Research on Non-Viral Vectors in Gene Therapy Applications
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Non-Viral Vector Development History and Objectives
Gene therapy has evolved significantly since its conceptual inception in the 1970s, with non-viral vectors emerging as a promising alternative to viral delivery systems. The development of non-viral vectors began in earnest during the 1980s when researchers recognized the limitations and safety concerns associated with viral vectors, including immunogenicity, insertional mutagenesis, and limited payload capacity. Early non-viral approaches primarily utilized naked DNA and physical methods such as electroporation and gene gun technology, which offered simplicity but suffered from low transfection efficiency.
The 1990s witnessed the emergence of first-generation chemical carriers, particularly cationic lipids and polymers. Lipofection, introduced by Felgner in 1987, represented a significant breakthrough, leading to the development of lipid-based transfection reagents that are still widely used in laboratory settings. During this period, polyethylenimine (PEI) also emerged as a prominent polymer-based vector due to its "proton sponge effect," enhancing endosomal escape of genetic material.
By the early 2000s, research focus shifted toward addressing the key limitations of non-viral vectors: low transfection efficiency, poor targeting specificity, and transient gene expression. This era saw the development of more sophisticated delivery systems, including peptide-based vectors, dendrimers, and inorganic nanoparticles. The integration of targeting ligands and cell-penetrating peptides represented important advances in improving cellular uptake and specificity.
The past decade has witnessed remarkable progress in non-viral vector technology, driven by advances in materials science, nanotechnology, and molecular biology. Lipid nanoparticles (LNPs), which gained widespread recognition through their application in mRNA COVID-19 vaccines, represent one of the most successful non-viral delivery platforms to date. Concurrently, innovations in polymer chemistry have led to biodegradable, less toxic polymeric vectors with enhanced transfection capabilities.
The primary objectives of current non-viral vector research include achieving transfection efficiencies comparable to viral vectors while maintaining superior safety profiles, developing vectors capable of targeted delivery to specific tissues or cell types, enabling controlled and sustained gene expression, and designing scalable, cost-effective manufacturing processes. Additionally, researchers aim to overcome biological barriers such as serum stability, immune recognition, endosomal escape, and nuclear entry.
Looking forward, the field is moving toward multifunctional, stimuli-responsive vectors that can adapt to biological environments and precisely control the release of genetic cargo. The ultimate goal remains the development of safe, efficient, and versatile non-viral delivery systems that can facilitate the translation of gene therapy from promising concept to widespread clinical application across diverse disease indications.
The 1990s witnessed the emergence of first-generation chemical carriers, particularly cationic lipids and polymers. Lipofection, introduced by Felgner in 1987, represented a significant breakthrough, leading to the development of lipid-based transfection reagents that are still widely used in laboratory settings. During this period, polyethylenimine (PEI) also emerged as a prominent polymer-based vector due to its "proton sponge effect," enhancing endosomal escape of genetic material.
By the early 2000s, research focus shifted toward addressing the key limitations of non-viral vectors: low transfection efficiency, poor targeting specificity, and transient gene expression. This era saw the development of more sophisticated delivery systems, including peptide-based vectors, dendrimers, and inorganic nanoparticles. The integration of targeting ligands and cell-penetrating peptides represented important advances in improving cellular uptake and specificity.
The past decade has witnessed remarkable progress in non-viral vector technology, driven by advances in materials science, nanotechnology, and molecular biology. Lipid nanoparticles (LNPs), which gained widespread recognition through their application in mRNA COVID-19 vaccines, represent one of the most successful non-viral delivery platforms to date. Concurrently, innovations in polymer chemistry have led to biodegradable, less toxic polymeric vectors with enhanced transfection capabilities.
The primary objectives of current non-viral vector research include achieving transfection efficiencies comparable to viral vectors while maintaining superior safety profiles, developing vectors capable of targeted delivery to specific tissues or cell types, enabling controlled and sustained gene expression, and designing scalable, cost-effective manufacturing processes. Additionally, researchers aim to overcome biological barriers such as serum stability, immune recognition, endosomal escape, and nuclear entry.
Looking forward, the field is moving toward multifunctional, stimuli-responsive vectors that can adapt to biological environments and precisely control the release of genetic cargo. The ultimate goal remains the development of safe, efficient, and versatile non-viral delivery systems that can facilitate the translation of gene therapy from promising concept to widespread clinical application across diverse disease indications.
Market Analysis for Non-Viral Gene Therapy Solutions
The non-viral vector gene therapy market is experiencing significant growth, driven by increasing concerns over the safety profiles of viral vectors and advancements in non-viral delivery technologies. Current market valuations place the global non-viral gene therapy sector at approximately $2.5 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 21.3% through 2030. This robust growth trajectory reflects the expanding therapeutic applications and technological innovations in the field.
Demand for non-viral gene therapy solutions is particularly strong in oncology, rare genetic disorders, and chronic diseases where conventional treatments show limited efficacy. Oncology applications currently dominate the market share at 38%, followed by genetic disorders at 27% and cardiovascular diseases at 15%. The remaining market segments include neurological disorders, infectious diseases, and other therapeutic areas.
Regional analysis reveals North America as the leading market for non-viral gene therapy solutions, accounting for approximately 42% of global revenue. Europe follows with 28% market share, while Asia-Pacific represents the fastest-growing region with a projected CAGR of 25.7% over the next five years. This growth in Asia-Pacific is primarily attributed to increasing healthcare expenditure, expanding research infrastructure, and favorable regulatory environments in countries like China, Japan, and South Korea.
Key market drivers include the lower immunogenicity of non-viral vectors compared to viral alternatives, reduced manufacturing complexity and costs, improved safety profiles, and greater packaging capacity for larger genetic payloads. Additionally, the potential for repeat administration without triggering immune responses represents a significant advantage for chronic disease management applications.
Market challenges primarily revolve around lower transfection efficiency compared to viral vectors, limited in vivo stability, and difficulties in targeted delivery to specific tissues. These technical limitations have historically restricted the widespread adoption of non-viral approaches, though recent innovations are progressively addressing these barriers.
End-user segmentation shows academic and research institutions currently leading adoption at 45%, followed by pharmaceutical and biotechnology companies at 38%, and hospitals and clinical centers at 17%. However, the pharmaceutical sector is expected to demonstrate the highest growth rate as more non-viral gene therapy candidates advance through clinical pipelines toward commercialization.
The reimbursement landscape remains complex, with significant variations across different healthcare systems. While some advanced markets have established pathways for novel gene therapies, many regions lack clear frameworks for non-viral approaches, potentially limiting market penetration despite their cost advantages over viral vector alternatives.
Demand for non-viral gene therapy solutions is particularly strong in oncology, rare genetic disorders, and chronic diseases where conventional treatments show limited efficacy. Oncology applications currently dominate the market share at 38%, followed by genetic disorders at 27% and cardiovascular diseases at 15%. The remaining market segments include neurological disorders, infectious diseases, and other therapeutic areas.
Regional analysis reveals North America as the leading market for non-viral gene therapy solutions, accounting for approximately 42% of global revenue. Europe follows with 28% market share, while Asia-Pacific represents the fastest-growing region with a projected CAGR of 25.7% over the next five years. This growth in Asia-Pacific is primarily attributed to increasing healthcare expenditure, expanding research infrastructure, and favorable regulatory environments in countries like China, Japan, and South Korea.
Key market drivers include the lower immunogenicity of non-viral vectors compared to viral alternatives, reduced manufacturing complexity and costs, improved safety profiles, and greater packaging capacity for larger genetic payloads. Additionally, the potential for repeat administration without triggering immune responses represents a significant advantage for chronic disease management applications.
Market challenges primarily revolve around lower transfection efficiency compared to viral vectors, limited in vivo stability, and difficulties in targeted delivery to specific tissues. These technical limitations have historically restricted the widespread adoption of non-viral approaches, though recent innovations are progressively addressing these barriers.
End-user segmentation shows academic and research institutions currently leading adoption at 45%, followed by pharmaceutical and biotechnology companies at 38%, and hospitals and clinical centers at 17%. However, the pharmaceutical sector is expected to demonstrate the highest growth rate as more non-viral gene therapy candidates advance through clinical pipelines toward commercialization.
The reimbursement landscape remains complex, with significant variations across different healthcare systems. While some advanced markets have established pathways for novel gene therapies, many regions lack clear frameworks for non-viral approaches, potentially limiting market penetration despite their cost advantages over viral vector alternatives.
Current Challenges in Non-Viral Vector Technology
Despite significant advancements in non-viral vector technology for gene therapy applications, several critical challenges continue to impede widespread clinical adoption. The primary obstacle remains transfection efficiency, with non-viral vectors consistently demonstrating lower gene delivery rates compared to their viral counterparts. This efficiency gap is particularly pronounced in vivo, where biological barriers including serum protein interactions, nuclease degradation, and cellular uptake mechanisms significantly reduce effective payload delivery to target tissues.
Cytotoxicity presents another substantial challenge, especially with lipid and polymer-based vectors that can trigger inflammatory responses and oxidative stress. These adverse reactions not only compromise patient safety but also limit the maximum dosage that can be administered, further constraining therapeutic efficacy. The delicate balance between enhancing transfection efficiency and minimizing toxicity remains a central technical dilemma.
The stability of non-viral vectors in biological environments represents a significant hurdle. Many current formulations exhibit poor circulation half-lives and premature payload release, resulting in suboptimal therapeutic outcomes. Additionally, the colloidal stability of these vectors often deteriorates during storage, creating substantial manufacturing and distribution challenges that impede commercial viability.
Targeting specificity remains inadequately addressed in most non-viral vector systems. Without precise tissue targeting mechanisms, these vectors distribute broadly throughout the body, reducing therapeutic concentration at intended sites while increasing off-target effects. This limitation is particularly problematic for treating specific tissues like the central nervous system, where crossing the blood-brain barrier adds another layer of complexity.
Manufacturing scalability presents formidable technical barriers, with many promising laboratory-scale vectors failing to translate to industrial production. Issues including batch-to-batch variability, complex purification requirements, and difficulties in maintaining consistent physicochemical properties during scale-up have hindered commercialization efforts. The regulatory pathway for novel non-viral vectors also remains challenging, with limited precedent for approval compared to established viral vector platforms.
Payload capacity constraints further limit applications, particularly for larger therapeutic genes or multi-gene delivery systems. While non-viral vectors theoretically offer unlimited cargo capacity, practical formulation challenges often emerge when incorporating larger genetic payloads, affecting stability, cellular uptake, and overall transfection efficiency.
The transient expression profile of most non-viral vector systems restricts their utility in conditions requiring long-term therapeutic gene expression. Unlike some viral vectors that can integrate into the host genome or persist as episomes, non-viral vectors typically provide only temporary transgene expression, necessitating repeated administrations that compound safety concerns and treatment costs.
Cytotoxicity presents another substantial challenge, especially with lipid and polymer-based vectors that can trigger inflammatory responses and oxidative stress. These adverse reactions not only compromise patient safety but also limit the maximum dosage that can be administered, further constraining therapeutic efficacy. The delicate balance between enhancing transfection efficiency and minimizing toxicity remains a central technical dilemma.
The stability of non-viral vectors in biological environments represents a significant hurdle. Many current formulations exhibit poor circulation half-lives and premature payload release, resulting in suboptimal therapeutic outcomes. Additionally, the colloidal stability of these vectors often deteriorates during storage, creating substantial manufacturing and distribution challenges that impede commercial viability.
Targeting specificity remains inadequately addressed in most non-viral vector systems. Without precise tissue targeting mechanisms, these vectors distribute broadly throughout the body, reducing therapeutic concentration at intended sites while increasing off-target effects. This limitation is particularly problematic for treating specific tissues like the central nervous system, where crossing the blood-brain barrier adds another layer of complexity.
Manufacturing scalability presents formidable technical barriers, with many promising laboratory-scale vectors failing to translate to industrial production. Issues including batch-to-batch variability, complex purification requirements, and difficulties in maintaining consistent physicochemical properties during scale-up have hindered commercialization efforts. The regulatory pathway for novel non-viral vectors also remains challenging, with limited precedent for approval compared to established viral vector platforms.
Payload capacity constraints further limit applications, particularly for larger therapeutic genes or multi-gene delivery systems. While non-viral vectors theoretically offer unlimited cargo capacity, practical formulation challenges often emerge when incorporating larger genetic payloads, affecting stability, cellular uptake, and overall transfection efficiency.
The transient expression profile of most non-viral vector systems restricts their utility in conditions requiring long-term therapeutic gene expression. Unlike some viral vectors that can integrate into the host genome or persist as episomes, non-viral vectors typically provide only temporary transgene expression, necessitating repeated administrations that compound safety concerns and treatment costs.
Current Non-Viral Vector Delivery Approaches
01 Lipid-based non-viral vectors
Lipid-based vectors are widely used non-viral delivery systems for gene therapy and drug delivery. These include liposomes, lipid nanoparticles (LNPs), and lipoplexes that can encapsulate nucleic acids or therapeutic agents. The lipid composition typically includes cationic lipids that interact with negatively charged nucleic acids, neutral helper lipids to enhance stability, and sometimes PEG-lipids for improved circulation. These systems offer advantages such as reduced immunogenicity, versatility in cargo capacity, and potential for targeted delivery.- Lipid-based non-viral vectors: Lipid-based delivery systems are widely used as non-viral vectors for gene therapy and drug delivery. These include liposomes, lipid nanoparticles (LNPs), and lipoplexes that can encapsulate nucleic acids such as DNA, RNA, or siRNA. The lipid composition typically includes cationic lipids that interact with negatively charged nucleic acids, helper lipids to enhance stability, and sometimes PEG-lipids for improved circulation. These vectors offer advantages such as reduced immunogenicity, larger payload capacity, and easier manufacturing compared to viral vectors.
- Polymer-based non-viral vectors: Polymer-based delivery systems utilize synthetic or natural polymers to condense and protect nucleic acids for cellular delivery. Common polymers include polyethylenimine (PEI), poly(L-lysine), chitosan, and PLGA. These polymers form complexes with nucleic acids through electrostatic interactions, creating polyplexes that protect the genetic material from degradation and facilitate cellular uptake. Polymer-based vectors can be modified with targeting ligands to enhance delivery to specific cell types and tissues, offering versatility in design and application for gene therapy.
- Peptide and protein-based non-viral vectors: Peptide and protein-based vectors utilize cell-penetrating peptides, nuclear localization signals, and other bioactive peptides to enhance the delivery of nucleic acids into cells. These vectors can overcome cellular barriers through specific interactions with cell surface receptors or membrane components. Engineered proteins and peptides can be designed to condense DNA/RNA, protect against nucleases, facilitate endosomal escape, and target specific cell types. Their biodegradability and potential for reduced toxicity make them attractive alternatives to synthetic delivery systems.
- Hybrid and composite non-viral vectors: Hybrid and composite vectors combine multiple delivery components to overcome the limitations of individual vector types. These systems may integrate lipids with polymers, peptides with inorganic nanoparticles, or other combinations to create multifunctional delivery platforms. The synergistic effects of different components can enhance transfection efficiency, reduce cytotoxicity, improve stability, and provide targeted delivery. Examples include lipopolyplexes, polymer-lipid hybrid nanoparticles, and peptide-modified liposomes that combine the advantages of different vector types.
- Inorganic nanoparticle-based non-viral vectors: Inorganic nanoparticles such as gold, silica, calcium phosphate, and magnetic nanoparticles serve as non-viral vectors for gene delivery. These materials offer unique properties including surface functionalization capabilities, controlled size distribution, and stimulus-responsive features. Some inorganic nanoparticles also provide imaging capabilities for tracking delivery or additional therapeutic functions like photothermal therapy. Their rigid structure can protect nucleic acids from degradation, while their surface can be modified with targeting ligands, polymers, or other functional groups to enhance delivery efficiency.
02 Polymer-based non-viral vectors
Polymer-based vectors utilize synthetic or natural polymers to condense and deliver genetic material or therapeutic agents. Common polymers include polyethylenimine (PEI), poly(L-lysine), chitosan, and PLGA. These polymers typically contain positive charges that interact with negatively charged nucleic acids to form polyplexes. Polymer vectors can be engineered with specific properties such as biodegradability, stimuli-responsiveness, and surface modifications for targeted delivery. They offer advantages including ease of manufacturing, stability, and the ability to carry large genetic payloads.Expand Specific Solutions03 Peptide and protein-based non-viral vectors
Peptide and protein-based vectors utilize cell-penetrating peptides, nuclear localization signals, and other functional peptides to facilitate cellular uptake and intracellular trafficking of therapeutic cargoes. These vectors can be designed to overcome specific cellular barriers and target particular cell types or tissues. Protein-based vectors may include engineered protein cages, virus-like particles without viral genetic material, and recombinant proteins designed for delivery purposes. These systems offer advantages such as biocompatibility, biodegradability, and the ability to incorporate multiple functional domains.Expand Specific Solutions04 Hybrid and composite non-viral vectors
Hybrid and composite vectors combine multiple delivery components to overcome limitations of single-component systems. These may include lipid-polymer hybrid nanoparticles, polymer-peptide conjugates, or inorganic-organic composites. By integrating different materials, these vectors can achieve synergistic effects such as improved stability, enhanced cellular uptake, controlled release, and reduced toxicity. Hybrid systems often incorporate targeting ligands, stimuli-responsive elements, and other functional components to enhance delivery efficiency and specificity.Expand Specific Solutions05 Inorganic non-viral vectors
Inorganic non-viral vectors utilize materials such as gold nanoparticles, quantum dots, silica nanoparticles, carbon nanotubes, and calcium phosphate to deliver therapeutic agents. These vectors often offer unique physical properties such as optical, magnetic, or thermal characteristics that can be exploited for tracking, imaging, or triggered release. Inorganic vectors can be surface-modified with functional groups to improve biocompatibility, reduce toxicity, and enhance cellular uptake. They typically provide high stability and the potential for multifunctional applications in both diagnostics and therapeutics.Expand Specific Solutions
Key Industry Players in Non-Viral Vector Development
The non-viral vector gene therapy field is currently in a growth phase, with increasing market adoption despite being less mature than viral vector approaches. The global market is expanding rapidly, projected to reach significant value as companies seek safer alternatives to viral delivery systems. Academic institutions like MIT, Johns Hopkins University, and Zhejiang University are driving fundamental research, while specialized companies including Generation Bio, Rampart Bioscience, and Helixmith are advancing clinical applications. The technology landscape shows varying maturity levels, with lipid nanoparticle systems (developed by companies like EnGeneIC and Generation Bio) being more advanced than polymer-based and hybrid systems. Collaborations between academia and industry are accelerating development, with companies like Charles River Laboratories providing essential testing services for the emerging field.
EnGeneIC Molecular Delivery Pty Ltd.
Technical Solution: EnGeneIC has developed the EnGeneIC Dream Vector (EDV™), a novel non-viral delivery platform based on bacterial-derived nanocells. These non-living, bacterially-derived minicells (approximately 400nm in diameter) can be loaded with various therapeutic payloads including plasmid DNA, siRNA, miRNA, and small molecule drugs. The EDV™ platform features bispecific antibodies attached to the nanocell surface that target specific receptors on cancer cells or other target tissues, enabling precise delivery. The bacterial cell wall provides natural protection for the genetic payload, while the surface modifications allow for targeted delivery to specific tissues. EnGeneIC has demonstrated successful transfection in multiple cancer models with reduced systemic toxicity compared to conventional delivery methods[4][5]. The platform has advanced to clinical trials for cancer applications, showing promising safety profiles and preliminary efficacy. Their manufacturing process allows for standardized, scalable production of these nanocells with consistent size distribution and loading capacity.
Strengths: Versatile platform capable of delivering various nucleic acid types; targeted delivery reducing off-target effects; established manufacturing protocols; demonstrated safety in early clinical trials; potential for combination therapy with simultaneous delivery of multiple therapeutic agents. Weaknesses: Limited to certain cell types with specific surface receptors; potential immunogenicity concerns despite modifications; relatively large particle size may limit tissue penetration; primarily focused on oncology applications with less data in other therapeutic areas.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed several cutting-edge non-viral gene delivery technologies, most notably their polymer-based delivery systems and lipid nanoparticle platforms. Their poly(beta-amino ester) (PBAE) library represents a significant advancement in polymer-based gene delivery, with hundreds of structurally distinct polymers optimized for different tissue targets. These biodegradable polymers self-assemble with nucleic acids to form nanoparticles that facilitate cellular uptake and endosomal escape. MIT has also pioneered ionizable lipid nanoparticles (LNPs) with novel lipid structures that enhance transfection efficiency while minimizing toxicity. Their combinatorial chemistry approach has generated libraries of lipid-like materials ("lipidoids") with superior delivery properties[6][7]. Additionally, MIT researchers have developed layer-by-layer (LbL) nanoparticles that incorporate multiple functional components to overcome biological barriers. These systems have demonstrated successful gene delivery to challenging targets including the central nervous system, lungs, and immune cells in preclinical models, with transfection efficiencies approaching those of viral vectors in some tissues.
Strengths: Highly customizable delivery systems adaptable to different tissue targets; biodegradable materials with favorable safety profiles; potential for large-scale, cost-effective manufacturing; ability to deliver various nucleic acid types including DNA, mRNA, and siRNA. Weaknesses: Complex formulation processes requiring precise control of physicochemical properties; potential batch-to-batch variability; generally lower transfection efficiency than viral vectors in vivo; limited duration of gene expression compared to integrating viral vectors.
Critical Patents and Innovations in Non-Viral Vectors
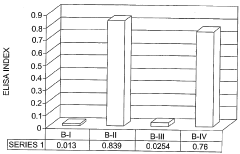
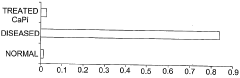
A method using inorganic nanoparticles as non-viral vectors for gene therapy
PatentWO2005123142A1
Innovation
- Encapsulating genetic material in inorganic nanoparticles such as calcium phosphate (CaPi), magnesium phosphate (MgPi), or manganese phosphate (MnPi) nanoparticles of less than 100 nm in diameter, which provide high transfection efficiency and protect the genetic material, allowing for targeted and efficient delivery to specific cells.
Dendrimers as non-viral vehicles for gene therapy
PatentInactiveUS20130210887A1
Innovation
- Development of novel compounds of general formula (I) and (II) as non-viral vehicles, which are synthesized using polymers or dendrimers derived from polyphenylenevinylene or polyphenyleneethylidene, conjugated with aromatic rings and heterocyclic bases, capable of forming complexes with genetic materials like DNA, RNA, or siRNA for targeted gene delivery.
Safety and Immunogenicity Considerations
Safety and immunogenicity considerations represent critical factors in the development and application of non-viral vectors for gene therapy. Unlike viral vectors, non-viral delivery systems generally exhibit lower immunogenicity profiles, making them potentially safer alternatives for clinical applications. However, they are not without safety concerns that require thorough evaluation and mitigation strategies.
The immune response to non-viral vectors varies significantly based on their composition and design. Lipid-based vectors, particularly lipid nanoparticles (LNPs), may trigger complement activation and inflammatory responses when administered systemically. Recent studies have demonstrated that modifications to lipid structures and formulation processes can substantially reduce these immunogenic effects, enhancing their safety profile for repeated administrations.
Polymer-based vectors present different immunological challenges. Cationic polymers like polyethylenimine (PEI) can induce cytotoxicity at higher concentrations due to their positive charge density. This has prompted the development of biodegradable polymers and hybrid systems that maintain transfection efficiency while reducing toxicity. The incorporation of polyethylene glycol (PEGylation) has proven effective in creating "stealth" properties that reduce recognition by the immune system.
Cytotoxicity remains a significant concern with many non-viral vectors. The mechanisms underlying vector-induced cell damage include membrane disruption, mitochondrial impairment, and oxidative stress. Advanced in vitro and in vivo screening methodologies have been established to evaluate these effects across different cell types and tissues, allowing for more predictive safety assessments prior to clinical translation.
The potential for off-target effects presents another safety consideration. Non-specific delivery can result in transgene expression in unintended tissues, leading to adverse effects. Targeting strategies incorporating tissue-specific ligands, responsive elements, and physical targeting methods have shown promise in enhancing delivery specificity and reducing off-target effects.
Long-term safety profiles of non-viral vectors require further investigation. While these vectors typically do not integrate into the host genome (reducing insertional mutagenesis risks), questions remain regarding the persistence of vector components in tissues and potential long-term immunological memory. Regulatory frameworks increasingly require comprehensive long-term safety monitoring for gene therapy products utilizing non-viral delivery systems.
Standardization of safety assessment protocols specifically tailored for non-viral vectors represents an ongoing challenge. The diversity of non-viral vector designs necessitates adaptable yet rigorous evaluation frameworks that can accurately predict clinical safety outcomes. International collaborative efforts are underway to establish consensus guidelines that address the unique safety considerations of these delivery systems.
The immune response to non-viral vectors varies significantly based on their composition and design. Lipid-based vectors, particularly lipid nanoparticles (LNPs), may trigger complement activation and inflammatory responses when administered systemically. Recent studies have demonstrated that modifications to lipid structures and formulation processes can substantially reduce these immunogenic effects, enhancing their safety profile for repeated administrations.
Polymer-based vectors present different immunological challenges. Cationic polymers like polyethylenimine (PEI) can induce cytotoxicity at higher concentrations due to their positive charge density. This has prompted the development of biodegradable polymers and hybrid systems that maintain transfection efficiency while reducing toxicity. The incorporation of polyethylene glycol (PEGylation) has proven effective in creating "stealth" properties that reduce recognition by the immune system.
Cytotoxicity remains a significant concern with many non-viral vectors. The mechanisms underlying vector-induced cell damage include membrane disruption, mitochondrial impairment, and oxidative stress. Advanced in vitro and in vivo screening methodologies have been established to evaluate these effects across different cell types and tissues, allowing for more predictive safety assessments prior to clinical translation.
The potential for off-target effects presents another safety consideration. Non-specific delivery can result in transgene expression in unintended tissues, leading to adverse effects. Targeting strategies incorporating tissue-specific ligands, responsive elements, and physical targeting methods have shown promise in enhancing delivery specificity and reducing off-target effects.
Long-term safety profiles of non-viral vectors require further investigation. While these vectors typically do not integrate into the host genome (reducing insertional mutagenesis risks), questions remain regarding the persistence of vector components in tissues and potential long-term immunological memory. Regulatory frameworks increasingly require comprehensive long-term safety monitoring for gene therapy products utilizing non-viral delivery systems.
Standardization of safety assessment protocols specifically tailored for non-viral vectors represents an ongoing challenge. The diversity of non-viral vector designs necessitates adaptable yet rigorous evaluation frameworks that can accurately predict clinical safety outcomes. International collaborative efforts are underway to establish consensus guidelines that address the unique safety considerations of these delivery systems.
Regulatory Pathway for Non-Viral Gene Therapies
The regulatory landscape for non-viral gene therapies presents a complex framework that differs significantly from traditional pharmaceutical approval pathways. The U.S. Food and Drug Administration (FDA) categorizes gene therapies as biological products, requiring Investigational New Drug (IND) applications before clinical trials can commence. For non-viral vectors specifically, regulatory bodies evaluate both the delivery system and the genetic payload as an integrated therapeutic entity.
The European Medicines Agency (EMA) classifies non-viral gene therapies under Advanced Therapy Medicinal Products (ATMPs), with specific guidelines outlined in Regulation EC No 1394/2007. This framework requires centralized authorization procedures and specialized scientific committee review through the Committee for Advanced Therapies (CAT).
Safety considerations dominate regulatory scrutiny for non-viral vectors, with particular emphasis on immunogenicity profiles, biodistribution patterns, and potential for genomic integration. Notably, non-viral vectors often face less stringent safety requirements compared to viral counterparts due to their reduced immunogenicity and lower risk of insertional mutagenesis.
Clinical trial design for non-viral gene therapies typically follows a phased approach, with Phase I focusing on safety and dosing, Phase II on preliminary efficacy, and Phase III on confirmatory efficacy. Regulatory agencies increasingly accept adaptive trial designs that allow for protocol modifications based on interim results, particularly beneficial for novel non-viral delivery technologies.
Manufacturing considerations present unique regulatory challenges, with Chemistry, Manufacturing, and Controls (CMC) documentation requiring extensive characterization of both the genetic material and delivery system. Quality control measures must demonstrate batch-to-batch consistency, stability, and purity of the final product.
Accelerated approval pathways exist for gene therapies addressing serious conditions with unmet medical needs. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer expedited review processes, reduced application fees, and enhanced regulatory support.
Post-marketing surveillance requirements for non-viral gene therapies typically include long-term follow-up studies ranging from 5 to 15 years, depending on the persistence of the genetic modification and potential delayed adverse effects. Risk management plans must address theoretical concerns about delayed oncogenesis or unexpected genetic alterations.
International harmonization efforts through the International Council for Harmonisation (ICH) aim to standardize regulatory requirements across major markets, though significant regional differences persist in the evaluation of non-viral gene therapy products.
The European Medicines Agency (EMA) classifies non-viral gene therapies under Advanced Therapy Medicinal Products (ATMPs), with specific guidelines outlined in Regulation EC No 1394/2007. This framework requires centralized authorization procedures and specialized scientific committee review through the Committee for Advanced Therapies (CAT).
Safety considerations dominate regulatory scrutiny for non-viral vectors, with particular emphasis on immunogenicity profiles, biodistribution patterns, and potential for genomic integration. Notably, non-viral vectors often face less stringent safety requirements compared to viral counterparts due to their reduced immunogenicity and lower risk of insertional mutagenesis.
Clinical trial design for non-viral gene therapies typically follows a phased approach, with Phase I focusing on safety and dosing, Phase II on preliminary efficacy, and Phase III on confirmatory efficacy. Regulatory agencies increasingly accept adaptive trial designs that allow for protocol modifications based on interim results, particularly beneficial for novel non-viral delivery technologies.
Manufacturing considerations present unique regulatory challenges, with Chemistry, Manufacturing, and Controls (CMC) documentation requiring extensive characterization of both the genetic material and delivery system. Quality control measures must demonstrate batch-to-batch consistency, stability, and purity of the final product.
Accelerated approval pathways exist for gene therapies addressing serious conditions with unmet medical needs. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer expedited review processes, reduced application fees, and enhanced regulatory support.
Post-marketing surveillance requirements for non-viral gene therapies typically include long-term follow-up studies ranging from 5 to 15 years, depending on the persistence of the genetic modification and potential delayed adverse effects. Risk management plans must address theoretical concerns about delayed oncogenesis or unexpected genetic alterations.
International harmonization efforts through the International Council for Harmonisation (ICH) aim to standardize regulatory requirements across major markets, though significant regional differences persist in the evaluation of non-viral gene therapy products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!