Gene Therapy for Hemophilia: A Closer Look at Mechanisms
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hemophilia Gene Therapy Background and Objectives
Hemophilia has been recognized as a hereditary bleeding disorder for centuries, with the first modern description dating back to the early 1800s. The condition is characterized by deficiencies in specific clotting factors - Factor VIII in Hemophilia A and Factor IX in Hemophilia B - resulting in impaired blood coagulation and increased bleeding risk. Traditional management has evolved from blood transfusions to plasma-derived clotting factors and eventually to recombinant factor concentrates, which have significantly improved patients' quality of life but still require regular infusions.
Gene therapy represents a paradigm shift in hemophilia treatment by addressing the root genetic cause rather than merely managing symptoms. The fundamental objective of hemophilia gene therapy is to enable patients' cells to produce functional clotting factors autonomously, potentially eliminating the need for regular factor replacement therapy. This approach aims to provide sustained therapeutic factor levels through a single or limited number of treatments, dramatically reducing treatment burden and improving quality of life.
The technological evolution of gene therapy vectors has been crucial to current progress. Early attempts using retroviral and adenoviral vectors faced significant challenges with immune responses and transient expression. The breakthrough came with adeno-associated viral (AAV) vectors, which demonstrate lower immunogenicity and the ability to transduce non-dividing cells, particularly hepatocytes - the natural site of factor production.
Current hemophilia gene therapy approaches primarily focus on liver-directed gene transfer using AAV vectors carrying functional factor VIII or IX genes. The field has witnessed remarkable progress, with several clinical trials demonstrating sustained factor expression for years following a single treatment. Notable milestones include the first approved gene therapy for hemophilia B (Hemgenix) in 2022, marking a historic advancement in treatment options.
Despite these achievements, significant challenges remain in the development of hemophilia gene therapy. These include pre-existing immunity to AAV vectors in many patients, limited packaging capacity of AAV vectors (particularly challenging for the large factor VIII gene), potential hepatotoxicity, and durability of expression over a patient's lifetime. Additionally, questions regarding optimal timing of intervention, long-term safety, and accessibility of these high-cost therapies require further investigation.
The ultimate goal of hemophilia gene therapy research extends beyond achieving factor levels that prevent spontaneous bleeding to establishing normal hemostasis. Researchers aim to develop solutions applicable to all hemophilia patients, including those with inhibitors, pre-existing AAV immunity, and pediatric populations currently excluded from trials. The field is progressing toward more efficient vectors, improved expression cassettes, and alternative delivery approaches to overcome current limitations.
Gene therapy represents a paradigm shift in hemophilia treatment by addressing the root genetic cause rather than merely managing symptoms. The fundamental objective of hemophilia gene therapy is to enable patients' cells to produce functional clotting factors autonomously, potentially eliminating the need for regular factor replacement therapy. This approach aims to provide sustained therapeutic factor levels through a single or limited number of treatments, dramatically reducing treatment burden and improving quality of life.
The technological evolution of gene therapy vectors has been crucial to current progress. Early attempts using retroviral and adenoviral vectors faced significant challenges with immune responses and transient expression. The breakthrough came with adeno-associated viral (AAV) vectors, which demonstrate lower immunogenicity and the ability to transduce non-dividing cells, particularly hepatocytes - the natural site of factor production.
Current hemophilia gene therapy approaches primarily focus on liver-directed gene transfer using AAV vectors carrying functional factor VIII or IX genes. The field has witnessed remarkable progress, with several clinical trials demonstrating sustained factor expression for years following a single treatment. Notable milestones include the first approved gene therapy for hemophilia B (Hemgenix) in 2022, marking a historic advancement in treatment options.
Despite these achievements, significant challenges remain in the development of hemophilia gene therapy. These include pre-existing immunity to AAV vectors in many patients, limited packaging capacity of AAV vectors (particularly challenging for the large factor VIII gene), potential hepatotoxicity, and durability of expression over a patient's lifetime. Additionally, questions regarding optimal timing of intervention, long-term safety, and accessibility of these high-cost therapies require further investigation.
The ultimate goal of hemophilia gene therapy research extends beyond achieving factor levels that prevent spontaneous bleeding to establishing normal hemostasis. Researchers aim to develop solutions applicable to all hemophilia patients, including those with inhibitors, pre-existing AAV immunity, and pediatric populations currently excluded from trials. The field is progressing toward more efficient vectors, improved expression cassettes, and alternative delivery approaches to overcome current limitations.
Market Analysis for Hemophilia Gene Therapy Solutions
The global hemophilia gene therapy market is experiencing unprecedented growth, driven by significant advancements in genetic engineering technologies and increasing prevalence of hemophilia worldwide. Currently valued at approximately 11.98 billion USD in 2023, the market is projected to reach 17.92 billion USD by 2030, representing a compound annual growth rate (CAGR) of 5.9% during the forecast period.
Hemophilia A dominates the market share, accounting for roughly 80-85% of cases globally, while Hemophilia B represents the remaining 15-20%. This distribution directly influences investment patterns in gene therapy research and development, with proportionally higher resources allocated to Hemophilia A solutions.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30%, Asia-Pacific at 20%, and the rest of the world at 5%. The United States specifically represents the largest single country market due to its advanced healthcare infrastructure, substantial research funding, and favorable regulatory environment for gene therapy approvals.
Key market drivers include the high cost burden of traditional factor replacement therapies, which can exceed 300,000 USD annually per patient for severe cases, creating strong economic incentives for one-time curative approaches. Additionally, patient advocacy groups are increasingly demanding longer-lasting solutions that reduce treatment burden and improve quality of life.
Market restraints primarily revolve around the extremely high cost of gene therapies, with current approved treatments priced between 2-3 million USD per patient. Reimbursement challenges persist as healthcare systems struggle to accommodate these high one-time costs despite potential long-term savings. Safety concerns regarding immune responses to viral vectors and potential oncogenicity risks also temper market growth.
The competitive landscape features pharmaceutical giants like Pfizer, Bayer, and Novo Nordisk alongside specialized biotech companies such as BioMarin, Spark Therapeutics (Roche), and uniQure. Recent strategic partnerships between large pharmaceutical companies and gene therapy specialists indicate market consolidation trends, with larger entities acquiring specialized technological capabilities.
Patient segmentation reveals that severe hemophilia patients (factor levels <1%) represent the primary target population for gene therapy, constituting approximately 60% of the addressable market. Moderate patients (factor levels 1-5%) represent a secondary but growing segment as therapy safety profiles improve.
Future market evolution will likely feature tiered pricing models, outcomes-based reimbursement structures, and expanded geographic accessibility as manufacturing processes become more efficient and scalable.
Hemophilia A dominates the market share, accounting for roughly 80-85% of cases globally, while Hemophilia B represents the remaining 15-20%. This distribution directly influences investment patterns in gene therapy research and development, with proportionally higher resources allocated to Hemophilia A solutions.
Geographically, North America leads the market with approximately 45% share, followed by Europe at 30%, Asia-Pacific at 20%, and the rest of the world at 5%. The United States specifically represents the largest single country market due to its advanced healthcare infrastructure, substantial research funding, and favorable regulatory environment for gene therapy approvals.
Key market drivers include the high cost burden of traditional factor replacement therapies, which can exceed 300,000 USD annually per patient for severe cases, creating strong economic incentives for one-time curative approaches. Additionally, patient advocacy groups are increasingly demanding longer-lasting solutions that reduce treatment burden and improve quality of life.
Market restraints primarily revolve around the extremely high cost of gene therapies, with current approved treatments priced between 2-3 million USD per patient. Reimbursement challenges persist as healthcare systems struggle to accommodate these high one-time costs despite potential long-term savings. Safety concerns regarding immune responses to viral vectors and potential oncogenicity risks also temper market growth.
The competitive landscape features pharmaceutical giants like Pfizer, Bayer, and Novo Nordisk alongside specialized biotech companies such as BioMarin, Spark Therapeutics (Roche), and uniQure. Recent strategic partnerships between large pharmaceutical companies and gene therapy specialists indicate market consolidation trends, with larger entities acquiring specialized technological capabilities.
Patient segmentation reveals that severe hemophilia patients (factor levels <1%) represent the primary target population for gene therapy, constituting approximately 60% of the addressable market. Moderate patients (factor levels 1-5%) represent a secondary but growing segment as therapy safety profiles improve.
Future market evolution will likely feature tiered pricing models, outcomes-based reimbursement structures, and expanded geographic accessibility as manufacturing processes become more efficient and scalable.
Current Landscape and Challenges in Hemophilia Gene Therapy
Hemophilia gene therapy has witnessed remarkable progress over the past decade, with several clinical trials demonstrating promising results. Currently, there are over 20 active clinical trials globally investigating various gene therapy approaches for both hemophilia A and B. The landscape is dominated by adeno-associated virus (AAV) vector-based therapies, which have shown the most consistent results in delivering functional factor VIII or IX genes to hepatocytes.
The FDA's approval of Hemgenix (etranacogene dezaparvovec) in 2022 marked a significant milestone as the first gene therapy for hemophilia B. This therapy utilizes an AAV5 vector to deliver a functional copy of the factor IX gene, demonstrating sustained factor IX activity levels in treated patients. For hemophilia A, BioMarin's Roctavian (valoctocogene roxaparvovec) received conditional approval in Europe in 2022, while continuing its regulatory journey in other regions.
Despite these advancements, several critical challenges persist in hemophilia gene therapy. Pre-existing immunity to AAV vectors remains a significant barrier, with approximately 30-50% of potential patients having neutralizing antibodies that exclude them from current therapies. This immunological challenge limits the eligible patient population and raises questions about equitable access to these breakthrough treatments.
Vector dose-dependent hepatotoxicity presents another major concern, with elevated liver enzymes observed in multiple clinical trials. This toxicity often necessitates immunosuppressive regimens, adding complexity to patient management and raising long-term safety concerns. The durability of therapeutic effect also remains uncertain, with some studies showing declining factor expression over time, particularly in hemophilia A trials.
The large size of the factor VIII gene creates specific challenges for hemophilia A therapies, requiring either vector optimization or the use of truncated gene variants, which may affect protein functionality. Manufacturing scalability presents additional hurdles, with current production methods struggling to meet potential global demand while maintaining consistent quality and purity standards.
Cost considerations further complicate the landscape, with current gene therapies priced between $2-3 million per treatment. This raises significant concerns about healthcare system sustainability and patient access, particularly in resource-limited settings. Regulatory frameworks continue to evolve as agencies develop appropriate assessment metrics for these novel one-time treatments.
Geographically, research and clinical trials remain concentrated in North America, Europe, and select Asian countries, creating disparities in global access. The technical expertise and infrastructure required for gene therapy administration further exacerbate these regional inequalities, highlighting the need for capacity building in underserved regions.
The FDA's approval of Hemgenix (etranacogene dezaparvovec) in 2022 marked a significant milestone as the first gene therapy for hemophilia B. This therapy utilizes an AAV5 vector to deliver a functional copy of the factor IX gene, demonstrating sustained factor IX activity levels in treated patients. For hemophilia A, BioMarin's Roctavian (valoctocogene roxaparvovec) received conditional approval in Europe in 2022, while continuing its regulatory journey in other regions.
Despite these advancements, several critical challenges persist in hemophilia gene therapy. Pre-existing immunity to AAV vectors remains a significant barrier, with approximately 30-50% of potential patients having neutralizing antibodies that exclude them from current therapies. This immunological challenge limits the eligible patient population and raises questions about equitable access to these breakthrough treatments.
Vector dose-dependent hepatotoxicity presents another major concern, with elevated liver enzymes observed in multiple clinical trials. This toxicity often necessitates immunosuppressive regimens, adding complexity to patient management and raising long-term safety concerns. The durability of therapeutic effect also remains uncertain, with some studies showing declining factor expression over time, particularly in hemophilia A trials.
The large size of the factor VIII gene creates specific challenges for hemophilia A therapies, requiring either vector optimization or the use of truncated gene variants, which may affect protein functionality. Manufacturing scalability presents additional hurdles, with current production methods struggling to meet potential global demand while maintaining consistent quality and purity standards.
Cost considerations further complicate the landscape, with current gene therapies priced between $2-3 million per treatment. This raises significant concerns about healthcare system sustainability and patient access, particularly in resource-limited settings. Regulatory frameworks continue to evolve as agencies develop appropriate assessment metrics for these novel one-time treatments.
Geographically, research and clinical trials remain concentrated in North America, Europe, and select Asian countries, creating disparities in global access. The technical expertise and infrastructure required for gene therapy administration further exacerbate these regional inequalities, highlighting the need for capacity building in underserved regions.
Current Vector Delivery Systems and Therapeutic Approaches
01 AAV-based gene therapy vectors for hemophilia
Adeno-associated virus (AAV) vectors are widely used in gene therapy for hemophilia due to their safety profile and efficiency in delivering therapeutic genes. These vectors can be engineered to target specific tissues, particularly the liver, which is the primary site for clotting factor production. AAV-mediated gene transfer enables sustained expression of clotting factors VIII or IX, potentially providing long-term treatment for hemophilia patients with minimal immune response.- AAV-based gene therapy vectors for hemophilia: Adeno-associated virus (AAV) vectors are widely used in gene therapy approaches for hemophilia due to their safety profile and efficiency in delivering therapeutic genes. These vectors can be engineered to carry genes encoding clotting factors such as Factor VIII for hemophilia A or Factor IX for hemophilia B. The AAV vectors target liver cells, enabling them to produce the missing clotting factors continuously, potentially providing long-term therapeutic benefits with a single administration.
- Lentiviral and retroviral vector systems for hemophilia gene therapy: Lentiviral and retroviral vectors offer an alternative approach for hemophilia gene therapy, particularly beneficial for their ability to integrate into the host genome, potentially providing long-term expression of therapeutic genes. These vectors can be modified to enhance safety and targeting specificity, reducing the risk of insertional mutagenesis. They are especially useful for ex vivo gene therapy approaches where patient cells are modified outside the body before reinfusion, allowing for stable expression of clotting factors.
- CRISPR/Cas9 gene editing for hemophilia treatment: CRISPR/Cas9 technology represents a cutting-edge approach for treating hemophilia by directly correcting mutations in the patient's genome. This precision gene editing system can target specific mutations in the F8 or F9 genes responsible for hemophilia A and B, respectively. The technology allows for permanent correction of the genetic defect, potentially eliminating the need for repeated treatments. Various delivery methods, including viral vectors and lipid nanoparticles, are being developed to efficiently introduce the CRISPR/Cas9 components into target cells.
- Non-viral delivery systems for hemophilia gene therapy: Non-viral delivery systems offer advantages for hemophilia gene therapy including reduced immunogenicity and larger packaging capacity compared to viral vectors. These systems include lipid nanoparticles, polymeric carriers, and physical methods such as electroporation. They can deliver therapeutic genes encoding clotting factors or gene editing components to target cells. Recent advances in non-viral delivery technologies have improved transfection efficiency and targeting specificity, making them increasingly viable alternatives to viral vectors for hemophilia treatment.
- Immune tolerance induction strategies in hemophilia gene therapy: Immune responses to gene therapy vectors or the expressed clotting factors can significantly reduce treatment efficacy in hemophilia patients. Various strategies are being developed to induce immune tolerance, including immunomodulatory drugs, engineered regulatory T cells, and liver-directed gene expression to promote tolerance. Additionally, vector capsid modifications and optimization of transgene sequences can help evade immune detection. These approaches aim to prevent the formation of inhibitory antibodies against therapeutic clotting factors and enable successful long-term gene therapy outcomes.
02 Lentiviral and retroviral vector systems
Lentiviral and retroviral vectors offer an alternative approach for hemophilia gene therapy by integrating therapeutic genes into the host genome, potentially providing lifelong expression of clotting factors. These vector systems are particularly useful for ex vivo gene therapy approaches where patient cells are modified outside the body before reinfusion. The integration capability allows for stable transgene expression in dividing cells, though careful design is necessary to minimize insertional mutagenesis risks.Expand Specific Solutions03 CRISPR/Cas9 gene editing for hemophilia treatment
CRISPR/Cas9 technology represents a cutting-edge approach for treating hemophilia by directly correcting mutations in the F8 or F9 genes. This precision gene editing technique can repair specific genetic defects responsible for hemophilia, potentially restoring normal clotting factor production. The technology allows for targeted modification of hepatocytes to enable endogenous production of functional clotting factors, offering the possibility of a permanent cure rather than just symptom management.Expand Specific Solutions04 Non-viral delivery systems for hemophilia gene therapy
Non-viral delivery systems, including lipid nanoparticles, polymeric carriers, and physical methods like electroporation, offer advantages for hemophilia gene therapy such as reduced immunogenicity and larger packaging capacity compared to viral vectors. These systems can deliver therapeutic genes or mRNA encoding clotting factors to hepatocytes, providing transient expression that may require repeated administration but with potentially improved safety profiles. Recent advances in non-viral delivery technologies have significantly improved transfection efficiency and targeting specificity.Expand Specific Solutions05 Immune tolerance induction strategies
Immune responses to introduced clotting factors or gene therapy vectors represent a significant challenge in hemophilia treatment. Various strategies have been developed to induce immune tolerance, including co-delivery of immunomodulatory molecules, transient immunosuppression during gene therapy administration, liver-directed gene expression to promote tolerance, and engineered vector capsids with reduced immunogenicity. These approaches aim to prevent the formation of inhibitory antibodies against therapeutic clotting factors, enhancing the long-term efficacy of gene therapy treatments.Expand Specific Solutions
Key Stakeholders in Hemophilia Gene Therapy Development
Gene therapy for hemophilia is currently in a transitional phase from experimental to commercial applications, with the market expected to grow significantly from its current valuation of approximately $1 billion to over $5 billion by 2028. The technology has reached mid-level maturity, with several key players advancing clinical trials and regulatory approvals. Companies like uniQure, Sangamo Therapeutics, and Pfizer are leading with late-stage clinical trials, while Takeda Pharmaceutical and CSL Behring have made significant investments in platform development. Academic institutions including St. Jude Children's Research Hospital and University of Pennsylvania contribute foundational research. The competitive landscape is intensifying as bluebird bio and Editas Medicine leverage gene editing technologies to improve treatment efficacy, while established pharmaceutical companies like Baxalta (Takeda) are strategically positioning through acquisitions and partnerships to capture market share in this emerging therapeutic area.
uniQure biopharma BV
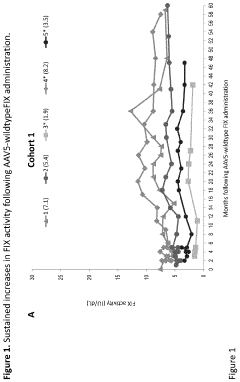
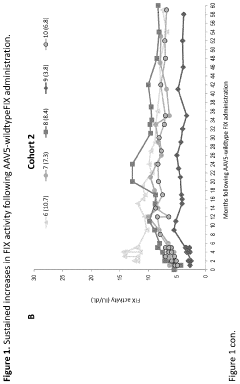
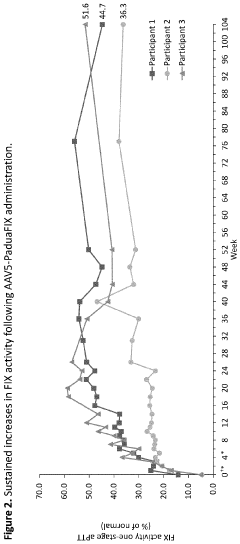
Technical Solution: uniQure has developed AMT-061 (etranacogene dezaparvovec), an AAV5-based gene therapy for hemophilia B that delivers a highly functional variant of human Factor IX (FIX-Padua). This therapy utilizes a codon-optimized gene cassette containing the FIX-Padua variant with a single amino acid substitution (R338L) that results in approximately 8-9 times higher FIX activity levels than wild-type FIX[1]. The AAV5 vector serotype was specifically chosen for its favorable immunological profile and ability to transduce liver cells efficiently, even in patients with pre-existing neutralizing antibodies to other AAV serotypes[2]. uniQure's manufacturing process employs an insect cell-based baculovirus expression system that allows for scalable production with consistent quality. Clinical trials have demonstrated sustained FIX activity levels of approximately 40% of normal, significantly reducing annual bleeding rates and virtually eliminating the need for FIX replacement therapy in most patients[3].
Strengths: Superior transduction efficiency in liver cells with AAV5 vectors; ability to treat patients with pre-existing antibodies to other AAV serotypes; scalable manufacturing process; demonstrated long-term expression of therapeutic FIX levels. Weaknesses: Potential immune responses to AAV capsid proteins may still occur in some patients; high production costs; limited redosing capability due to development of neutralizing antibodies after initial treatment.
Sangamo Therapeutics, Inc.
Technical Solution: Sangamo Therapeutics has pioneered a genome editing approach for hemophilia using zinc finger nuclease (ZFN) technology. Their platform, SB-FIX, employs a targeted in vivo genome editing strategy that precisely integrates a functional Factor IX gene into the albumin locus of liver cells[1]. This approach leverages the liver's natural albumin production machinery, allowing the therapeutic gene to benefit from the strong albumin promoter while maintaining physiological regulation. The ZFN components and donor DNA template are delivered via AAV vectors, creating a targeted double-strand break in the albumin locus that facilitates integration of the functional Factor IX gene through homology-directed repair[2]. This site-specific integration is designed to provide stable, lifelong expression of Factor IX without disrupting essential genes or risking insertional mutagenesis. Sangamo's technology also incorporates proprietary modifications to enhance specificity and reduce off-target effects, addressing a key safety concern in genome editing approaches[3].
Strengths: Site-specific integration provides potential for permanent correction without vector dilution during liver cell turnover; leverages strong albumin promoter for robust expression; avoids random integration risks; potentially allows for physiological regulation of Factor IX. Weaknesses: More complex delivery requirements compared to conventional AAV gene therapy; potential for off-target editing despite improvements in specificity; lower editing efficiency compared to viral vector-mediated gene transfer; requires optimization of both ZFN and donor template delivery.
Critical Mechanisms and Breakthrough Technologies
Methods and means for the prevention and/or treatment of hemophilic arthropathy in hemophilia
PatentPendingUS20240181082A1
Innovation
- Gene therapy using a nucleic acid-encoded coagulation factor, such as Factor IX, delivered via viral vectors like AAV, aims to maintain constant activity levels above the threshold of less severe forms of hemophilia, reducing joint damage by flattening peaks and troughs, thereby arresting or slowing the progression of hemophilic arthropathy.
Gene therapy of hemophilia a using viral vectors encoding recombinant fviii variants with increased expression
PatentPendingUS20240269241A1
Innovation
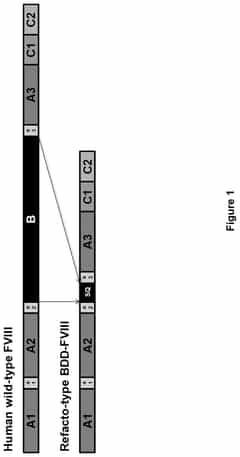
- Development of codon-altered Factor VIII variants with high sequence identity to CS04-HC and CS04-LC nucleotide sequences, including a linker sequence that replaces the native B-domain, for improved packaging and delivery via gene therapy vectors, enhancing expression and virion packaging.
Safety and Immunogenicity Considerations
Safety considerations remain paramount in the development of gene therapy for hemophilia. The administration of viral vectors, particularly adeno-associated viruses (AAVs), can trigger immune responses that potentially compromise both safety and efficacy. Clinical trials have documented various immunological reactions, ranging from mild inflammatory responses to severe systemic reactions requiring immediate medical intervention. These immune responses often target either the viral capsid proteins or the transgene product itself, creating significant barriers to successful treatment outcomes.
The development of neutralizing antibodies against viral vectors represents a critical challenge in hemophilia gene therapy. These antibodies can substantially reduce transduction efficiency and prevent successful gene delivery to target tissues. Studies indicate that approximately 30-50% of the general population possesses pre-existing neutralizing antibodies against common AAV serotypes, potentially excluding a significant portion of patients from current gene therapy approaches. Furthermore, vector-induced immune responses can lead to the elimination of transduced cells, resulting in diminished therapeutic protein expression over time.
Liver toxicity has emerged as another significant safety concern in hemophilia gene therapy trials. Elevated liver enzymes have been observed in multiple clinical studies, indicating potential hepatocellular damage following vector administration. This toxicity appears dose-dependent and may necessitate immunosuppressive treatment to mitigate adverse effects. Long-term monitoring protocols have become standard practice to detect delayed hepatotoxicity that might manifest months or years after treatment.
Insertional mutagenesis, though less common with AAV vectors compared to retroviral vectors, remains a theoretical concern requiring vigilant long-term surveillance. While AAVs predominantly exist episomally, integration events have been documented, particularly in dividing cells or at sites of DNA damage. The potential for vector integration to disrupt tumor suppressor genes or activate oncogenes necessitates comprehensive genotoxicity assessments and extended patient monitoring strategies.
Immunosuppressive regimens have been implemented in clinical protocols to mitigate immune responses, typically involving corticosteroids and sometimes additional immunomodulatory agents. These regimens have demonstrated partial success in controlling immune reactions but introduce additional risks associated with immunosuppression. Optimizing these protocols to balance immune suppression with minimal side effects represents an ongoing challenge in the field.
Novel approaches to overcome immunogenicity include vector engineering to reduce capsid antigenicity, development of alternative serotypes with lower prevalence of pre-existing immunity, and implementation of tolerogenic strategies to prevent immune responses against the therapeutic transgene product. Empty capsid decoys and transient immunomodulation techniques are also being explored as potential solutions to enhance the safety profile of hemophilia gene therapy.
The development of neutralizing antibodies against viral vectors represents a critical challenge in hemophilia gene therapy. These antibodies can substantially reduce transduction efficiency and prevent successful gene delivery to target tissues. Studies indicate that approximately 30-50% of the general population possesses pre-existing neutralizing antibodies against common AAV serotypes, potentially excluding a significant portion of patients from current gene therapy approaches. Furthermore, vector-induced immune responses can lead to the elimination of transduced cells, resulting in diminished therapeutic protein expression over time.
Liver toxicity has emerged as another significant safety concern in hemophilia gene therapy trials. Elevated liver enzymes have been observed in multiple clinical studies, indicating potential hepatocellular damage following vector administration. This toxicity appears dose-dependent and may necessitate immunosuppressive treatment to mitigate adverse effects. Long-term monitoring protocols have become standard practice to detect delayed hepatotoxicity that might manifest months or years after treatment.
Insertional mutagenesis, though less common with AAV vectors compared to retroviral vectors, remains a theoretical concern requiring vigilant long-term surveillance. While AAVs predominantly exist episomally, integration events have been documented, particularly in dividing cells or at sites of DNA damage. The potential for vector integration to disrupt tumor suppressor genes or activate oncogenes necessitates comprehensive genotoxicity assessments and extended patient monitoring strategies.
Immunosuppressive regimens have been implemented in clinical protocols to mitigate immune responses, typically involving corticosteroids and sometimes additional immunomodulatory agents. These regimens have demonstrated partial success in controlling immune reactions but introduce additional risks associated with immunosuppression. Optimizing these protocols to balance immune suppression with minimal side effects represents an ongoing challenge in the field.
Novel approaches to overcome immunogenicity include vector engineering to reduce capsid antigenicity, development of alternative serotypes with lower prevalence of pre-existing immunity, and implementation of tolerogenic strategies to prevent immune responses against the therapeutic transgene product. Empty capsid decoys and transient immunomodulation techniques are also being explored as potential solutions to enhance the safety profile of hemophilia gene therapy.
Long-term Efficacy and Patient Monitoring Strategies
The long-term efficacy of gene therapy for hemophilia represents a critical consideration in treatment evaluation, with current clinical data suggesting persistence of therapeutic effect ranging from 5-8 years in the most successful trials. This duration, while promising, falls short of the lifelong cure initially envisioned, necessitating robust monitoring strategies to track treatment efficacy over extended timeframes.
Monitoring protocols typically include regular assessment of factor levels (VIII or IX) at decreasing intervals, beginning with weekly measurements post-administration, then monthly for the first year, and quarterly thereafter. These measurements provide crucial data on the stability of transgene expression and help identify early signs of efficacy waning.
Comprehensive coagulation profiles, including thrombin generation assays and rotational thromboelastometry, offer deeper insights into hemostatic function beyond simple factor level measurements. These advanced assays can detect subtle changes in coagulation dynamics that may precede clinically significant bleeding events.
Immunological surveillance constitutes another vital component of long-term monitoring, focusing on antibody development against the viral vector or the transgene product. Evidence suggests that neutralizing antibodies may develop in approximately 30% of patients within 3-5 years post-treatment, potentially compromising therapeutic efficacy.
Liver health monitoring through regular enzyme panels, imaging studies, and in some cases, periodic biopsies, helps assess the long-term safety of hepatocyte-directed gene therapy. Emerging data indicates that transaminase elevations may occur years after treatment, necessitating vigilance beyond the initial post-administration period.
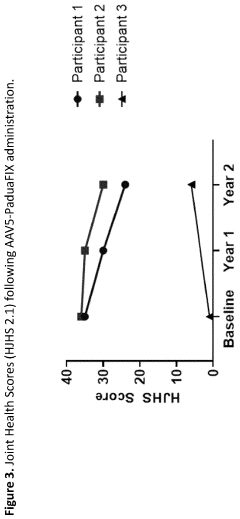
Quality of life assessments using validated instruments such as the Hemophilia Quality of Life Questionnaire (Haem-A-QoL) provide patient-centered outcomes data that complement clinical measurements. These assessments have demonstrated sustained improvements in physical functioning and reduced anxiety about bleeding events for up to 5 years post-therapy.
The development of biomarkers predictive of long-term efficacy remains an active area of research. Preliminary studies suggest that early post-treatment patterns of vector genome copy numbers in peripheral blood and specific microRNA signatures may correlate with sustained transgene expression.
Economic analyses indicate that despite high initial costs, gene therapy may achieve cost-effectiveness thresholds if therapeutic effects persist beyond 8-10 years. This economic consideration underscores the importance of developing reliable predictors of long-term efficacy to inform treatment decisions and healthcare resource allocation.
Monitoring protocols typically include regular assessment of factor levels (VIII or IX) at decreasing intervals, beginning with weekly measurements post-administration, then monthly for the first year, and quarterly thereafter. These measurements provide crucial data on the stability of transgene expression and help identify early signs of efficacy waning.
Comprehensive coagulation profiles, including thrombin generation assays and rotational thromboelastometry, offer deeper insights into hemostatic function beyond simple factor level measurements. These advanced assays can detect subtle changes in coagulation dynamics that may precede clinically significant bleeding events.
Immunological surveillance constitutes another vital component of long-term monitoring, focusing on antibody development against the viral vector or the transgene product. Evidence suggests that neutralizing antibodies may develop in approximately 30% of patients within 3-5 years post-treatment, potentially compromising therapeutic efficacy.
Liver health monitoring through regular enzyme panels, imaging studies, and in some cases, periodic biopsies, helps assess the long-term safety of hepatocyte-directed gene therapy. Emerging data indicates that transaminase elevations may occur years after treatment, necessitating vigilance beyond the initial post-administration period.
Quality of life assessments using validated instruments such as the Hemophilia Quality of Life Questionnaire (Haem-A-QoL) provide patient-centered outcomes data that complement clinical measurements. These assessments have demonstrated sustained improvements in physical functioning and reduced anxiety about bleeding events for up to 5 years post-therapy.
The development of biomarkers predictive of long-term efficacy remains an active area of research. Preliminary studies suggest that early post-treatment patterns of vector genome copy numbers in peripheral blood and specific microRNA signatures may correlate with sustained transgene expression.
Economic analyses indicate that despite high initial costs, gene therapy may achieve cost-effectiveness thresholds if therapeutic effects persist beyond 8-10 years. This economic consideration underscores the importance of developing reliable predictors of long-term efficacy to inform treatment decisions and healthcare resource allocation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!