Analyzing the Genetic Precision of Current Gene Therapy Technologies
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy Evolution and Objectives
Gene therapy has evolved significantly since its conceptual inception in the 1970s, transforming from theoretical possibility to clinical reality. The field's development can be traced through several distinct phases, beginning with early recombinant DNA experiments and progressing through viral vector development, genome editing technologies, and the current era of precision genetic medicine. This evolutionary trajectory has been marked by both breakthrough successes and sobering setbacks that have collectively shaped our understanding of genetic intervention strategies.
The initial phase of gene therapy development (1970s-1990s) focused primarily on establishing fundamental techniques for gene transfer and expression. Scientists pioneered methods to introduce functional genes into cells to compensate for defective or missing genes. This period was characterized by optimism but limited by rudimentary delivery systems and incomplete understanding of genetic regulation mechanisms.
A pivotal shift occurred in the late 1990s and early 2000s following several high-profile adverse events, including the death of Jesse Gelsinger in 1999 due to an immune reaction to a viral vector. These events prompted a reevaluation of safety protocols and vector design, leading to a temporary slowdown in clinical applications while fundamental research continued to advance.
The field experienced revitalization with the emergence of more sophisticated viral vectors (particularly adeno-associated viruses and lentiviruses) and the development of genome editing technologies such as zinc-finger nucleases, TALENs, and ultimately CRISPR-Cas systems. These innovations dramatically expanded the precision and scope of genetic interventions, enabling not only gene addition but also gene correction, repression, and enhancement.
Current gene therapy technologies aim to achieve unprecedented levels of genetic precision through several complementary objectives. Primary among these is enhancing targeting specificity—ensuring therapeutic genetic material reaches intended cells and tissues while minimizing off-target effects. Equally important is improving editing accuracy at the genomic level, reducing unintended modifications that could lead to cellular dysfunction or oncogenesis.
Additional objectives include developing more efficient delivery systems capable of overcoming biological barriers, extending therapeutic durability to reduce the need for repeated interventions, and scaling manufacturing processes to improve accessibility and reduce costs. Researchers are also focused on minimizing immunogenicity to prevent adverse immune responses that can neutralize therapeutic vectors or trigger systemic inflammation.
The convergence of these technological advances and research objectives has culminated in several FDA-approved gene therapies for conditions including inherited retinal dystrophies, spinal muscular atrophy, and certain forms of cancer. These successes represent important milestones while highlighting the continued need for refinement in precision, safety, and accessibility as the field continues to mature.
The initial phase of gene therapy development (1970s-1990s) focused primarily on establishing fundamental techniques for gene transfer and expression. Scientists pioneered methods to introduce functional genes into cells to compensate for defective or missing genes. This period was characterized by optimism but limited by rudimentary delivery systems and incomplete understanding of genetic regulation mechanisms.
A pivotal shift occurred in the late 1990s and early 2000s following several high-profile adverse events, including the death of Jesse Gelsinger in 1999 due to an immune reaction to a viral vector. These events prompted a reevaluation of safety protocols and vector design, leading to a temporary slowdown in clinical applications while fundamental research continued to advance.
The field experienced revitalization with the emergence of more sophisticated viral vectors (particularly adeno-associated viruses and lentiviruses) and the development of genome editing technologies such as zinc-finger nucleases, TALENs, and ultimately CRISPR-Cas systems. These innovations dramatically expanded the precision and scope of genetic interventions, enabling not only gene addition but also gene correction, repression, and enhancement.
Current gene therapy technologies aim to achieve unprecedented levels of genetic precision through several complementary objectives. Primary among these is enhancing targeting specificity—ensuring therapeutic genetic material reaches intended cells and tissues while minimizing off-target effects. Equally important is improving editing accuracy at the genomic level, reducing unintended modifications that could lead to cellular dysfunction or oncogenesis.
Additional objectives include developing more efficient delivery systems capable of overcoming biological barriers, extending therapeutic durability to reduce the need for repeated interventions, and scaling manufacturing processes to improve accessibility and reduce costs. Researchers are also focused on minimizing immunogenicity to prevent adverse immune responses that can neutralize therapeutic vectors or trigger systemic inflammation.
The convergence of these technological advances and research objectives has culminated in several FDA-approved gene therapies for conditions including inherited retinal dystrophies, spinal muscular atrophy, and certain forms of cancer. These successes represent important milestones while highlighting the continued need for refinement in precision, safety, and accessibility as the field continues to mature.
Market Analysis for Gene Therapy Applications
The gene therapy market has experienced remarkable growth in recent years, with global valuations reaching $5.8 billion in 2022 and projections indicating expansion to $25.3 billion by 2030, representing a compound annual growth rate of 20.4%. This accelerated growth is primarily driven by increasing prevalence of genetic disorders, advancements in delivery technologies, and substantial investments from pharmaceutical companies and venture capital firms seeking to capitalize on breakthrough treatments.
Oncology represents the largest application segment, accounting for approximately 32% of the current gene therapy market. This dominance stems from the pressing need for alternative treatment modalities for various cancers and the promising results demonstrated in clinical trials. Following closely is the rare disease segment, which despite affecting smaller patient populations, commands premium pricing due to limited treatment alternatives and favorable regulatory pathways.
Regional analysis reveals North America as the dominant market, holding 48% of global share, attributed to robust research infrastructure, favorable reimbursement policies, and presence of key industry players. Europe follows at 28%, with Asia-Pacific emerging as the fastest-growing region due to increasing healthcare expenditure, improving regulatory frameworks, and expanding clinical trial activities particularly in China, Japan, and South Korea.
The market structure exhibits oligopolistic characteristics with significant barriers to entry, including high development costs, complex manufacturing requirements, and stringent regulatory oversight. Current pricing models reflect this complexity, with approved therapies commanding between $373,000 to $2.1 million per treatment course, raising significant concerns about accessibility and healthcare system sustainability.
Reimbursement landscapes vary considerably across regions, with innovative payment models emerging to address the high upfront costs. These include outcomes-based agreements, installment plans, and risk-sharing arrangements between manufacturers and payers. The sustainability of these models remains uncertain as more gene therapies receive approval.
Patient access challenges persist despite clinical promise, with only 30% of eligible patients receiving approved gene therapies in developed markets. This gap is substantially wider in emerging economies where infrastructure limitations and affordability concerns create significant market penetration challenges.
Future market dynamics will likely be shaped by technological advancements improving precision and reducing manufacturing costs, expanded indications beyond rare diseases, and evolving regulatory frameworks. The entry of biosimilar gene therapies following patent expirations of pioneer products could potentially reshape pricing structures and expand market access in the latter half of this decade.
Oncology represents the largest application segment, accounting for approximately 32% of the current gene therapy market. This dominance stems from the pressing need for alternative treatment modalities for various cancers and the promising results demonstrated in clinical trials. Following closely is the rare disease segment, which despite affecting smaller patient populations, commands premium pricing due to limited treatment alternatives and favorable regulatory pathways.
Regional analysis reveals North America as the dominant market, holding 48% of global share, attributed to robust research infrastructure, favorable reimbursement policies, and presence of key industry players. Europe follows at 28%, with Asia-Pacific emerging as the fastest-growing region due to increasing healthcare expenditure, improving regulatory frameworks, and expanding clinical trial activities particularly in China, Japan, and South Korea.
The market structure exhibits oligopolistic characteristics with significant barriers to entry, including high development costs, complex manufacturing requirements, and stringent regulatory oversight. Current pricing models reflect this complexity, with approved therapies commanding between $373,000 to $2.1 million per treatment course, raising significant concerns about accessibility and healthcare system sustainability.
Reimbursement landscapes vary considerably across regions, with innovative payment models emerging to address the high upfront costs. These include outcomes-based agreements, installment plans, and risk-sharing arrangements between manufacturers and payers. The sustainability of these models remains uncertain as more gene therapies receive approval.
Patient access challenges persist despite clinical promise, with only 30% of eligible patients receiving approved gene therapies in developed markets. This gap is substantially wider in emerging economies where infrastructure limitations and affordability concerns create significant market penetration challenges.
Future market dynamics will likely be shaped by technological advancements improving precision and reducing manufacturing costs, expanded indications beyond rare diseases, and evolving regulatory frameworks. The entry of biosimilar gene therapies following patent expirations of pioneer products could potentially reshape pricing structures and expand market access in the latter half of this decade.
Current Precision Challenges in Gene Therapy
Despite significant advancements in gene therapy technologies, current approaches face substantial precision challenges that limit their therapeutic potential and clinical application. The primary challenge remains the accuracy of gene editing tools like CRISPR-Cas9, which still exhibit off-target effects where unintended genomic sites are modified. Studies indicate off-target rates ranging from 0.1% to 5% depending on the specific system and target sequence, potentially causing unpredictable mutations that could lead to cellular dysfunction or oncogenesis.
Delivery precision represents another critical limitation, as current vector systems—including viral vectors like AAV and lentivirus—demonstrate variable transduction efficiencies across different tissue types. This inconsistency results in non-uniform therapeutic gene expression, with some tissues receiving insufficient genetic material while others experience potentially toxic overexpression. The blood-brain barrier particularly exemplifies this challenge, with less than 1% of systemically administered vectors typically reaching neural tissues.
Dosage control presents significant precision hurdles, as the relationship between vector dose and therapeutic effect often follows non-linear patterns. The therapeutic window—the range between minimal effective dose and toxicity threshold—remains narrow for many gene therapy applications, complicating clinical translation. Current technologies struggle to achieve consistent expression levels across treated cells, with coefficient of variation often exceeding 200% in expression profiles.
Integration site specificity constitutes another precision challenge, particularly for integrating vector systems. While non-integrating vectors avoid insertional mutagenesis risks, they provide only transient expression in dividing cells. Conversely, integrating vectors can provide durable expression but may disrupt essential genes or activate oncogenes. Current targeting technologies achieve desired integration sites with only 20-40% efficiency in optimal conditions.
Immunological responses further compromise precision, as pre-existing or treatment-induced immunity against delivery vectors can dramatically alter biodistribution and expression profiles. Studies show that neutralizing antibodies can reduce transduction efficiency by 50-95%, creating significant inter-patient variability that challenges standardized dosing protocols.
Temporal control limitations also affect precision, as most current systems lack robust mechanisms to regulate transgene expression over time. Once delivered, expression typically follows an unpredictable course, with some patients experiencing gradual decline while others maintain stable levels. The inability to modulate expression in response to changing physiological needs represents a significant barrier to precision therapeutics.
These multifaceted precision challenges collectively impede the widespread clinical implementation of gene therapy, necessitating technological innovations that enhance targeting accuracy, delivery specificity, dosage control, and expression regulation to realize the full potential of genetic medicine.
Delivery precision represents another critical limitation, as current vector systems—including viral vectors like AAV and lentivirus—demonstrate variable transduction efficiencies across different tissue types. This inconsistency results in non-uniform therapeutic gene expression, with some tissues receiving insufficient genetic material while others experience potentially toxic overexpression. The blood-brain barrier particularly exemplifies this challenge, with less than 1% of systemically administered vectors typically reaching neural tissues.
Dosage control presents significant precision hurdles, as the relationship between vector dose and therapeutic effect often follows non-linear patterns. The therapeutic window—the range between minimal effective dose and toxicity threshold—remains narrow for many gene therapy applications, complicating clinical translation. Current technologies struggle to achieve consistent expression levels across treated cells, with coefficient of variation often exceeding 200% in expression profiles.
Integration site specificity constitutes another precision challenge, particularly for integrating vector systems. While non-integrating vectors avoid insertional mutagenesis risks, they provide only transient expression in dividing cells. Conversely, integrating vectors can provide durable expression but may disrupt essential genes or activate oncogenes. Current targeting technologies achieve desired integration sites with only 20-40% efficiency in optimal conditions.
Immunological responses further compromise precision, as pre-existing or treatment-induced immunity against delivery vectors can dramatically alter biodistribution and expression profiles. Studies show that neutralizing antibodies can reduce transduction efficiency by 50-95%, creating significant inter-patient variability that challenges standardized dosing protocols.
Temporal control limitations also affect precision, as most current systems lack robust mechanisms to regulate transgene expression over time. Once delivered, expression typically follows an unpredictable course, with some patients experiencing gradual decline while others maintain stable levels. The inability to modulate expression in response to changing physiological needs represents a significant barrier to precision therapeutics.
These multifaceted precision challenges collectively impede the widespread clinical implementation of gene therapy, necessitating technological innovations that enhance targeting accuracy, delivery specificity, dosage control, and expression regulation to realize the full potential of genetic medicine.
Current Gene Editing Methodologies
01 CRISPR-Cas gene editing technologies
CRISPR-Cas systems represent a revolutionary approach to genetic precision therapy, allowing for targeted modification of DNA sequences. These systems utilize guide RNAs to direct Cas nucleases to specific genomic locations, enabling precise editing, deletion, or insertion of genetic material. Recent advancements have improved the specificity and efficiency of CRISPR-based therapies, reducing off-target effects and enhancing their potential for treating genetic disorders through precise genomic modifications.- CRISPR-Cas gene editing systems for precision therapy: CRISPR-Cas gene editing technology enables precise modification of genetic material for therapeutic applications. These systems can target specific DNA sequences to correct mutations, delete harmful genes, or insert beneficial ones. The technology allows for customized treatment of genetic disorders by making precise changes to the genome with minimal off-target effects, representing a significant advancement in genetic precision medicine.
- Viral vector delivery systems for gene therapy: Viral vectors serve as efficient delivery vehicles for therapeutic genetic material. These systems utilize modified viruses such as adeno-associated viruses (AAVs), lentiviruses, and retroviruses to transport genes into target cells. The vectors can be engineered for tissue specificity and improved safety profiles, enabling precise delivery of genetic payloads to affected tissues while minimizing systemic exposure and immune responses.
- Non-viral gene delivery technologies: Non-viral delivery methods offer alternatives to viral vectors for gene therapy applications. These include lipid nanoparticles, polymeric carriers, and physical methods like electroporation. Such approaches can reduce immunogenicity concerns while maintaining delivery efficiency. Advanced formulations enable targeted delivery to specific tissues and cell types, improving the precision of genetic interventions while potentially offering better safety profiles and manufacturing scalability.
- Precision targeting and genetic screening technologies: Advanced screening and targeting technologies enable identification of specific genetic targets for therapeutic intervention. These include high-throughput sequencing, bioinformatics tools, and computational modeling to identify disease-causing mutations with high accuracy. Precision targeting approaches allow for patient-specific therapy design based on individual genetic profiles, enabling personalized treatment strategies that address the exact genetic cause of disease.
- Gene expression regulation and control systems: Technologies for precise regulation of gene expression provide temporal and spatial control over therapeutic genetic interventions. These include inducible promoters, RNA interference, antisense oligonucleotides, and synthetic biology approaches. Such systems allow for adjustable gene expression levels, conditional activation, or silencing of therapeutic genes, enhancing the safety and efficacy of gene therapy by enabling fine-tuned control over the introduced genetic material.
02 Viral vector delivery systems for gene therapy
Viral vectors serve as efficient delivery vehicles for genetic material in precision gene therapy applications. These systems, including adeno-associated viruses (AAVs), lentiviruses, and retroviruses, have been engineered to safely transport therapeutic genes to target cells with high specificity. Recent innovations focus on improving tissue tropism, reducing immunogenicity, and enhancing transduction efficiency to enable more precise targeting of specific cell populations while minimizing off-target effects and safety concerns.Expand Specific Solutions03 Non-viral gene delivery technologies
Non-viral delivery systems represent an emerging approach for genetic precision therapies, offering advantages in safety, manufacturing, and reduced immunogenicity. These technologies include lipid nanoparticles, polymeric carriers, and physical methods such as electroporation and sonoporation. Recent developments have focused on improving cellular uptake, endosomal escape, and targeted delivery to specific tissues, enhancing the precision of genetic material delivery while minimizing systemic distribution and potential side effects.Expand Specific Solutions04 Precision gene regulation and expression control
Advanced technologies for precise control of gene expression enable fine-tuned regulation of therapeutic genes in target tissues. These approaches include inducible promoters, synthetic biology circuits, and RNA-based regulatory elements that respond to specific stimuli or cellular conditions. Such precision control mechanisms allow for temporal and spatial regulation of therapeutic gene expression, enhancing safety profiles and therapeutic efficacy by limiting expression to desired tissues and timeframes.Expand Specific Solutions05 Genomic integration and site-specific recombination
Site-specific genomic integration technologies enable precise insertion of therapeutic genes at predetermined locations in the genome, minimizing risks associated with random integration. These approaches utilize recombinases, transposases, and engineered nucleases to direct integration to safe harbor sites or to correct mutations at their endogenous loci. Recent advances have improved the specificity and efficiency of these systems, reducing genotoxicity risks while enabling durable expression of therapeutic genes through stable genomic integration.Expand Specific Solutions
Leading Companies in Gene Therapy Field
The gene therapy technology landscape is currently in a transitional phase between early commercialization and broader clinical adoption, with a global market expected to reach $20 billion by 2027. Technical maturity varies significantly across approaches, with companies demonstrating different levels of advancement. Sangamo Therapeutics leads in zinc finger nuclease technology, while academic institutions like MIT and Harvard drive fundamental research innovations. Guardant Health and Immunai are pioneering precision diagnostics essential for treatment efficacy. Pharmaceutical giants like Bayer are investing heavily in clinical translation, while specialized firms such as Regel Therapeutics and Scipher Medicine are developing novel regulatory approaches and companion diagnostics. The competitive landscape reflects a complex ecosystem where strategic partnerships between biotechnology innovators and established healthcare institutions are increasingly critical for commercial success.
Sangamo Therapeutics, Inc.
Technical Solution: Sangamo Therapeutics has pioneered zinc finger nuclease (ZFN) technology for gene therapy applications, offering highly precise genome editing capabilities. Their proprietary ZFN platform enables targeted DNA modifications with specificity down to a single base pair, allowing for gene knockout, insertion, or correction. The company has developed a comprehensive pipeline targeting genetic diseases including hemophilia, Fabry disease, and sickle cell disease. Sangamo's technology utilizes engineered zinc finger proteins that can recognize specific DNA sequences and, when fused with nuclease domains, create double-strand breaks at predetermined locations. This approach allows for homology-directed repair mechanisms to introduce therapeutic genetic modifications with minimal off-target effects. Recent advancements include the development of in vivo genome editing approaches that deliver ZFNs directly to affected tissues, eliminating the need for ex vivo cell manipulation in some applications.
Strengths: Exceptional DNA-binding specificity with customizable zinc finger arrays; established safety profile in clinical trials; versatility across multiple therapeutic areas. Weaknesses: Complex manufacturing process; potential immunogenicity concerns; competition from CRISPR-based approaches that may offer simpler design and implementation.
President & Fellows of Harvard College
Technical Solution: Harvard's gene therapy research program has developed advanced base editing technology that enables precise single nucleotide changes without creating double-strand breaks in DNA. This approach utilizes modified CRISPR systems where the Cas9 protein is engineered to nick rather than cut DNA, while being coupled with deaminase enzymes that can convert one nucleotide to another. Harvard researchers have further refined this technology with the development of prime editing, which combines a catalytically impaired Cas9 with an engineered reverse transcriptase and a prime editing guide RNA (pegRNA). This system can perform targeted insertions, deletions, and all possible base-to-base conversions with minimal off-target effects. The technology has demonstrated efficacy in correcting disease-causing mutations for conditions like sickle cell disease, Tay-Sachs, and certain forms of blindness. Harvard's platform also includes advanced delivery systems using engineered viral vectors and lipid nanoparticles that enhance targeting specificity to affected tissues while minimizing immunogenicity.
Strengths: Exceptional precision with minimal off-target effects; versatility in performing multiple types of genetic modifications; strong intellectual property portfolio. Weaknesses: Complex delivery requirements; potential limitations in editing efficiency compared to traditional CRISPR systems; challenges in scaling production for clinical applications.
Key Innovations in Genetic Precision
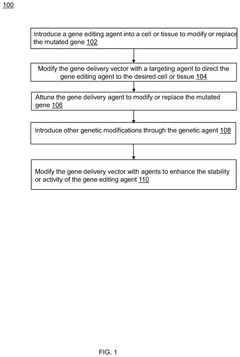
Method for gene editing using gene therapy
PatentPendingUS20240358854A1
Innovation
- A method for gene editing using a modified gene delivery vector with a targeting agent to direct the gene editing agent to specific cells or tissues, utilizing CRISPR-Cas9 or Zinc Finger Nucleases, and incorporating stability enhancers to ensure precise and efficient genetic modifications.
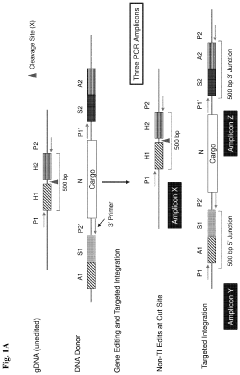
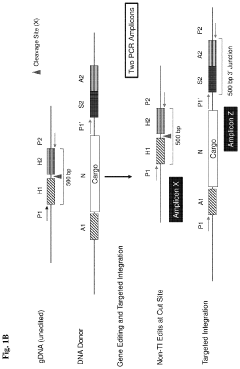
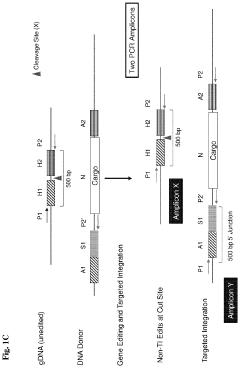
Systems and methods for targeted integration and genome editing and detection thereof using integrated priming sites
PatentActiveUS11866726B2
Innovation
- The use of donor templates with embedded primer binding sites that allow for homologous recombination, enabling the generation of amplicons for quantitative analysis of targeted integration and fidelity assessment through PCR reactions.
Regulatory Framework for Gene Therapy
The regulatory landscape for gene therapy has evolved significantly over the past three decades, reflecting both scientific advancements and societal concerns. In the United States, the Food and Drug Administration (FDA) has established a comprehensive framework through the Center for Biologics Evaluation and Research (CBER), which oversees the approval process for gene therapy products. This process typically involves rigorous preclinical testing, followed by phased clinical trials that assess safety, efficacy, and long-term effects.
The European Medicines Agency (EMA) has developed parallel but distinct regulatory pathways, including the Advanced Therapy Medicinal Products (ATMP) classification specifically designed for gene and cell therapies. The EMA's Committee for Advanced Therapies (CAT) provides specialized scientific expertise during the evaluation process, emphasizing the unique considerations required for genetic interventions.
In Asia, regulatory approaches vary significantly. Japan has implemented an expedited approval pathway through its Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approvals for promising therapies. China's National Medical Products Administration (NMPA) has recently strengthened its oversight of gene therapy research and commercialization, particularly focusing on genetic precision and off-target effects.
Global harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which aims to standardize safety and efficacy requirements across regions. However, significant regulatory divergences persist, creating challenges for multinational clinical trials and product approvals.
The precision of genetic modifications has become a central regulatory concern, with authorities increasingly requiring comprehensive data on off-target effects and long-term genetic stability. The FDA's 2020 guidance specifically addresses the need for improved analytical methods to detect unintended genomic alterations, while the EMA has emphasized the importance of long-term follow-up studies to monitor delayed adverse effects.
Ethical considerations are formally integrated into regulatory frameworks worldwide, with particular attention to germline modifications, informed consent processes, and genetic privacy. The World Health Organization's advisory committee on human genome editing has established global governance principles that influence national regulatory approaches.
As gene therapy technologies advance toward greater precision, regulatory frameworks continue to evolve, balancing the need for innovation with patient safety. The emergence of CRISPR-based therapies has prompted specific regulatory guidance focused on the unique precision challenges and potential off-target effects associated with these cutting-edge approaches.
The European Medicines Agency (EMA) has developed parallel but distinct regulatory pathways, including the Advanced Therapy Medicinal Products (ATMP) classification specifically designed for gene and cell therapies. The EMA's Committee for Advanced Therapies (CAT) provides specialized scientific expertise during the evaluation process, emphasizing the unique considerations required for genetic interventions.
In Asia, regulatory approaches vary significantly. Japan has implemented an expedited approval pathway through its Pharmaceuticals and Medical Devices Agency (PMDA), allowing conditional and time-limited approvals for promising therapies. China's National Medical Products Administration (NMPA) has recently strengthened its oversight of gene therapy research and commercialization, particularly focusing on genetic precision and off-target effects.
Global harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which aims to standardize safety and efficacy requirements across regions. However, significant regulatory divergences persist, creating challenges for multinational clinical trials and product approvals.
The precision of genetic modifications has become a central regulatory concern, with authorities increasingly requiring comprehensive data on off-target effects and long-term genetic stability. The FDA's 2020 guidance specifically addresses the need for improved analytical methods to detect unintended genomic alterations, while the EMA has emphasized the importance of long-term follow-up studies to monitor delayed adverse effects.
Ethical considerations are formally integrated into regulatory frameworks worldwide, with particular attention to germline modifications, informed consent processes, and genetic privacy. The World Health Organization's advisory committee on human genome editing has established global governance principles that influence national regulatory approaches.
As gene therapy technologies advance toward greater precision, regulatory frameworks continue to evolve, balancing the need for innovation with patient safety. The emergence of CRISPR-based therapies has prompted specific regulatory guidance focused on the unique precision challenges and potential off-target effects associated with these cutting-edge approaches.
Ethical Implications of Genetic Modification
The ethical landscape surrounding genetic modification technologies has evolved significantly with the advancement of gene therapy precision. As these technologies become increasingly capable of targeted genetic alterations, society faces profound ethical questions that extend beyond technical capabilities to fundamental aspects of human identity and social structure.
The principle of informed consent presents a significant ethical challenge in gene therapy applications. Current precision technologies often involve complex mechanisms that are difficult to explain comprehensively to patients or parents making decisions for minors. This raises questions about whether truly informed consent is possible when the long-term implications of genetic modifications remain partially understood, particularly with somatic versus germline modifications.
Justice and accessibility concerns emerge prominently in ethical discussions. The high cost of precision gene therapies creates potential disparities in treatment availability, potentially widening health inequalities between socioeconomic groups. This raises fundamental questions about whether advanced genetic treatments should be considered luxury interventions or essential healthcare that societies must make universally accessible.
The concept of human identity faces reconsideration in light of genetic modification capabilities. As precision increases in gene therapy technologies, the boundary between treatment and enhancement becomes increasingly blurred. This prompts essential questions about whether certain genetic modifications constitute legitimate medical interventions or represent attempts to "perfect" human biology beyond natural parameters.
Regulatory frameworks currently struggle to keep pace with rapid technological advancements. Different jurisdictions apply varying ethical standards to genetic modification research and applications, creating inconsistent oversight globally. This regulatory fragmentation potentially enables "ethics shopping," where researchers pursue controversial genetic modification work in regions with less stringent ethical requirements.
The intergenerational implications of genetic modifications, particularly germline alterations, present unique ethical challenges without historical precedent. Current precision technologies that affect heritable traits create modifications that will impact individuals who cannot consent to these changes, raising questions about intergenerational justice and responsibility.
Religious and cultural perspectives add further complexity to the ethical landscape. Many traditional belief systems hold specific views on the sanctity of human genetic makeup and the appropriate boundaries of scientific intervention. These perspectives must be acknowledged and respected in developing ethical frameworks for genetic modification technologies, even as scientific capabilities continue to advance.
The principle of informed consent presents a significant ethical challenge in gene therapy applications. Current precision technologies often involve complex mechanisms that are difficult to explain comprehensively to patients or parents making decisions for minors. This raises questions about whether truly informed consent is possible when the long-term implications of genetic modifications remain partially understood, particularly with somatic versus germline modifications.
Justice and accessibility concerns emerge prominently in ethical discussions. The high cost of precision gene therapies creates potential disparities in treatment availability, potentially widening health inequalities between socioeconomic groups. This raises fundamental questions about whether advanced genetic treatments should be considered luxury interventions or essential healthcare that societies must make universally accessible.
The concept of human identity faces reconsideration in light of genetic modification capabilities. As precision increases in gene therapy technologies, the boundary between treatment and enhancement becomes increasingly blurred. This prompts essential questions about whether certain genetic modifications constitute legitimate medical interventions or represent attempts to "perfect" human biology beyond natural parameters.
Regulatory frameworks currently struggle to keep pace with rapid technological advancements. Different jurisdictions apply varying ethical standards to genetic modification research and applications, creating inconsistent oversight globally. This regulatory fragmentation potentially enables "ethics shopping," where researchers pursue controversial genetic modification work in regions with less stringent ethical requirements.
The intergenerational implications of genetic modifications, particularly germline alterations, present unique ethical challenges without historical precedent. Current precision technologies that affect heritable traits create modifications that will impact individuals who cannot consent to these changes, raising questions about intergenerational justice and responsibility.
Religious and cultural perspectives add further complexity to the ethical landscape. Many traditional belief systems hold specific views on the sanctity of human genetic makeup and the appropriate boundaries of scientific intervention. These perspectives must be acknowledged and respected in developing ethical frameworks for genetic modification technologies, even as scientific capabilities continue to advance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!