How do Gene Therapy Strategies Vary Across Different Cancers?
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cancer Gene Therapy Evolution and Objectives
Gene therapy for cancer has evolved significantly since its conceptual inception in the 1970s. Initially, the field focused on simple gene replacement strategies, attempting to correct mutations in tumor suppressor genes like p53. By the 1990s, researchers began exploring viral vectors for gene delivery, marking the first clinical trials for cancer gene therapy. These early attempts faced significant challenges including immune responses to viral vectors, inefficient gene delivery, and limited understanding of cancer genomics.
The evolution accelerated in the early 2000s with the completion of the Human Genome Project, providing unprecedented insights into cancer genetics. This period saw the development of more sophisticated approaches, including suicide gene therapy, oncolytic virotherapy, and immunomodulatory gene therapy. The approval of Gendicine in China in 2003, the world's first commercialized gene therapy for head and neck squamous cell carcinoma, represented a significant milestone.
Recent advancements have been driven by breakthroughs in delivery technologies, particularly CRISPR-Cas9 gene editing, which has revolutionized the precision with which genetic modifications can be made. The development of CAR-T cell therapies, exemplified by Kymriah and Yescarta approvals in 2017, demonstrated the potential of ex vivo gene modification approaches for hematological malignancies.
The primary objective of cancer gene therapy is to develop targeted treatments that can overcome the limitations of conventional cancer therapies, including chemotherapy resistance, radiation toxicity, and surgical limitations. Specifically, gene therapy aims to selectively eliminate cancer cells while sparing normal tissues, address metastatic disease that is often untreatable with conventional approaches, and overcome therapy resistance mechanisms.
Current research objectives focus on expanding the applicability of gene therapy across diverse cancer types, particularly solid tumors which have proven more challenging than hematological malignancies. Researchers are working to enhance delivery efficiency to tumor sites, develop strategies to overcome the heterogeneous nature of solid tumors, and create combination approaches that target multiple cancer pathways simultaneously.
Long-term objectives include developing personalized gene therapy approaches based on individual tumor genomic profiles, creating "off-the-shelf" allogeneic cell therapies that eliminate the need for patient-specific manufacturing, and reducing the prohibitive costs associated with current gene therapy treatments to improve accessibility. The field is also exploring novel targets beyond traditional oncogenes, including tumor microenvironment factors, metabolic pathways, and epigenetic regulators.
The evolution accelerated in the early 2000s with the completion of the Human Genome Project, providing unprecedented insights into cancer genetics. This period saw the development of more sophisticated approaches, including suicide gene therapy, oncolytic virotherapy, and immunomodulatory gene therapy. The approval of Gendicine in China in 2003, the world's first commercialized gene therapy for head and neck squamous cell carcinoma, represented a significant milestone.
Recent advancements have been driven by breakthroughs in delivery technologies, particularly CRISPR-Cas9 gene editing, which has revolutionized the precision with which genetic modifications can be made. The development of CAR-T cell therapies, exemplified by Kymriah and Yescarta approvals in 2017, demonstrated the potential of ex vivo gene modification approaches for hematological malignancies.
The primary objective of cancer gene therapy is to develop targeted treatments that can overcome the limitations of conventional cancer therapies, including chemotherapy resistance, radiation toxicity, and surgical limitations. Specifically, gene therapy aims to selectively eliminate cancer cells while sparing normal tissues, address metastatic disease that is often untreatable with conventional approaches, and overcome therapy resistance mechanisms.
Current research objectives focus on expanding the applicability of gene therapy across diverse cancer types, particularly solid tumors which have proven more challenging than hematological malignancies. Researchers are working to enhance delivery efficiency to tumor sites, develop strategies to overcome the heterogeneous nature of solid tumors, and create combination approaches that target multiple cancer pathways simultaneously.
Long-term objectives include developing personalized gene therapy approaches based on individual tumor genomic profiles, creating "off-the-shelf" allogeneic cell therapies that eliminate the need for patient-specific manufacturing, and reducing the prohibitive costs associated with current gene therapy treatments to improve accessibility. The field is also exploring novel targets beyond traditional oncogenes, including tumor microenvironment factors, metabolic pathways, and epigenetic regulators.
Market Analysis of Cancer Gene Therapy Demand
The global market for cancer gene therapy has witnessed substantial growth in recent years, driven by increasing cancer prevalence and advancements in genetic engineering technologies. Current market valuations place the cancer gene therapy sector at approximately 2.5 billion USD in 2023, with projections indicating a compound annual growth rate of 20-25% over the next decade. This remarkable growth trajectory reflects the expanding clinical applications and increasing acceptance of gene therapy approaches among oncologists and patients alike.
Market demand analysis reveals significant variations across different cancer types. Hematological malignancies, particularly leukemias and lymphomas, currently dominate the gene therapy market share, accounting for nearly 40% of ongoing clinical trials and approved therapies. This concentration stems from the relative accessibility of blood cells for ex vivo manipulation and the established success of CAR-T cell therapies in these indications.
Solid tumors represent the largest potential market segment due to their higher incidence rates, with lung, breast, colorectal, and prostate cancers generating the strongest commercial interest. However, delivery challenges to solid tumor sites have limited market penetration compared to hematological applications. Industry forecasts suggest that breakthrough delivery technologies could unlock a market potential exceeding 15 billion USD by 2030 for solid tumor gene therapies alone.
Regional market analysis demonstrates notable disparities in demand patterns. North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The dominance of developed markets reflects their robust healthcare infrastructure, higher reimbursement capabilities, and greater access to specialized treatment centers. However, emerging markets in Asia, particularly China and South Korea, are experiencing the fastest growth rates as they rapidly expand their biotechnology capabilities and healthcare coverage.
Payer dynamics significantly influence market demand, with high therapy costs presenting substantial barriers to widespread adoption. The average cost of approved cancer gene therapies ranges from 300,000 to 2 million USD per treatment course, necessitating innovative payment models. Value-based agreements and outcomes-based reimbursement structures are gaining traction as mechanisms to balance market access with financial sustainability.
Patient demographics further shape market demand patterns. Pediatric cancers represent a smaller but critically important segment with distinct regulatory advantages, including orphan drug designations and accelerated approval pathways. Meanwhile, the aging global population is expanding the potential patient pool for adult-onset cancers, creating sustained long-term demand growth for gene therapy solutions tailored to geriatric patients with considerations for comorbidities and reduced organ function.
Market demand analysis reveals significant variations across different cancer types. Hematological malignancies, particularly leukemias and lymphomas, currently dominate the gene therapy market share, accounting for nearly 40% of ongoing clinical trials and approved therapies. This concentration stems from the relative accessibility of blood cells for ex vivo manipulation and the established success of CAR-T cell therapies in these indications.
Solid tumors represent the largest potential market segment due to their higher incidence rates, with lung, breast, colorectal, and prostate cancers generating the strongest commercial interest. However, delivery challenges to solid tumor sites have limited market penetration compared to hematological applications. Industry forecasts suggest that breakthrough delivery technologies could unlock a market potential exceeding 15 billion USD by 2030 for solid tumor gene therapies alone.
Regional market analysis demonstrates notable disparities in demand patterns. North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The dominance of developed markets reflects their robust healthcare infrastructure, higher reimbursement capabilities, and greater access to specialized treatment centers. However, emerging markets in Asia, particularly China and South Korea, are experiencing the fastest growth rates as they rapidly expand their biotechnology capabilities and healthcare coverage.
Payer dynamics significantly influence market demand, with high therapy costs presenting substantial barriers to widespread adoption. The average cost of approved cancer gene therapies ranges from 300,000 to 2 million USD per treatment course, necessitating innovative payment models. Value-based agreements and outcomes-based reimbursement structures are gaining traction as mechanisms to balance market access with financial sustainability.
Patient demographics further shape market demand patterns. Pediatric cancers represent a smaller but critically important segment with distinct regulatory advantages, including orphan drug designations and accelerated approval pathways. Meanwhile, the aging global population is expanding the potential patient pool for adult-onset cancers, creating sustained long-term demand growth for gene therapy solutions tailored to geriatric patients with considerations for comorbidities and reduced organ function.
Current Gene Therapy Approaches and Limitations
Gene therapy for cancer has evolved into a diverse field with multiple strategic approaches tailored to specific cancer types. Currently, viral vectors remain the predominant delivery system, with adenoviruses, retroviruses, and adeno-associated viruses (AAVs) being the most widely utilized. Each vector system offers distinct advantages: adenoviruses provide high transduction efficiency but face immunogenicity challenges; retroviruses enable stable gene integration but risk insertional mutagenesis; while AAVs offer prolonged expression with minimal immunogenicity but have limited payload capacity.
The primary gene therapy strategies currently employed include suicide gene therapy, oncolytic virotherapy, immunomodulatory approaches, and tumor suppressor gene restoration. Suicide gene therapy introduces genes encoding enzymes that convert non-toxic prodrugs into cytotoxic compounds within cancer cells, with the HSV-TK/ganciclovir system being particularly prominent in glioblastoma trials. Oncolytic virotherapy utilizes viruses engineered to selectively replicate in and destroy cancer cells, exemplified by Talimogene laherparepvec (T-VEC), FDA-approved for melanoma treatment.
Immunomodulatory approaches have gained significant traction, particularly CAR-T cell therapies which have demonstrated remarkable efficacy against hematological malignancies. Kymriah and Yescarta represent breakthrough approvals in this category. However, their effectiveness against solid tumors remains limited due to tumor microenvironment immunosuppression and antigen heterogeneity. Gene editing technologies like CRISPR-Cas9 are increasingly being incorporated to enhance CAR-T cell persistence and functionality.
Despite these advances, gene therapy faces substantial limitations across cancer types. Delivery efficiency remains a critical challenge, particularly for solid tumors where physical barriers impede vector penetration. Vector immunogenicity continues to restrict repeated dosing potential, while off-target effects pose safety concerns. The transient nature of gene expression in many approaches necessitates repeated treatments, and manufacturing complexities contribute to prohibitive costs, limiting widespread clinical implementation.
Cancer heterogeneity presents perhaps the most formidable obstacle, as genetic diversity within tumors enables resistance development. This is particularly evident in aggressive cancers like pancreatic and triple-negative breast cancer, where single-target approaches frequently fail. Additionally, the tumor microenvironment's immunosuppressive nature often neutralizes therapeutic effects, especially in immunologically "cold" tumors like prostate cancer.
Regulatory hurdles and standardization challenges further complicate development pathways, with varying requirements across global jurisdictions creating additional barriers to clinical translation. These limitations collectively highlight the need for combination approaches and cancer-specific optimization strategies to advance gene therapy's clinical potential.
The primary gene therapy strategies currently employed include suicide gene therapy, oncolytic virotherapy, immunomodulatory approaches, and tumor suppressor gene restoration. Suicide gene therapy introduces genes encoding enzymes that convert non-toxic prodrugs into cytotoxic compounds within cancer cells, with the HSV-TK/ganciclovir system being particularly prominent in glioblastoma trials. Oncolytic virotherapy utilizes viruses engineered to selectively replicate in and destroy cancer cells, exemplified by Talimogene laherparepvec (T-VEC), FDA-approved for melanoma treatment.
Immunomodulatory approaches have gained significant traction, particularly CAR-T cell therapies which have demonstrated remarkable efficacy against hematological malignancies. Kymriah and Yescarta represent breakthrough approvals in this category. However, their effectiveness against solid tumors remains limited due to tumor microenvironment immunosuppression and antigen heterogeneity. Gene editing technologies like CRISPR-Cas9 are increasingly being incorporated to enhance CAR-T cell persistence and functionality.
Despite these advances, gene therapy faces substantial limitations across cancer types. Delivery efficiency remains a critical challenge, particularly for solid tumors where physical barriers impede vector penetration. Vector immunogenicity continues to restrict repeated dosing potential, while off-target effects pose safety concerns. The transient nature of gene expression in many approaches necessitates repeated treatments, and manufacturing complexities contribute to prohibitive costs, limiting widespread clinical implementation.
Cancer heterogeneity presents perhaps the most formidable obstacle, as genetic diversity within tumors enables resistance development. This is particularly evident in aggressive cancers like pancreatic and triple-negative breast cancer, where single-target approaches frequently fail. Additionally, the tumor microenvironment's immunosuppressive nature often neutralizes therapeutic effects, especially in immunologically "cold" tumors like prostate cancer.
Regulatory hurdles and standardization challenges further complicate development pathways, with varying requirements across global jurisdictions creating additional barriers to clinical translation. These limitations collectively highlight the need for combination approaches and cancer-specific optimization strategies to advance gene therapy's clinical potential.
Established Gene Therapy Protocols by Cancer Type
01 Viral vector-based gene delivery systems
Viral vectors are engineered to deliver therapeutic genes to target cells for the treatment of genetic disorders. These systems utilize modified viruses such as adenoviruses, lentiviruses, and adeno-associated viruses (AAVs) that can efficiently transduce cells while minimizing immune responses. The viral vectors are designed to carry specific therapeutic genes that can replace defective genes, introduce new genes, or modify existing gene expression patterns in target tissues, offering potential treatments for inherited disorders, cancer, and other diseases.- Viral vector-based gene delivery systems: Viral vectors are commonly used in gene therapy to deliver therapeutic genes to target cells. These vectors include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can efficiently transduce cells and integrate genetic material into the host genome. The selection of appropriate viral vectors depends on factors such as target tissue specificity, payload capacity, and immune response considerations. These delivery systems are crucial for treating genetic disorders by introducing functional copies of defective genes.
- CRISPR-Cas gene editing technologies: CRISPR-Cas systems represent a revolutionary approach to gene therapy by enabling precise editing of the genome. This technology allows for the correction of genetic mutations, deletion of disease-causing sequences, or insertion of therapeutic genes. The CRISPR-Cas9 and newer variants like Cas12 and Cas13 provide versatile tools for treating genetic disorders at their source. Therapeutic strategies include ex vivo modification of patient cells followed by reinfusion or direct in vivo delivery of gene editing components to target tissues.
- Non-viral gene delivery methods: Non-viral gene delivery systems offer advantages in terms of safety, reduced immunogenicity, and ease of manufacturing compared to viral vectors. These methods include lipid nanoparticles, polymeric carriers, and physical techniques such as electroporation and sonoporation. Lipid nanoparticles have gained prominence for delivering mRNA therapeutics, while polymer-based systems provide versatility in targeting specific tissues. These approaches are particularly valuable for repeated administration therapies where immune responses to viral vectors might limit efficacy.
- Cell-based gene therapy approaches: Cell-based gene therapy involves genetic modification of cells ex vivo before transplantation into patients. This approach includes CAR-T cell therapy for cancer treatment, where T cells are engineered to express chimeric antigen receptors targeting tumor cells. Other applications include stem cell gene therapy for inherited disorders, where hematopoietic stem cells are modified to express functional copies of defective genes. These strategies combine cellular therapy with genetic modification to create living therapeutic agents that can provide long-term treatment effects.
- RNA-based therapeutic strategies: RNA-based gene therapies utilize various RNA molecules to modulate gene expression without altering the genome. These include antisense oligonucleotides that block translation of disease-causing proteins, small interfering RNAs (siRNAs) that promote degradation of specific mRNAs, and mRNA therapeutics that deliver instructions for producing therapeutic proteins. RNA therapeutics offer advantages in terms of transient expression, reduced risk of permanent genomic alterations, and the ability to target previously undruggable disease pathways.
02 CRISPR-Cas gene editing technologies
CRISPR-Cas gene editing represents a revolutionary approach to gene therapy by enabling precise modification of genetic sequences. This technology utilizes guide RNAs to direct Cas nucleases to specific DNA targets, allowing for gene knockout, insertion, or correction of mutations. Therapeutic strategies include ex vivo editing of patient cells followed by reinfusion, or direct in vivo delivery of CRISPR components to affected tissues. This approach offers potential treatments for monogenic disorders, cancer, and infectious diseases by addressing the genetic root causes rather than just managing symptoms.Expand Specific Solutions03 Non-viral gene delivery methods
Non-viral gene delivery systems provide alternatives to viral vectors with potential advantages in safety, manufacturing, and reduced immunogenicity. These methods include lipid nanoparticles, polymeric carriers, physical methods like electroporation, and hybrid delivery systems. Non-viral approaches can deliver various genetic payloads including DNA, mRNA, and oligonucleotides to target cells. While traditionally less efficient than viral vectors, advances in carrier design and formulation have significantly improved transfection efficiency, making these approaches increasingly viable for clinical applications in gene therapy.Expand Specific Solutions04 RNA-based therapeutic approaches
RNA-based gene therapies utilize various RNA molecules to modulate gene expression without altering the genome. These approaches include mRNA delivery for temporary protein expression, antisense oligonucleotides to modify RNA splicing or block translation, small interfering RNAs (siRNAs) for gene silencing, and RNA aptamers as therapeutic agents. RNA therapeutics offer advantages including transient expression, reduced risk of genomic integration, and the ability to target previously undruggable disease pathways. These strategies have shown promise for treating genetic disorders, cancer, and infectious diseases.Expand Specific Solutions05 Cell-based gene therapy strategies
Cell-based gene therapy involves genetic modification of cells ex vivo before reintroduction into patients. This approach includes CAR-T cell therapy for cancer, where T cells are engineered to express chimeric antigen receptors targeting tumor cells; hematopoietic stem cell gene therapy for blood disorders; and induced pluripotent stem cell-based approaches. These strategies allow for precise control of the genetic modification process, screening of modified cells before administration, and the potential to create persistent therapeutic cell populations that can provide long-term clinical benefits.Expand Specific Solutions
Leading Organizations in Cancer Gene Therapy
Gene therapy for cancer is evolving rapidly across a competitive landscape currently in the early growth phase. The global market is expanding significantly, projected to reach approximately $20 billion by 2030, with varying approaches tailored to specific cancer types. Technical maturity varies considerably across cancer indications, with solid tumors presenting greater delivery challenges than hematological malignancies. Leading players like Guardant Health are pioneering liquid biopsy technologies, while academic powerhouses (Yale University, Boston University) drive fundamental research. Pharmaceutical companies including Vertex Pharmaceuticals and GW Pharmaceuticals are advancing clinical applications, with Arrowhead Pharmaceuticals developing RNA-based delivery systems. Oxford Biomedica and Gennao Bio are focusing on viral and non-viral vector technologies respectively, demonstrating the diverse technical approaches being pursued across different cancer types.
The Regents of the University of California
Technical Solution: The University of California system has pioneered diverse gene therapy approaches across multiple cancer types. Their researchers have developed CRISPR-based therapies targeting oncogenic drivers in various solid tumors, achieving significant tumor regression in preclinical models. For glioblastoma, they've engineered neural stem cells as delivery vehicles for therapeutic genes, exploiting their natural tumor-homing abilities to overcome the blood-brain barrier. Their breast cancer program utilizes adeno-associated viral vectors delivering tumor suppressor genes with tumor-specific promoters, achieving selective expression in malignant cells. UC researchers have also developed innovative CAR-T cell therapies with logic-gated activation requiring recognition of multiple tumor antigens, reducing off-target effects. Their "hit-and-run" gene therapy approach uses transient gene expression systems that modify the tumor microenvironment without requiring permanent genetic integration. Additionally, they've pioneered in situ vaccination strategies using oncolytic viruses that deliver immunostimulatory genes directly to tumors, converting "cold" tumors to "hot" immunologically responsive ones.
Strengths: Broad research capabilities spanning multiple cancer types and gene therapy modalities; strong translational pipeline from basic discovery to clinical application; innovative approaches to overcome delivery barriers. Weaknesses: Decentralized research across multiple campuses may slow commercialization; complex academic licensing arrangements can complicate industry partnerships; limited internal manufacturing capabilities compared to dedicated biotech companies.
Oxford Biomedica (UK) Ltd.
Technical Solution: Oxford Biomedica has pioneered lentiviral vector-based gene therapy approaches for cancer treatment. Their LentiVector® platform delivers therapeutic genes with high efficiency and sustained expression. For cancer applications, they've developed tumor-targeted vectors that selectively deliver genes to cancer cells while sparing healthy tissue. Their approach includes engineering vectors with tumor-specific promoters that activate only in cancer cells and incorporating microRNA target sequences that de-target vector expression in non-cancer cells. Oxford Biomedica has demonstrated success with CAR-T cell therapies, where their vectors deliver genes encoding chimeric antigen receptors to T cells, enabling them to recognize and destroy cancer cells. Their manufacturing process ensures consistent vector quality with minimal batch-to-batch variation, critical for clinical applications. Recent innovations include their TRiP (Transgene Repression in vector Production) system that enhances vector yield and quality for complex therapeutic payloads.
Strengths: Industry-leading lentiviral vector technology with proven clinical applications; robust manufacturing capabilities ensuring consistent vector quality; versatile platform adaptable to different cancer types. Weaknesses: Lentiviral vectors have payload size limitations; potential for insertional mutagenesis requires careful safety monitoring; manufacturing complexity leads to higher production costs compared to some competing delivery systems.
Key Technological Innovations in Gene Delivery
Contemporaneous, heterogeneously-oriented, multi-targeted therapeutic modification and/or modulation of disease by administration of sulfur-containing, amino acid-specific small molecules
PatentInactiveUS20170007561A1
Innovation
- The use of sulfur-containing, amino acid-specific small molecules that can contemporaneously modify and/or modulate multiple target molecules, such as protein tyrosine kinases and DNA synthesis proteins, to address the complex interactions in cancer cells, thereby improving treatment outcomes.
Polynucleotide constructs and uses thereof
PatentInactiveEP1115877B1
Innovation
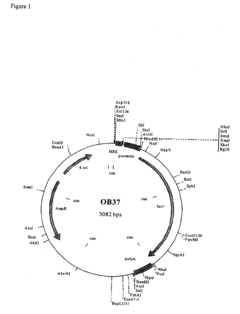
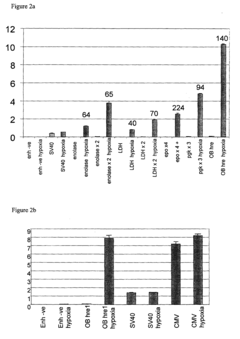
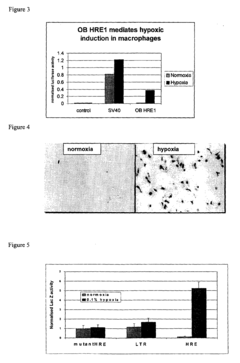
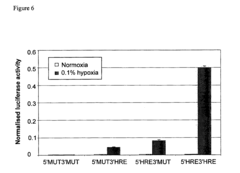
- A polynucleotide construct comprising three repeats of the mouse phosphoglycerate kinase (PGK) hypoxia response element (HRE) operably linked to a strong viral promoter, such as SV40 or MLV, drives high transcription levels under hypoxia with low basal levels under normoxic conditions, and is used in lentiviral vectors responsive to hypoxia and hypoxia-mimicking agents.
Regulatory Framework for Gene Therapy Approval
The regulatory landscape for gene therapy in oncology presents a complex framework that varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) has established a specialized pathway for gene therapy products through the Center for Biologics Evaluation and Research (CBER), requiring rigorous preclinical testing, phased clinical trials, and long-term follow-up studies that may extend 15 years post-treatment. This reflects the unique safety considerations associated with genetic modifications in cancer treatment.
The European Medicines Agency (EMA) employs a centralized approval procedure for gene therapies, classifying them as Advanced Therapy Medicinal Products (ATMPs). The EMA's Committee for Advanced Therapies (CAT) provides scientific assessments of these products, with particular emphasis on risk management plans for cancer-specific gene therapies. Unlike conventional pharmaceuticals, gene therapies for cancer often receive accelerated assessment due to their potential to address unmet medical needs in oncology.
Japan has implemented a conditional and time-limited approval system through the Pharmaceuticals and Medical Devices Agency (PMDA), allowing promising gene therapies for cancer to reach patients more quickly based on early-phase clinical data. This approach acknowledges the urgent need for innovative treatments in aggressive cancers while requiring post-market surveillance to confirm long-term safety and efficacy.
Regulatory frameworks increasingly recognize the heterogeneity of cancer types, with tailored requirements for different oncological applications. For instance, solid tumor gene therapies face different delivery challenges compared to hematological malignancies, necessitating specific safety and efficacy endpoints. Regulatory agencies have developed tumor-specific guidance documents that address the unique considerations for gene therapy in breast cancer versus glioblastoma or leukemia.
Harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) aim to standardize aspects of gene therapy regulation across regions, though significant differences remain. The ICH has published guidelines specifically addressing the quality, safety, and efficacy considerations for gene therapy products, including those targeting various cancer types.
Regulatory challenges specific to cancer gene therapies include demonstrating durable responses, managing insertional mutagenesis risks, and addressing potential off-target effects that may vary by cancer type. Agencies have developed specialized frameworks for monitoring these risks, with requirements for comprehensive genomic analyses and long-term patient registries that track outcomes across different cancer populations.
The European Medicines Agency (EMA) employs a centralized approval procedure for gene therapies, classifying them as Advanced Therapy Medicinal Products (ATMPs). The EMA's Committee for Advanced Therapies (CAT) provides scientific assessments of these products, with particular emphasis on risk management plans for cancer-specific gene therapies. Unlike conventional pharmaceuticals, gene therapies for cancer often receive accelerated assessment due to their potential to address unmet medical needs in oncology.
Japan has implemented a conditional and time-limited approval system through the Pharmaceuticals and Medical Devices Agency (PMDA), allowing promising gene therapies for cancer to reach patients more quickly based on early-phase clinical data. This approach acknowledges the urgent need for innovative treatments in aggressive cancers while requiring post-market surveillance to confirm long-term safety and efficacy.
Regulatory frameworks increasingly recognize the heterogeneity of cancer types, with tailored requirements for different oncological applications. For instance, solid tumor gene therapies face different delivery challenges compared to hematological malignancies, necessitating specific safety and efficacy endpoints. Regulatory agencies have developed tumor-specific guidance documents that address the unique considerations for gene therapy in breast cancer versus glioblastoma or leukemia.
Harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) aim to standardize aspects of gene therapy regulation across regions, though significant differences remain. The ICH has published guidelines specifically addressing the quality, safety, and efficacy considerations for gene therapy products, including those targeting various cancer types.
Regulatory challenges specific to cancer gene therapies include demonstrating durable responses, managing insertional mutagenesis risks, and addressing potential off-target effects that may vary by cancer type. Agencies have developed specialized frameworks for monitoring these risks, with requirements for comprehensive genomic analyses and long-term patient registries that track outcomes across different cancer populations.
Personalized Medicine Integration with Gene Therapy
The integration of personalized medicine with gene therapy represents a transformative approach in cancer treatment, enabling tailored interventions based on individual genetic profiles. This convergence allows oncologists to move beyond the traditional "one-size-fits-all" treatment paradigm toward precision-based therapeutic strategies that maximize efficacy while minimizing adverse effects.
Gene therapy's personalization begins with comprehensive genomic profiling of both the patient and their specific cancer type. Advanced sequencing technologies now permit rapid identification of driver mutations, gene expression patterns, and tumor microenvironment characteristics that influence treatment response. This genetic information serves as the foundation for designing customized gene therapy vectors and selecting appropriate therapeutic genes.
Machine learning algorithms have significantly enhanced the personalization process by analyzing vast datasets to predict treatment outcomes based on genetic biomarkers. These computational tools can identify subtle patterns in genomic data that human analysis might miss, allowing for more precise matching of gene therapy approaches to individual cancer profiles. The integration of artificial intelligence continues to refine these predictive models, improving treatment selection accuracy.
Pharmacogenomics plays a crucial role in personalizing gene therapy by identifying genetic variants that affect drug metabolism and response. This knowledge helps determine optimal dosing strategies and anticipate potential adverse reactions, further tailoring the therapeutic approach to the individual patient. The combination of gene therapy with pharmacogenomic insights creates a powerful framework for truly personalized cancer treatment.
Patient-derived organoids and xenograft models have emerged as valuable tools for testing personalized gene therapy approaches before clinical application. These models, created using a patient's own cancer cells, allow researchers to evaluate multiple gene therapy strategies in a laboratory setting that closely mimics the actual tumor environment, providing critical insights into potential efficacy.
The clinical implementation of personalized gene therapy requires sophisticated delivery systems capable of targeting specific genetic alterations. Advances in vector technology, including engineered viral vectors and nanoparticle-based delivery systems, now enable precise delivery of therapeutic genetic material to cancer cells while sparing healthy tissues, further enhancing the personalization aspect of treatment.
Regulatory frameworks are evolving to accommodate the personalized nature of gene therapy, with regulatory agencies developing new approval pathways for individualized treatments. These frameworks balance the need for rigorous safety assessment with the recognition that personalized therapies may require different evaluation metrics than traditional pharmaceutical products.
Gene therapy's personalization begins with comprehensive genomic profiling of both the patient and their specific cancer type. Advanced sequencing technologies now permit rapid identification of driver mutations, gene expression patterns, and tumor microenvironment characteristics that influence treatment response. This genetic information serves as the foundation for designing customized gene therapy vectors and selecting appropriate therapeutic genes.
Machine learning algorithms have significantly enhanced the personalization process by analyzing vast datasets to predict treatment outcomes based on genetic biomarkers. These computational tools can identify subtle patterns in genomic data that human analysis might miss, allowing for more precise matching of gene therapy approaches to individual cancer profiles. The integration of artificial intelligence continues to refine these predictive models, improving treatment selection accuracy.
Pharmacogenomics plays a crucial role in personalizing gene therapy by identifying genetic variants that affect drug metabolism and response. This knowledge helps determine optimal dosing strategies and anticipate potential adverse reactions, further tailoring the therapeutic approach to the individual patient. The combination of gene therapy with pharmacogenomic insights creates a powerful framework for truly personalized cancer treatment.
Patient-derived organoids and xenograft models have emerged as valuable tools for testing personalized gene therapy approaches before clinical application. These models, created using a patient's own cancer cells, allow researchers to evaluate multiple gene therapy strategies in a laboratory setting that closely mimics the actual tumor environment, providing critical insights into potential efficacy.
The clinical implementation of personalized gene therapy requires sophisticated delivery systems capable of targeting specific genetic alterations. Advances in vector technology, including engineered viral vectors and nanoparticle-based delivery systems, now enable precise delivery of therapeutic genetic material to cancer cells while sparing healthy tissues, further enhancing the personalization aspect of treatment.
Regulatory frameworks are evolving to accommodate the personalized nature of gene therapy, with regulatory agencies developing new approval pathways for individualized treatments. These frameworks balance the need for rigorous safety assessment with the recognition that personalized therapies may require different evaluation metrics than traditional pharmaceutical products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!