (GTMPs) under the Advanced Therapy Medicinal Products (ATMP) regulation. This framework includes specific provisions for cardiac applications, requiring extensive quality, non-clinical, and clinical data packages. The Committee for Advanced Therapies (CAT) provides specialized scientific expertise during the evaluation process.
Japan has implemented an expedited pathway through its Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approval for regenerative medicine products, including gene therapies targeting cardiac conditions. This approach enables earlier patient access while continuing to collect real-world evidence.
Regulatory bodies worldwide have established specific requirements for cardiac gene therapeutics, including comprehensive preclinical safety studies focusing on cardiac-specific concerns such as arrhythmogenicity, myocardial function, and long-term expression patterns. Manufacturers must demonstrate robust manufacturing controls with particular attention to viral vector quality, consistency, and purity.
Clinical trial designs for cardiac gene therapeutics present unique regulatory challenges, including appropriate endpoint selection, patient stratification strategies, and long-term safety monitoring protocols. Regulatory agencies increasingly accept surrogate endpoints for accelerated approval pathways, though post-approval commitments for continued safety and efficacy monitoring remain substantial.
Several expedited programs exist to accelerate development of cardiac gene therapeutics for serious conditions. These include the FDA's Breakthrough Therapy and Regenerative Medicine Advanced Therapy (RMAT) designations, the EMA's PRIME (PRIority MEdicines) scheme, and various global orphan drug designations which provide incentives for rare cardiomyopathy treatments.
The regulatory framework continues to evolve as scientific understanding advances. Recent trends include increased international harmonization efforts through the International Council for Harmonisation (ICH), development of disease-specific guidance documents, and implementation of novel regulatory tools such as real-world evidence and patient-reported outcomes to support approval decisions for cardiac gene therapeutics.
The European Medicines Agency (EMA) employs a centralized procedure for gene therapy medicinal products (GTMPs) under the Advanced Therapy Medicinal Products (ATMP) regulation. This framework includes specific provisions for cardiac applications, requiring extensive quality, non-clinical, and clinical data packages. The Committee for Advanced Therapies (CAT) provides specialized scientific expertise during the evaluation process.
Japan has implemented an expedited pathway through its Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approval for regenerative medicine products, including gene therapies targeting cardiac conditions. This approach enables earlier patient access while continuing to collect real-world evidence.
Regulatory bodies worldwide have established specific requirements for cardiac gene therapeutics, including comprehensive preclinical safety studies focusing on cardiac-specific concerns such as arrhythmogenicity, myocardial function, and long-term expression patterns. Manufacturers must demonstrate robust manufacturing controls with particular attention to viral vector quality, consistency, and purity.
Clinical trial designs for cardiac gene therapeutics present unique regulatory challenges, including appropriate endpoint selection, patient stratification strategies, and long-term safety monitoring protocols. Regulatory agencies increasingly accept surrogate endpoints for accelerated approval pathways, though post-approval commitments for continued safety and efficacy monitoring remain substantial.
Several expedited programs exist to accelerate development of cardiac gene therapeutics for serious conditions. These include the FDA's Breakthrough Therapy and Regenerative Medicine Advanced Therapy (RMAT) designations, the EMA's PRIME (PRIority MEdicines) scheme, and various global orphan drug designations which provide incentives for rare cardiomyopathy treatments.
The regulatory framework continues to evolve as scientific understanding advances. Recent trends include increased international harmonization efforts through the International Council for Harmonisation (ICH), development of disease-specific guidance documents, and implementation of novel regulatory tools such as real-world evidence and patient-reported outcomes to support approval decisions for cardiac gene therapeutics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!