Understanding the Technical Mechanisms Behind Retroviral Vectors in Gene Therapy
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Retroviral Vector Evolution and Therapeutic Objectives
Retroviral vectors have evolved significantly since their initial development in the 1980s, transforming from basic gene delivery tools to sophisticated therapeutic agents. The evolution began with simple gamma-retroviral vectors derived from murine leukemia virus (MLV), which were the first viral vectors used in human gene therapy trials. These early vectors, while groundbreaking, had significant limitations including insertional mutagenesis risks and inability to transduce non-dividing cells.
The field experienced a paradigm shift with the development of lentiviral vectors in the mid-1990s, derived primarily from HIV-1. These vectors offered crucial advantages over gamma-retroviruses, including the ability to transduce non-dividing cells and improved safety profiles through self-inactivating (SIN) designs. This advancement expanded the therapeutic potential to previously inaccessible target tissues such as neurons, hepatocytes, and hematopoietic stem cells.
Technical evolution has focused on several key objectives: enhancing safety, improving transduction efficiency, increasing payload capacity, and enabling targeted delivery. Safety improvements have been particularly critical following adverse events in early clinical trials, leading to the development of third-generation lentiviral vectors with multiple safety features including split packaging systems and deletion of enhancer elements in the viral LTRs.
Vector pseudotyping represents another significant evolutionary milestone, allowing retroviral vectors to be packaged with envelope proteins from different viruses. This innovation has dramatically expanded tropism and enabled targeting of specific cell types. The VSV-G envelope, for instance, has become widely used due to its broad tropism and stability during ultracentrifugation.
Recent technical advancements include the development of integration-deficient lentiviral vectors (IDLVs) for applications requiring transient gene expression and non-integrating approaches. Additionally, site-directed integration systems using engineered integrases aim to reduce genotoxicity by directing vector integration to "safe harbor" genomic sites.
The therapeutic objectives driving retroviral vector evolution have expanded from treating monogenic disorders to addressing complex diseases including cancer, neurodegenerative disorders, and infectious diseases. In oncology, retroviral vectors have enabled breakthrough CAR-T cell therapies, while in infectious disease they're being explored for HIV cure strategies through gene editing approaches.
Looking forward, the field is moving toward precision medicine applications with patient-specific vector designs and combinatorial approaches integrating gene addition, gene editing, and immunomodulation. The ultimate objective remains developing vectors with optimal safety profiles, targeted delivery capabilities, regulated expression, and scalable manufacturing processes to address unmet medical needs across diverse disease indications.
The field experienced a paradigm shift with the development of lentiviral vectors in the mid-1990s, derived primarily from HIV-1. These vectors offered crucial advantages over gamma-retroviruses, including the ability to transduce non-dividing cells and improved safety profiles through self-inactivating (SIN) designs. This advancement expanded the therapeutic potential to previously inaccessible target tissues such as neurons, hepatocytes, and hematopoietic stem cells.
Technical evolution has focused on several key objectives: enhancing safety, improving transduction efficiency, increasing payload capacity, and enabling targeted delivery. Safety improvements have been particularly critical following adverse events in early clinical trials, leading to the development of third-generation lentiviral vectors with multiple safety features including split packaging systems and deletion of enhancer elements in the viral LTRs.
Vector pseudotyping represents another significant evolutionary milestone, allowing retroviral vectors to be packaged with envelope proteins from different viruses. This innovation has dramatically expanded tropism and enabled targeting of specific cell types. The VSV-G envelope, for instance, has become widely used due to its broad tropism and stability during ultracentrifugation.
Recent technical advancements include the development of integration-deficient lentiviral vectors (IDLVs) for applications requiring transient gene expression and non-integrating approaches. Additionally, site-directed integration systems using engineered integrases aim to reduce genotoxicity by directing vector integration to "safe harbor" genomic sites.
The therapeutic objectives driving retroviral vector evolution have expanded from treating monogenic disorders to addressing complex diseases including cancer, neurodegenerative disorders, and infectious diseases. In oncology, retroviral vectors have enabled breakthrough CAR-T cell therapies, while in infectious disease they're being explored for HIV cure strategies through gene editing approaches.
Looking forward, the field is moving toward precision medicine applications with patient-specific vector designs and combinatorial approaches integrating gene addition, gene editing, and immunomodulation. The ultimate objective remains developing vectors with optimal safety profiles, targeted delivery capabilities, regulated expression, and scalable manufacturing processes to address unmet medical needs across diverse disease indications.
Clinical Demand Analysis for Gene Therapy Applications
Gene therapy has emerged as a revolutionary approach to treating genetic disorders by addressing the root cause rather than merely managing symptoms. The clinical demand for gene therapy applications has grown exponentially over the past decade, driven by increasing prevalence of genetic disorders and limitations of conventional treatments. According to recent market analyses, approximately 10,000 diseases are caused by single-gene defects, affecting hundreds of millions of people worldwide, creating a substantial unmet medical need.
Retroviral vector-based gene therapies have shown particular promise in treating previously incurable conditions. The successful treatment of severe combined immunodeficiency (SCID) using retroviral vectors demonstrated clinical efficacy, establishing proof-of-concept for this approach. This breakthrough has catalyzed interest in applying similar techniques to other monogenic disorders such as hemophilia, sickle cell anemia, and various lysosomal storage diseases.
Market research indicates that the global gene therapy market size was valued at $7.6 billion in 2022 and is projected to grow at a compound annual growth rate of 19.2% through 2030. Retroviral vector technologies represent a significant segment of this market, with increasing investment from pharmaceutical companies and biotechnology firms seeking to develop novel therapeutic applications.
Healthcare providers have expressed growing interest in gene therapy solutions due to their potential for long-term efficacy or even curative outcomes. A survey of specialists treating genetic disorders revealed that 78% believe gene therapy will become standard care for certain conditions within the next decade, highlighting the clinical demand for these innovative treatments.
Patient advocacy groups have also become powerful drivers of demand, lobbying for accelerated development and approval pathways for gene therapies. Their influence has contributed to regulatory adaptations, including the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation, which expedites review of promising cell and gene therapies.
Despite the enthusiasm, several factors constrain broader clinical adoption of retroviral vector-based therapies. Cost remains a significant barrier, with current approved gene therapies priced between $375,000 and $2.1 million per treatment. Additionally, manufacturing challenges, limited treatment centers with necessary expertise, and concerns about long-term safety profiles temper immediate market growth.
The clinical demand landscape is further shaped by geographical disparities in healthcare infrastructure and regulatory frameworks. While North America and Europe lead in clinical trials and approved therapies, emerging markets are showing increasing interest as technology transfer and local manufacturing capabilities develop.
Retroviral vector-based gene therapies have shown particular promise in treating previously incurable conditions. The successful treatment of severe combined immunodeficiency (SCID) using retroviral vectors demonstrated clinical efficacy, establishing proof-of-concept for this approach. This breakthrough has catalyzed interest in applying similar techniques to other monogenic disorders such as hemophilia, sickle cell anemia, and various lysosomal storage diseases.
Market research indicates that the global gene therapy market size was valued at $7.6 billion in 2022 and is projected to grow at a compound annual growth rate of 19.2% through 2030. Retroviral vector technologies represent a significant segment of this market, with increasing investment from pharmaceutical companies and biotechnology firms seeking to develop novel therapeutic applications.
Healthcare providers have expressed growing interest in gene therapy solutions due to their potential for long-term efficacy or even curative outcomes. A survey of specialists treating genetic disorders revealed that 78% believe gene therapy will become standard care for certain conditions within the next decade, highlighting the clinical demand for these innovative treatments.
Patient advocacy groups have also become powerful drivers of demand, lobbying for accelerated development and approval pathways for gene therapies. Their influence has contributed to regulatory adaptations, including the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation, which expedites review of promising cell and gene therapies.
Despite the enthusiasm, several factors constrain broader clinical adoption of retroviral vector-based therapies. Cost remains a significant barrier, with current approved gene therapies priced between $375,000 and $2.1 million per treatment. Additionally, manufacturing challenges, limited treatment centers with necessary expertise, and concerns about long-term safety profiles temper immediate market growth.
The clinical demand landscape is further shaped by geographical disparities in healthcare infrastructure and regulatory frameworks. While North America and Europe lead in clinical trials and approved therapies, emerging markets are showing increasing interest as technology transfer and local manufacturing capabilities develop.
Current Limitations and Technical Barriers in Retroviral Vectors
Despite significant advancements in retroviral vector technology for gene therapy applications, several critical limitations and technical barriers continue to challenge researchers and clinicians. The integration pattern of retroviral vectors remains a primary concern, as these vectors insert genetic material semi-randomly into the host genome. This characteristic creates a substantial risk of insertional mutagenesis, which has been documented in clinical trials where patients developed leukemia following retroviral gene therapy. The unpredictable nature of integration sites makes it difficult to control gene expression and avoid disruption of essential cellular functions.
Vector capacity represents another significant limitation, with most retroviral vectors restricted to carrying genetic payloads of approximately 8-10 kb. This size constraint severely limits their utility for delivering larger therapeutic genes or complex regulatory elements necessary for sophisticated gene expression control. For conditions requiring the transfer of large genes, such as Duchenne muscular dystrophy, retroviral vectors often prove inadequate without significant modification.
Production challenges further complicate the clinical application of retroviral vectors. Manufacturing stable, high-titer vector preparations at clinical grade remains technically demanding and expensive. The requirement for specialized facilities and expertise creates bottlenecks in translational research and increases the cost of gene therapy treatments. Additionally, batch-to-batch variability in vector quality can impact treatment efficacy and safety profiles.
Immunogenicity issues present another barrier, as patients may develop immune responses against vector components or the transgene products. Pre-existing immunity to viral proteins can neutralize vectors before they reach target cells, while immune reactions against transduced cells can eliminate therapeutic effects. This immune recognition significantly limits the potential for repeated administrations of retroviral vectors in patients requiring ongoing treatment.
The requirement for cell division represents a fundamental limitation of traditional retroviral vectors, which can only transduce actively dividing cells. This restriction severely limits their application for targeting post-mitotic tissues such as neurons, cardiomyocytes, or mature hepatocytes. While lentiviral vectors have partially overcome this limitation, they introduce additional safety concerns related to their HIV-derived components.
Long-term expression stability remains problematic, with gene silencing frequently observed over time. Epigenetic modifications of the integrated provirus can lead to gradual reduction in transgene expression, diminishing therapeutic efficacy. The mechanisms controlling this silencing are not fully understood, making it difficult to design vectors that maintain consistent expression levels throughout a patient's lifetime.
Vector capacity represents another significant limitation, with most retroviral vectors restricted to carrying genetic payloads of approximately 8-10 kb. This size constraint severely limits their utility for delivering larger therapeutic genes or complex regulatory elements necessary for sophisticated gene expression control. For conditions requiring the transfer of large genes, such as Duchenne muscular dystrophy, retroviral vectors often prove inadequate without significant modification.
Production challenges further complicate the clinical application of retroviral vectors. Manufacturing stable, high-titer vector preparations at clinical grade remains technically demanding and expensive. The requirement for specialized facilities and expertise creates bottlenecks in translational research and increases the cost of gene therapy treatments. Additionally, batch-to-batch variability in vector quality can impact treatment efficacy and safety profiles.
Immunogenicity issues present another barrier, as patients may develop immune responses against vector components or the transgene products. Pre-existing immunity to viral proteins can neutralize vectors before they reach target cells, while immune reactions against transduced cells can eliminate therapeutic effects. This immune recognition significantly limits the potential for repeated administrations of retroviral vectors in patients requiring ongoing treatment.
The requirement for cell division represents a fundamental limitation of traditional retroviral vectors, which can only transduce actively dividing cells. This restriction severely limits their application for targeting post-mitotic tissues such as neurons, cardiomyocytes, or mature hepatocytes. While lentiviral vectors have partially overcome this limitation, they introduce additional safety concerns related to their HIV-derived components.
Long-term expression stability remains problematic, with gene silencing frequently observed over time. Epigenetic modifications of the integrated provirus can lead to gradual reduction in transgene expression, diminishing therapeutic efficacy. The mechanisms controlling this silencing are not fully understood, making it difficult to design vectors that maintain consistent expression levels throughout a patient's lifetime.
Contemporary Retroviral Vector Design Strategies
01 Basic structure and components of retroviral vectors
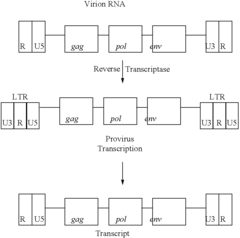
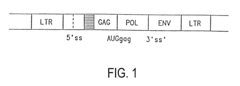
Retroviral vectors are derived from retroviruses and contain essential components for gene delivery. These vectors typically include long terminal repeats (LTRs), packaging signals, and transgene expression cassettes. The basic structure allows for integration of genetic material into the host genome, providing stable gene expression. These vectors can be engineered to carry therapeutic genes while removing viral genes responsible for pathogenicity, making them useful tools for gene therapy applications.- Basic structure and components of retroviral vectors: Retroviral vectors are derived from retroviruses and contain essential components including long terminal repeats (LTRs), packaging signal, and transgene expression cassettes. These vectors are designed to efficiently deliver genetic material into target cells by utilizing the natural infection mechanism of retroviruses. The basic structure typically includes viral genes necessary for replication replaced with therapeutic genes or other genetic elements of interest, while maintaining the viral elements required for packaging and integration.
- Packaging systems and production methods: Advanced packaging systems for retroviral vectors involve separating viral components onto multiple plasmids to enhance safety and prevent recombination events that could generate replication-competent retroviruses. These systems typically include packaging cell lines that stably express viral structural proteins (gag, pol, env) while the vector carries the therapeutic gene and packaging signal. Various production methods have been developed to increase viral titer and stability, including optimization of culture conditions, purification techniques, and concentration methods.
- Tropism modification and pseudotyping: Retroviral vector tropism can be modified through pseudotyping, which involves replacing the native viral envelope proteins with envelope proteins from other viruses. This technique allows for targeting specific cell types and expanding the host range of the vector. Common envelope proteins used for pseudotyping include vesicular stomatitis virus glycoprotein (VSV-G), which provides broad tropism and stability during purification. Other modifications include incorporating cell-specific ligands or antibodies into the envelope to enhance targeting specificity.
- Integration mechanisms and safety features: Retroviral vectors integrate their genetic material into the host cell genome, allowing for stable long-term expression of the transgene. The integration process is mediated by the viral integrase enzyme, which recognizes specific sequences in the viral LTRs. To address safety concerns related to insertional mutagenesis, modern vectors incorporate various safety features such as self-inactivating (SIN) designs with deleted promoter/enhancer elements in the LTRs, insulator sequences to prevent activation of neighboring genes, and site-directed integration systems to control integration sites.
- Lentiviral vectors as advanced retroviral systems: Lentiviral vectors represent an advanced subset of retroviral vectors derived from lentiviruses such as HIV. Unlike conventional retroviral vectors, lentiviral vectors can transduce both dividing and non-dividing cells, making them valuable for targeting terminally differentiated cells like neurons. These vectors have been engineered with multiple safety features including split-genome packaging systems and deletion of accessory genes not essential for gene transfer. Their ability to carry larger transgenes and provide long-term stable expression has made them increasingly important in gene therapy applications.
02 Packaging systems and production methods
Advanced packaging systems have been developed to produce retroviral vectors with high titers and improved safety profiles. These systems typically separate the packaging components from the vector genome to prevent generation of replication-competent retroviruses. Split-genome packaging designs use multiple plasmids to express viral proteins needed for vector production. Producer cell lines can be engineered to stably express packaging components, allowing for consistent and scalable vector manufacturing.Expand Specific Solutions03 Targeting and tropism modification
Retroviral vectors can be engineered to target specific cell types through modification of their envelope proteins. Pseudotyping involves replacing the native envelope with glycoproteins from other viruses to alter cellular tropism. Molecular engineering of the envelope can incorporate targeting ligands or antibody fragments to direct vectors to specific receptors. These modifications enhance the specificity of gene delivery and reduce off-target effects, improving both safety and efficacy for therapeutic applications.Expand Specific Solutions04 Self-inactivating and safety features
Self-inactivating (SIN) retroviral vectors contain deletions in the U3 region of the 3' LTR, which is copied to the 5' LTR during reverse transcription. This modification reduces the risk of insertional mutagenesis by inactivating the viral promoter after integration. Additional safety features include insulator elements to prevent interaction with neighboring genes, regulated expression systems, and suicide genes for selective elimination of transduced cells if necessary. These safety enhancements are critical for clinical applications of retroviral vector technology.Expand Specific Solutions05 Lentiviral vectors as advanced retroviral systems
Lentiviral vectors represent an advanced subset of retroviral vectors derived from lentiviruses such as HIV. Unlike conventional retroviral vectors, lentiviral systems can transduce non-dividing cells, significantly expanding their application range. They feature enhanced nuclear import mechanisms and accessory proteins that facilitate integration in quiescent cells. Modern lentiviral vectors incorporate multiple safety features including self-inactivating designs and minimal viral components, making them valuable tools for both research and therapeutic applications.Expand Specific Solutions
Leading Research Institutions and Biotech Companies in Gene Therapy
The retroviral vector gene therapy market is in a growth phase, with increasing clinical applications driving market expansion. The global market size for viral vectors in gene therapy is projected to reach several billion dollars by 2025, with retroviral vectors representing a significant segment. Technologically, retroviral vectors have reached moderate maturity, with established players like Oxford Biomedica and Takara Bio leading in lentiviral vector development. Major pharmaceutical companies including GlaxoSmithKline and Genentech have integrated these technologies into their therapeutic pipelines. Academic institutions such as St. Jude Children's Research Hospital and University of North Carolina contribute significantly to innovation, while specialized biotechs like Cell Genesys and Janssen Vaccines focus on vector optimization. The competitive landscape features collaboration between established pharmaceutical companies and specialized vector manufacturers to overcome remaining challenges in safety, manufacturing scale, and targeting efficiency.
Oxford Biomedica (UK) Ltd.
Technical Solution: Oxford Biomedica has developed the LentiVector® platform, a proprietary lentiviral vector-based gene delivery system specifically engineered for gene therapy applications. Their technology utilizes HIV-1 derived vectors with significant modifications to enhance safety, including the removal of approximately 60% of the HIV-1 genome and separation of vector components into multiple plasmids. The platform incorporates a self-inactivating (SIN) design that eliminates the viral promoter activity upon integration, reducing the risk of insertional mutagenesis. Their manufacturing process employs a serum-free suspension culture system that enables scalable production with consistent vector quality. The company has implemented a proprietary purification process that achieves high purity levels (>90%) while maintaining functional vector titers of 10^9-10^10 TU/mL. Oxford Biomedica's vectors demonstrate stable transgene expression for over 10 years in preclinical models and have been used in multiple approved therapies including Kymriah® for cancer treatment.
Strengths: Industry-leading lentiviral vector production capacity with established regulatory approval track record; proprietary purification technology achieving exceptional purity while maintaining high titers; demonstrated long-term expression stability in clinical applications. Weaknesses: Higher manufacturing complexity compared to some alternative vector systems; potential for limited packaging capacity (~8kb); relatively higher production costs compared to non-viral delivery systems.
Takara Bio, Inc.
Technical Solution: Takara Bio has developed the RetroNectin® Method, an innovative approach to retroviral vector-mediated gene transfer that significantly enhances transduction efficiency. Their technology utilizes a recombinant human fibronectin fragment (CH-296) that co-localizes retroviral particles and target cells through specific binding domains. The RetroNectin protein contains three functional domains: the cell-binding domain (C-domain) that binds integrin VLA-5, the heparin-binding domain (H-domain) that binds retroviral vectors, and the CS-1 sequence that binds integrin VLA-4. This co-localization mechanism increases the probability of vector-cell interaction, resulting in 3-10 fold higher transduction rates compared to conventional methods. Takara's system is particularly effective for hard-to-transduce cells like hematopoietic stem cells, T cells, and NK cells. Their manufacturing process includes proprietary stabilization technologies that extend vector half-life and maintain functional activity during freeze-thaw cycles. The company has also developed specialized culture vessels (RetroNectin Dish) pre-coated with the RetroNectin reagent to standardize the transduction process.
Strengths: Significantly enhanced transduction efficiency for difficult-to-transduce primary cells; reduced cytotoxicity compared to chemical transfection methods; compatible with both retroviral and lentiviral vector systems; well-established safety profile with extensive clinical use. Weaknesses: Requires additional processing steps compared to direct transduction methods; higher cost due to recombinant protein component; primarily enhances transduction rather than addressing fundamental vector limitations like immunogenicity.
Critical Patents and Breakthrough Publications in Vector Engineering
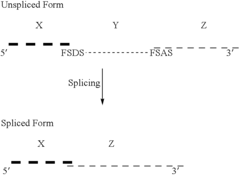
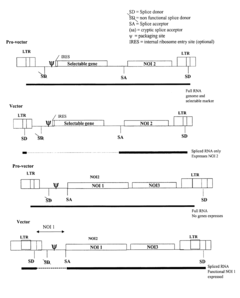
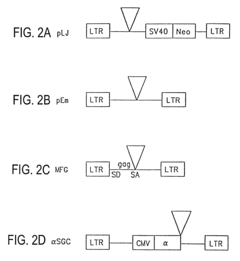
Retroviral vectors comprising a functional splice donor site and a functional splice acceptor site
PatentInactiveUS6808922B1
Innovation
- A novel retroviral vector system incorporating a functional splice donor and acceptor site configuration, derived from a retroviral pro-vector, allows for efficient splicing and expression of nucleotide sequences of interest, with safety features to prevent recombination and ensure targeted delivery.
Retroviral gene therapy vectors and therapeutic methods based thereon
PatentInactiveUS6544771B1
Innovation
- Development of novel retroviral vectors with specific structural features such as incomplete or defective gag, env, or pol genes, inclusion of splice donor and acceptor sites, and use of alpha-globin or cytomegalovirus enhancer sequences to control gene expression, and absence of selectable markers, allowing for high-level, long-term expression of therapeutic proteins in various cell types.
Biosafety and Regulatory Framework for Viral Vector-Based Therapies
The regulatory landscape for viral vector-based gene therapies has evolved significantly over the past two decades, reflecting growing understanding of safety concerns and therapeutic potential. Regulatory bodies worldwide have established comprehensive frameworks that address the unique challenges posed by retroviral vectors, with particular emphasis on insertional mutagenesis risks that emerged following early clinical trials in the early 2000s.
The FDA and EMA have developed specialized guidance documents specifically for viral vector-based therapies, requiring extensive preclinical testing focused on vector integration profiles, genotoxicity assessments, and long-term safety monitoring. These requirements typically include vector sequence analysis, integration site mapping, and assessment of potential oncogenic activation through insertional events.
Biosafety considerations for retroviral vectors encompass multiple dimensions, including laboratory containment requirements (typically BSL-2), environmental risk assessments, and patient monitoring protocols. Vector design has evolved to incorporate self-inactivating (SIN) configurations that reduce the risk of mobilization and recombination events, representing a critical advancement in safety engineering.
Manufacturing standards present unique challenges, with regulatory bodies requiring demonstration of consistent production processes that minimize the risk of replication-competent retroviruses (RCRs). Testing for RCRs has become a mandatory component of both manufacturing quality control and clinical monitoring protocols, with increasingly sensitive detection methods being developed and validated.
Patient follow-up requirements have been standardized across major regulatory jurisdictions, with long-term monitoring (often 15+ years) mandated for recipients of integrating vector therapies. These protocols focus on detecting delayed adverse events related to insertional mutagenesis, immune responses, and potential germline transmission risks.
International harmonization efforts have accelerated through initiatives like the International Pharmaceutical Regulators Programme (IPRP) Gene Therapy Working Group, which aims to standardize safety assessment approaches across different regulatory regions. However, significant regional variations persist in specific requirements for vector characterization, integration analysis methodologies, and acceptable risk thresholds.
Recent regulatory innovations include accelerated approval pathways for therapies addressing unmet medical needs, balanced with enhanced post-marketing surveillance requirements. The implementation of Risk Evaluation and Mitigation Strategies (REMS) has become increasingly common for vector-based therapies, reflecting the need for structured risk management throughout the product lifecycle.
The FDA and EMA have developed specialized guidance documents specifically for viral vector-based therapies, requiring extensive preclinical testing focused on vector integration profiles, genotoxicity assessments, and long-term safety monitoring. These requirements typically include vector sequence analysis, integration site mapping, and assessment of potential oncogenic activation through insertional events.
Biosafety considerations for retroviral vectors encompass multiple dimensions, including laboratory containment requirements (typically BSL-2), environmental risk assessments, and patient monitoring protocols. Vector design has evolved to incorporate self-inactivating (SIN) configurations that reduce the risk of mobilization and recombination events, representing a critical advancement in safety engineering.
Manufacturing standards present unique challenges, with regulatory bodies requiring demonstration of consistent production processes that minimize the risk of replication-competent retroviruses (RCRs). Testing for RCRs has become a mandatory component of both manufacturing quality control and clinical monitoring protocols, with increasingly sensitive detection methods being developed and validated.
Patient follow-up requirements have been standardized across major regulatory jurisdictions, with long-term monitoring (often 15+ years) mandated for recipients of integrating vector therapies. These protocols focus on detecting delayed adverse events related to insertional mutagenesis, immune responses, and potential germline transmission risks.
International harmonization efforts have accelerated through initiatives like the International Pharmaceutical Regulators Programme (IPRP) Gene Therapy Working Group, which aims to standardize safety assessment approaches across different regulatory regions. However, significant regional variations persist in specific requirements for vector characterization, integration analysis methodologies, and acceptable risk thresholds.
Recent regulatory innovations include accelerated approval pathways for therapies addressing unmet medical needs, balanced with enhanced post-marketing surveillance requirements. The implementation of Risk Evaluation and Mitigation Strategies (REMS) has become increasingly common for vector-based therapies, reflecting the need for structured risk management throughout the product lifecycle.
Manufacturing Scalability and Cost Considerations
The manufacturing of retroviral vectors for gene therapy presents significant challenges in terms of scalability and cost-effectiveness. Current production methods predominantly rely on transient transfection of plasmid DNA into producer cell lines, which becomes increasingly inefficient at larger scales. This limitation creates a bottleneck in the production pipeline, restricting the availability of viral vectors for clinical applications and contributing to the high costs associated with gene therapy treatments.
Scale-up challenges are particularly evident in the transition from laboratory-scale production to commercial manufacturing. The complex biological processes involved in viral vector production do not scale linearly, resulting in decreased yields and increased variability when production volumes increase. Additionally, the requirement for specialized facilities with high biosafety levels (BSL-2 or BSL-3) adds substantial capital expenditure to the manufacturing process.
Cost considerations for retroviral vector manufacturing extend beyond facility requirements. Raw materials, particularly plasmid DNA and transfection reagents, represent a significant portion of production costs. Current estimates suggest that raw materials alone can account for 30-40% of the total manufacturing cost. Furthermore, the extensive quality control testing required for clinical-grade vectors, including tests for replication-competent retroviruses, sterility, and potency, adds another layer of expense.
Labor costs also contribute substantially to the overall manufacturing expenses. The production of retroviral vectors requires highly skilled personnel with specialized training in biomanufacturing and virology. The labor-intensive nature of current production methods, coupled with the need for extensive documentation to meet regulatory requirements, further increases the cost burden.
Recent technological innovations aim to address these scalability and cost challenges. Stable producer cell lines, which eliminate the need for repeated transfections, show promise for more consistent and cost-effective production. Similarly, advances in bioreactor design and process intensification strategies are improving yields and reducing production times. Continuous manufacturing approaches, as opposed to traditional batch processing, offer potential for increased efficiency and reduced costs.
Despite these innovations, the cost of goods for retroviral vector production remains high, typically ranging from $10,000 to $30,000 per dose for clinical applications. This cost structure presents a significant barrier to widespread adoption of gene therapies and highlights the urgent need for continued investment in manufacturing technologies that can improve scalability while reducing production costs.
Scale-up challenges are particularly evident in the transition from laboratory-scale production to commercial manufacturing. The complex biological processes involved in viral vector production do not scale linearly, resulting in decreased yields and increased variability when production volumes increase. Additionally, the requirement for specialized facilities with high biosafety levels (BSL-2 or BSL-3) adds substantial capital expenditure to the manufacturing process.
Cost considerations for retroviral vector manufacturing extend beyond facility requirements. Raw materials, particularly plasmid DNA and transfection reagents, represent a significant portion of production costs. Current estimates suggest that raw materials alone can account for 30-40% of the total manufacturing cost. Furthermore, the extensive quality control testing required for clinical-grade vectors, including tests for replication-competent retroviruses, sterility, and potency, adds another layer of expense.
Labor costs also contribute substantially to the overall manufacturing expenses. The production of retroviral vectors requires highly skilled personnel with specialized training in biomanufacturing and virology. The labor-intensive nature of current production methods, coupled with the need for extensive documentation to meet regulatory requirements, further increases the cost burden.
Recent technological innovations aim to address these scalability and cost challenges. Stable producer cell lines, which eliminate the need for repeated transfections, show promise for more consistent and cost-effective production. Similarly, advances in bioreactor design and process intensification strategies are improving yields and reducing production times. Continuous manufacturing approaches, as opposed to traditional batch processing, offer potential for increased efficiency and reduced costs.
Despite these innovations, the cost of goods for retroviral vector production remains high, typically ranging from $10,000 to $30,000 per dose for clinical applications. This cost structure presents a significant barrier to widespread adoption of gene therapies and highlights the urgent need for continued investment in manufacturing technologies that can improve scalability while reducing production costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!