The Mechanism of Action Behind Anti-Tumor Gene Therapy Approaches
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy Evolution and Objectives
Gene therapy has evolved significantly since its conceptual inception in the 1970s, transforming from theoretical possibility to clinical reality in anti-tumor applications. The initial phase of gene therapy development (1970s-1990s) focused primarily on establishing fundamental concepts and developing basic viral vector systems. This period saw the first approved gene therapy clinical trial in 1990 for treating severe combined immunodeficiency, laying groundwork for future cancer applications.
The second evolutionary phase (1990s-2000s) witnessed critical advancements in vector technology and delivery systems, particularly with the refinement of adenoviral, retroviral, and later lentiviral vectors. During this period, researchers began exploring specific anti-tumor applications, though early clinical trials yielded mixed results due to safety concerns and limited efficacy.
The contemporary phase (2010s-present) has been characterized by revolutionary breakthroughs, including CRISPR-Cas9 gene editing technology, which has dramatically expanded precision capabilities for targeting cancer-specific genetic alterations. This period has also seen the emergence of CAR-T cell therapy as the first FDA-approved gene therapy for certain hematological malignancies, demonstrating remarkable response rates in previously treatment-resistant patients.
The current technical objectives in anti-tumor gene therapy center around several key areas. First, enhancing delivery efficiency to solid tumors remains a significant challenge, as the tumor microenvironment presents substantial barriers to effective gene transfer. Researchers aim to develop vectors capable of penetrating these physical and biological barriers while maintaining specificity for cancer cells.
Second, improving safety profiles by minimizing off-target effects and immunogenicity represents a critical objective. This includes developing more sophisticated regulatory elements to control transgene expression specifically within tumor cells and reducing vector-related immune responses.
Third, expanding the range of targetable cancers beyond the current successes in hematological malignancies to include common solid tumors such as breast, lung, and colorectal cancers. This requires overcoming the heterogeneity and complex microenvironments characteristic of solid tumors.
Finally, researchers are pursuing combination approaches that integrate gene therapy with conventional treatments or immunotherapies to achieve synergistic effects. The ultimate objective is to develop gene therapy platforms that can be personalized based on individual tumor genetic profiles, moving toward precision oncology where treatments target specific driver mutations or dysregulated pathways unique to each patient's cancer.
The second evolutionary phase (1990s-2000s) witnessed critical advancements in vector technology and delivery systems, particularly with the refinement of adenoviral, retroviral, and later lentiviral vectors. During this period, researchers began exploring specific anti-tumor applications, though early clinical trials yielded mixed results due to safety concerns and limited efficacy.
The contemporary phase (2010s-present) has been characterized by revolutionary breakthroughs, including CRISPR-Cas9 gene editing technology, which has dramatically expanded precision capabilities for targeting cancer-specific genetic alterations. This period has also seen the emergence of CAR-T cell therapy as the first FDA-approved gene therapy for certain hematological malignancies, demonstrating remarkable response rates in previously treatment-resistant patients.
The current technical objectives in anti-tumor gene therapy center around several key areas. First, enhancing delivery efficiency to solid tumors remains a significant challenge, as the tumor microenvironment presents substantial barriers to effective gene transfer. Researchers aim to develop vectors capable of penetrating these physical and biological barriers while maintaining specificity for cancer cells.
Second, improving safety profiles by minimizing off-target effects and immunogenicity represents a critical objective. This includes developing more sophisticated regulatory elements to control transgene expression specifically within tumor cells and reducing vector-related immune responses.
Third, expanding the range of targetable cancers beyond the current successes in hematological malignancies to include common solid tumors such as breast, lung, and colorectal cancers. This requires overcoming the heterogeneity and complex microenvironments characteristic of solid tumors.
Finally, researchers are pursuing combination approaches that integrate gene therapy with conventional treatments or immunotherapies to achieve synergistic effects. The ultimate objective is to develop gene therapy platforms that can be personalized based on individual tumor genetic profiles, moving toward precision oncology where treatments target specific driver mutations or dysregulated pathways unique to each patient's cancer.
Anti-Tumor Therapy Market Analysis
The global anti-tumor therapy market has witnessed substantial growth in recent years, with gene therapy approaches emerging as a promising segment. Currently valued at approximately 156 billion USD, the market is projected to reach 310 billion USD by 2028, representing a compound annual growth rate (CAGR) of 12.1%. This growth is primarily driven by the increasing prevalence of cancer worldwide, with over 19.3 million new cases reported in 2020 according to the World Health Organization.
Gene therapy specifically has shown remarkable market potential within this broader landscape. The anti-tumor gene therapy segment was valued at 7.2 billion USD in 2022 and is expected to grow at a CAGR of 18.7% through 2030, significantly outpacing traditional treatment modalities. This accelerated growth reflects the paradigm shift occurring in cancer treatment approaches, moving from conventional cytotoxic therapies toward more targeted molecular interventions.
Demand analysis reveals strong market pull factors across different geographic regions. North America currently dominates the market with approximately 45% share, followed by Europe (28%) and Asia-Pacific (20%). However, the highest growth rates are being observed in emerging markets, particularly China and India, where increasing healthcare expenditure and growing awareness about advanced treatment options are creating new market opportunities.
The reimbursement landscape for gene therapy approaches remains complex but is gradually evolving. In the United States, the Centers for Medicare & Medicaid Services has implemented new payment models specifically designed for cell and gene therapies, while the European Medicines Agency has established accelerated assessment procedures for these innovative treatments.
Patient demographics significantly influence market dynamics, with a growing aging population contributing to increased cancer incidence rates. Additionally, the rise of precision medicine and personalized treatment approaches has created distinct market segments based on biomarker profiles and genetic characteristics of tumors.
Competitive analysis indicates a fragmented market structure with several key players including Novartis, Gilead Sciences (Kite Pharma), Bristol Myers Squibb, and emerging biotech companies specializing in gene therapy platforms. Strategic partnerships between pharmaceutical companies and academic institutions have become increasingly common, facilitating technology transfer and accelerating commercialization pathways.
Market barriers include high treatment costs, manufacturing challenges, and regulatory hurdles. The average cost of gene therapy treatments ranges from $373,000 to over $2 million per patient, raising significant concerns about accessibility and healthcare system sustainability. Despite these challenges, investor confidence remains strong, with venture capital funding for gene therapy startups reaching record levels of $5.7 billion in 2021.
Gene therapy specifically has shown remarkable market potential within this broader landscape. The anti-tumor gene therapy segment was valued at 7.2 billion USD in 2022 and is expected to grow at a CAGR of 18.7% through 2030, significantly outpacing traditional treatment modalities. This accelerated growth reflects the paradigm shift occurring in cancer treatment approaches, moving from conventional cytotoxic therapies toward more targeted molecular interventions.
Demand analysis reveals strong market pull factors across different geographic regions. North America currently dominates the market with approximately 45% share, followed by Europe (28%) and Asia-Pacific (20%). However, the highest growth rates are being observed in emerging markets, particularly China and India, where increasing healthcare expenditure and growing awareness about advanced treatment options are creating new market opportunities.
The reimbursement landscape for gene therapy approaches remains complex but is gradually evolving. In the United States, the Centers for Medicare & Medicaid Services has implemented new payment models specifically designed for cell and gene therapies, while the European Medicines Agency has established accelerated assessment procedures for these innovative treatments.
Patient demographics significantly influence market dynamics, with a growing aging population contributing to increased cancer incidence rates. Additionally, the rise of precision medicine and personalized treatment approaches has created distinct market segments based on biomarker profiles and genetic characteristics of tumors.
Competitive analysis indicates a fragmented market structure with several key players including Novartis, Gilead Sciences (Kite Pharma), Bristol Myers Squibb, and emerging biotech companies specializing in gene therapy platforms. Strategic partnerships between pharmaceutical companies and academic institutions have become increasingly common, facilitating technology transfer and accelerating commercialization pathways.
Market barriers include high treatment costs, manufacturing challenges, and regulatory hurdles. The average cost of gene therapy treatments ranges from $373,000 to over $2 million per patient, raising significant concerns about accessibility and healthcare system sustainability. Despite these challenges, investor confidence remains strong, with venture capital funding for gene therapy startups reaching record levels of $5.7 billion in 2021.
Current Gene Therapy Landscape and Barriers
Gene therapy for cancer treatment has evolved significantly over the past two decades, transitioning from theoretical concepts to clinical applications. Currently, the landscape encompasses several major approaches including viral vector-mediated gene delivery, non-viral delivery systems, gene editing technologies like CRISPR-Cas9, and cell-based gene therapies such as CAR-T. These technologies have demonstrated promising results in clinical trials, particularly for hematological malignancies, with approved therapies like Kymriah and Yescarta showing remarkable response rates in patients with refractory leukemia and lymphoma.
Despite these advancements, significant barriers impede widespread implementation of anti-tumor gene therapies. Delivery efficiency remains a primary challenge, with many vectors struggling to reach sufficient concentrations in solid tumors due to physical barriers including abnormal vasculature, high interstitial pressure, and dense extracellular matrix. This challenge is particularly pronounced in solid tumors, explaining the current disparity in success rates between hematological and solid malignancies.
Immunogenicity presents another substantial obstacle, as both viral vectors and gene products can trigger immune responses that neutralize therapeutic effects or cause adverse reactions. This has limited repeated dosing regimens and restricted the patient populations that can safely receive certain therapies. Additionally, off-target effects continue to raise safety concerns, with potential for unintended genetic modifications leading to oncogenesis or disruption of essential cellular functions.
Manufacturing complexities and associated costs create accessibility barriers, with current approved therapies often exceeding $400,000 per treatment. The intricate production processes, quality control requirements, and cold chain logistics contribute to these prohibitive costs, limiting availability to specialized centers in developed countries.
Regulatory frameworks worldwide are still adapting to the unique challenges posed by gene therapies, creating inconsistent approval pathways and requirements across different regions. This regulatory uncertainty has slowed development pipelines and commercial deployment strategies for many promising approaches.
Tumor heterogeneity and adaptive resistance mechanisms further complicate therapeutic efficacy, as cancer cells can evolve to circumvent single-target approaches. This necessitates combination strategies or multi-target designs that add layers of complexity to both development and regulatory approval processes.
Recent technological innovations are beginning to address these barriers, including next-generation vectors with enhanced targeting capabilities, improved gene editing precision, and novel manufacturing platforms that may reduce production costs. However, these solutions remain in early development stages, indicating that while the field is advancing rapidly, significant challenges persist in translating promising laboratory findings into broadly applicable clinical solutions.
Despite these advancements, significant barriers impede widespread implementation of anti-tumor gene therapies. Delivery efficiency remains a primary challenge, with many vectors struggling to reach sufficient concentrations in solid tumors due to physical barriers including abnormal vasculature, high interstitial pressure, and dense extracellular matrix. This challenge is particularly pronounced in solid tumors, explaining the current disparity in success rates between hematological and solid malignancies.
Immunogenicity presents another substantial obstacle, as both viral vectors and gene products can trigger immune responses that neutralize therapeutic effects or cause adverse reactions. This has limited repeated dosing regimens and restricted the patient populations that can safely receive certain therapies. Additionally, off-target effects continue to raise safety concerns, with potential for unintended genetic modifications leading to oncogenesis or disruption of essential cellular functions.
Manufacturing complexities and associated costs create accessibility barriers, with current approved therapies often exceeding $400,000 per treatment. The intricate production processes, quality control requirements, and cold chain logistics contribute to these prohibitive costs, limiting availability to specialized centers in developed countries.
Regulatory frameworks worldwide are still adapting to the unique challenges posed by gene therapies, creating inconsistent approval pathways and requirements across different regions. This regulatory uncertainty has slowed development pipelines and commercial deployment strategies for many promising approaches.
Tumor heterogeneity and adaptive resistance mechanisms further complicate therapeutic efficacy, as cancer cells can evolve to circumvent single-target approaches. This necessitates combination strategies or multi-target designs that add layers of complexity to both development and regulatory approval processes.
Recent technological innovations are beginning to address these barriers, including next-generation vectors with enhanced targeting capabilities, improved gene editing precision, and novel manufacturing platforms that may reduce production costs. However, these solutions remain in early development stages, indicating that while the field is advancing rapidly, significant challenges persist in translating promising laboratory findings into broadly applicable clinical solutions.
Current Anti-Tumor Gene Therapy Mechanisms
01 Viral vector-mediated gene delivery
Viral vectors are engineered to deliver therapeutic genes to cancer cells. These vectors, including adenoviruses, retroviruses, and lentiviruses, can efficiently transduce target cells and express therapeutic genes. The delivered genes may encode tumor-suppressing proteins, immunomodulatory factors, or enzymes that convert prodrugs into active cytotoxic compounds within the tumor microenvironment. This approach allows for targeted gene delivery to cancer cells while minimizing effects on healthy tissues.- Viral vector-mediated gene delivery: Viral vectors are engineered to deliver therapeutic genes to cancer cells. These vectors, including adenoviruses, retroviruses, and lentiviruses, can efficiently transfer genetic material into target cells. Once inside, the therapeutic genes express proteins that can directly kill cancer cells, inhibit tumor growth, or stimulate immune responses against the tumor. This approach allows for targeted delivery of genetic material to specific cell types, enhancing therapeutic efficacy while minimizing side effects.
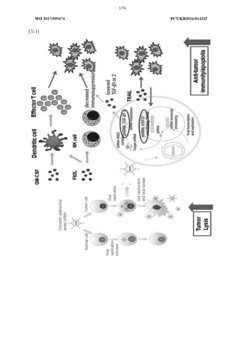
- Immune system modulation through gene therapy: Gene therapy can enhance anti-tumor immune responses by introducing genes that encode immunostimulatory molecules such as cytokines, chemokines, or co-stimulatory factors. These therapeutic genes can be delivered to tumor cells or immune cells, resulting in increased recognition and destruction of cancer cells by the immune system. This approach can overcome tumor-induced immunosuppression and create a more favorable microenvironment for immune cell activation and tumor cell elimination.
- Suicide gene therapy: Suicide gene therapy involves introducing genes into tumor cells that convert non-toxic prodrugs into toxic metabolites, leading to selective cancer cell death. Common suicide gene systems include the herpes simplex virus thymidine kinase (HSV-TK) with ganciclovir and cytosine deaminase with 5-fluorocytosine. This approach provides targeted cancer cell killing while sparing normal tissues, as the toxic metabolites are generated primarily within the genetically modified tumor cells, minimizing systemic toxicity.
- Tumor suppressor gene restoration: Many cancers develop due to inactivation or mutation of tumor suppressor genes. Gene therapy can restore normal function by delivering wild-type copies of these genes, such as p53, BRCA1, or RB, to cancer cells. The reintroduction of functional tumor suppressor genes can induce cell cycle arrest, apoptosis, or senescence in cancer cells, inhibiting tumor growth and progression. This approach targets the fundamental genetic alterations that drive cancer development and maintenance.
- CRISPR/Cas9-based gene editing for cancer therapy: CRISPR/Cas9 technology enables precise modification of cancer-related genes through targeted gene editing. This approach can be used to correct oncogenic mutations, disrupt overexpressed oncogenes, or enhance anti-tumor immunity. The high specificity of CRISPR/Cas9 allows for targeted genetic modifications with minimal off-target effects. This cutting-edge technology represents a promising direction for developing more effective and personalized cancer gene therapies.
02 Immune system modulation through gene therapy
Gene therapy can enhance anti-tumor immune responses by delivering genes that encode cytokines, chemokines, or immune checkpoint inhibitors. These therapeutic genes can stimulate immune cell recruitment and activation within the tumor microenvironment, overcome immunosuppression, and promote recognition and elimination of cancer cells by the immune system. This approach leverages the body's natural defense mechanisms to combat cancer and can potentially generate long-lasting anti-tumor immunity.Expand Specific Solutions03 Tumor suppressor gene restoration
Many cancers are characterized by mutations or deletions in tumor suppressor genes. Gene therapy approaches aim to restore the function of these genes by delivering functional copies to cancer cells. Key tumor suppressors targeted include p53, PTEN, and RB1, which regulate cell cycle, apoptosis, and DNA repair. Restoration of these genes can induce cancer cell death, inhibit proliferation, and sensitize tumors to conventional therapies like chemotherapy and radiation.Expand Specific Solutions04 Suicide gene therapy/prodrug activation
This approach involves delivering genes encoding enzymes that convert non-toxic prodrugs into cytotoxic compounds specifically within cancer cells. When the prodrug is administered systemically, it is activated only in cells expressing the therapeutic enzyme, resulting in selective killing of cancer cells while sparing normal tissues. Common suicide gene systems include herpes simplex virus thymidine kinase (HSV-TK) with ganciclovir and cytosine deaminase with 5-fluorocytosine. This strategy creates a targeted chemotherapy effect with reduced systemic toxicity.Expand Specific Solutions05 RNA interference and gene silencing
RNA interference (RNAi) technology uses small interfering RNAs (siRNAs), short hairpin RNAs (shRNAs), or microRNAs to silence the expression of oncogenes or other genes critical for tumor growth and survival. These RNA molecules can be delivered using viral vectors or nanoparticles and function by binding to complementary messenger RNA sequences, leading to their degradation or translational repression. This approach effectively downregulates cancer-promoting genes and pathways, inhibiting tumor growth, angiogenesis, and metastasis.Expand Specific Solutions
Leading Companies and Research Institutions
The anti-tumor gene therapy field is currently in a transitional phase from experimental to clinical application, with a growing market expected to reach significant value in the coming years. The competitive landscape features a diverse mix of academic institutions (Sichuan University, Peking University, Duke University), specialized biotechnology companies (Transgene SA, Oxford Biomedica, Arrowhead Pharmaceuticals), and research organizations (Mayo Foundation, CNRS). Technology maturity varies across approaches, with viral vector delivery systems (developed by Oxford Biomedica) being more established, while newer CRISPR-based therapies are emerging. Leading institutions like Johns Hopkins University and Mayo Foundation are advancing clinical trials, while Chinese universities (Fudan, Zhejiang) are rapidly increasing their patent portfolios and publications, indicating an increasingly global competitive environment.
Mayo Foundation for Medical Education & Research
Technical Solution: Mayo Foundation has developed a comprehensive platform for anti-tumor gene therapy centered around oncolytic virotherapy. Their flagship approach utilizes engineered measles viruses (MV) and vesicular stomatitis viruses (VSV) that selectively replicate in and destroy cancer cells while sparing normal tissues[2]. These viruses are genetically modified to express imaging reporters (such as sodium iodide symporter) that allow for non-invasive monitoring of viral spread and therapeutic efficacy through PET/CT imaging[3]. Mayo researchers have enhanced viral targeting through the incorporation of microRNA target sequences that prevent viral gene expression in normal cells while permitting it in cancer cells lacking specific microRNAs. Their platform also includes arming viruses with therapeutic transgenes including immunostimulatory cytokines (IL-12, GM-CSF), prodrug convertases, and immune checkpoint inhibitors to create multifunctional therapeutic agents[5]. A distinctive feature of Mayo's approach is their development of cell-carrier systems where mesenchymal stem cells or irradiated tumor cells are used to shield viruses from neutralizing antibodies and deliver them more effectively to tumor sites[7]. Recent innovations include the development of chimeric viruses that combine the advantages of different viral backbones to optimize tumor cell entry, replication, and immune stimulation.
Strengths: Extensive clinical trial experience with oncolytic virotherapy; sophisticated imaging capabilities for real-time monitoring of therapeutic efficacy; innovative cell-carrier systems that improve delivery; strong translational research infrastructure. Weaknesses: Pre-existing immunity against viral backbones may limit efficacy in some patients; challenges in achieving uniform virus distribution throughout solid tumors; potential for viral mutation requiring rigorous safety monitoring.
Transgene SA
Technical Solution: Transgene SA has developed a proprietary viral vector platform called Invir.IO™ specifically designed for oncolytic virus immunotherapy against cancer. Their approach centers on engineered oncolytic viruses (primarily vaccinia virus derivatives) that selectively infect and replicate within tumor cells, causing direct lysis while simultaneously stimulating anti-tumor immune responses[2]. These vectors are modified to express therapeutic transgenes such as cytokines, chemokines, and immune checkpoint inhibitors directly within the tumor microenvironment. Transgene's lead candidate, TG6002, is a vaccinia virus armed with the FCU1 gene that converts the non-toxic flucytosine into the chemotherapeutic 5-FU directly inside tumor cells[4]. Another innovative approach is their TG4050 personalized immunotherapy, which utilizes viral vectors to express patient-specific neoantigens identified through tumor sequencing, effectively creating individualized cancer vaccines[5]. Their vectors incorporate multiple safety features including thymidine kinase gene deletions that restrict viral replication to cancer cells with high thymidine levels.
Strengths: Dual mechanism of action combining direct oncolysis with immune activation; tumor-selective replication provides natural targeting; ability to deliver multiple therapeutic payloads simultaneously; established safety profile in clinical trials. Weaknesses: Pre-existing immunity to viral backbones may limit efficacy in some patients; manufacturing complexity for personalized therapies increases costs; potential for rapid clearance limiting therapeutic window.
Key Patents and Scientific Breakthroughs
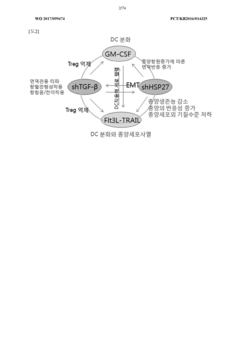
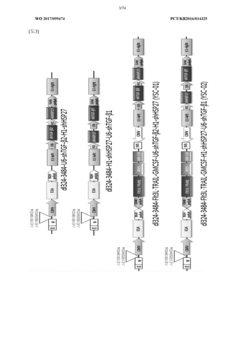
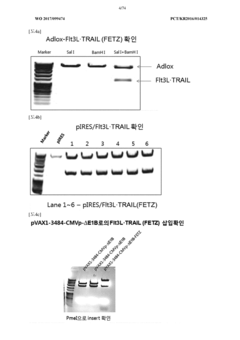
Antitumor composition comprising GM-CSF gene, FLT3l-trail fusion gene, shrna inhibiting TGF-Β expression, and shrna inhibiting HSP expression
PatentWO2017099474A1
Innovation
- A genetic anti-tumor composition comprising a granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, Flt3L-TRAIL fusion gene, and short hairpin RNA (shRNA) that inhibits TGF-β and HSP expression, delivered via a gene delivery vehicle to co-express these genes, thereby enhancing anti-tumor activity by promoting immune response and apoptosis in cancer cells.
Methods of Anti-tumor therapy
PatentInactiveUS20220154212A1
Innovation
- Administration of a priming dose of a vector containing a Fas-chimera gene linked to an endothelial cell-specific promoter before tumor surgery, potentially combined with post-surgical doses and chemotherapeutic agents like bevacizumab, to enhance anti-tumor response by targeting endothelial cells and inhibiting angiogenesis.
Regulatory Framework for Gene Therapy Approval
The regulatory landscape for gene therapy approval represents a complex and evolving framework designed to ensure safety, efficacy, and ethical implementation of these innovative treatments. In the United States, the Food and Drug Administration (FDA) has established a comprehensive regulatory pathway through the Center for Biologics Evaluation and Research (CBER), which oversees the approval process for anti-tumor gene therapies. This process typically requires extensive preclinical testing, followed by phased clinical trials demonstrating both safety and therapeutic efficacy.
The European Medicines Agency (EMA) has developed parallel but distinct regulatory mechanisms, including the Advanced Therapy Medicinal Products (ATMP) classification, which specifically addresses gene therapy products. Both regulatory bodies have implemented accelerated approval pathways for treatments targeting serious conditions with unmet medical needs, including many oncological applications of gene therapy.
Risk assessment protocols for anti-tumor gene therapies are particularly stringent, focusing on potential immunogenicity, insertional mutagenesis, and off-target effects. Long-term safety monitoring requirements often extend well beyond the approval phase, with manufacturers required to maintain post-marketing surveillance programs that may span decades.
Manufacturing standards present another critical regulatory consideration, with Good Manufacturing Practice (GMP) requirements specifically adapted for gene therapy production. These standards address unique challenges in viral vector production, quality control, and batch consistency that are essential for ensuring reproducible clinical outcomes in anti-tumor applications.
Ethical considerations are formally integrated into the regulatory framework through institutional review board (IRB) approvals and informed consent protocols. These mechanisms are particularly important for novel anti-tumor gene therapies where risk-benefit profiles may be less established than conventional treatments.
International harmonization efforts, including the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have made progress in standardizing certain aspects of gene therapy regulation across major markets. However, significant regional variations persist, creating challenges for global development programs targeting oncological applications.
Recent regulatory innovations include the implementation of Real-World Evidence (RWE) frameworks that allow for the incorporation of data collected outside traditional clinical trials. This approach is particularly valuable for rare cancers where conventional trial recruitment may be challenging, potentially accelerating the approval timeline for targeted gene therapies.
The European Medicines Agency (EMA) has developed parallel but distinct regulatory mechanisms, including the Advanced Therapy Medicinal Products (ATMP) classification, which specifically addresses gene therapy products. Both regulatory bodies have implemented accelerated approval pathways for treatments targeting serious conditions with unmet medical needs, including many oncological applications of gene therapy.
Risk assessment protocols for anti-tumor gene therapies are particularly stringent, focusing on potential immunogenicity, insertional mutagenesis, and off-target effects. Long-term safety monitoring requirements often extend well beyond the approval phase, with manufacturers required to maintain post-marketing surveillance programs that may span decades.
Manufacturing standards present another critical regulatory consideration, with Good Manufacturing Practice (GMP) requirements specifically adapted for gene therapy production. These standards address unique challenges in viral vector production, quality control, and batch consistency that are essential for ensuring reproducible clinical outcomes in anti-tumor applications.
Ethical considerations are formally integrated into the regulatory framework through institutional review board (IRB) approvals and informed consent protocols. These mechanisms are particularly important for novel anti-tumor gene therapies where risk-benefit profiles may be less established than conventional treatments.
International harmonization efforts, including the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have made progress in standardizing certain aspects of gene therapy regulation across major markets. However, significant regional variations persist, creating challenges for global development programs targeting oncological applications.
Recent regulatory innovations include the implementation of Real-World Evidence (RWE) frameworks that allow for the incorporation of data collected outside traditional clinical trials. This approach is particularly valuable for rare cancers where conventional trial recruitment may be challenging, potentially accelerating the approval timeline for targeted gene therapies.
Safety and Ethical Considerations
Gene therapy for cancer treatment represents a significant advancement in medical science, yet it brings forth substantial safety concerns and ethical considerations that must be carefully addressed. The administration of genetic material into human cells carries inherent risks, including immune system reactions that can range from mild inflammation to life-threatening cytokine storms. These adverse responses have been documented in clinical trials, most notably in the 1999 case of Jesse Gelsinger, whose death highlighted the potential severity of immune reactions to viral vectors.
Vector-related toxicity presents another critical safety challenge. Viral vectors, while efficient for gene delivery, may cause insertional mutagenesis, potentially activating oncogenes or disrupting tumor suppressor genes. Non-viral vectors, though generally safer, often demonstrate lower efficacy and may introduce different toxicity profiles that require thorough evaluation before clinical application.
Off-target effects remain a persistent concern in gene therapy approaches. Even with advanced targeting technologies, genetic material may affect unintended cells or tissues, potentially leading to unpredictable consequences. The long-term effects of genetic modifications, particularly those involving germline cells, remain largely unknown and represent a significant area of ongoing research and ethical debate.
Regulatory frameworks worldwide have evolved to address these safety concerns, with agencies like the FDA and EMA implementing stringent guidelines for gene therapy clinical trials. These include requirements for comprehensive preclinical safety data, rigorous monitoring protocols, and long-term follow-up of treated patients to detect delayed adverse effects.
From an ethical perspective, informed consent presents unique challenges in gene therapy trials. The complexity of these treatments makes it difficult for patients to fully comprehend the risks involved, particularly when standard treatments have failed and experimental gene therapies represent a last hope. This situation raises questions about the voluntariness and adequacy of consent.
Equity of access represents another significant ethical consideration. The high cost of developing and administering gene therapies may limit their availability to wealthy individuals or nations, potentially exacerbating existing healthcare disparities. This raises important questions about justice in healthcare resource allocation and the responsibility of developers and healthcare systems to ensure equitable access.
The potential for enhancement beyond therapeutic applications also raises profound ethical questions about the boundaries of medical intervention and the definition of disease versus human enhancement. As anti-tumor gene therapy technologies advance, the scientific community must engage in ongoing dialogue with ethicists, policymakers, and the public to navigate these complex issues responsibly.
Vector-related toxicity presents another critical safety challenge. Viral vectors, while efficient for gene delivery, may cause insertional mutagenesis, potentially activating oncogenes or disrupting tumor suppressor genes. Non-viral vectors, though generally safer, often demonstrate lower efficacy and may introduce different toxicity profiles that require thorough evaluation before clinical application.
Off-target effects remain a persistent concern in gene therapy approaches. Even with advanced targeting technologies, genetic material may affect unintended cells or tissues, potentially leading to unpredictable consequences. The long-term effects of genetic modifications, particularly those involving germline cells, remain largely unknown and represent a significant area of ongoing research and ethical debate.
Regulatory frameworks worldwide have evolved to address these safety concerns, with agencies like the FDA and EMA implementing stringent guidelines for gene therapy clinical trials. These include requirements for comprehensive preclinical safety data, rigorous monitoring protocols, and long-term follow-up of treated patients to detect delayed adverse effects.
From an ethical perspective, informed consent presents unique challenges in gene therapy trials. The complexity of these treatments makes it difficult for patients to fully comprehend the risks involved, particularly when standard treatments have failed and experimental gene therapies represent a last hope. This situation raises questions about the voluntariness and adequacy of consent.
Equity of access represents another significant ethical consideration. The high cost of developing and administering gene therapies may limit their availability to wealthy individuals or nations, potentially exacerbating existing healthcare disparities. This raises important questions about justice in healthcare resource allocation and the responsibility of developers and healthcare systems to ensure equitable access.
The potential for enhancement beyond therapeutic applications also raises profound ethical questions about the boundaries of medical intervention and the definition of disease versus human enhancement. As anti-tumor gene therapy technologies advance, the scientific community must engage in ongoing dialogue with ethicists, policymakers, and the public to navigate these complex issues responsibly.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!