Exploring Viral Vector Design for Targeted Gene Therapy Delivery
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Viral Vector Evolution and Gene Therapy Objectives
Viral vectors have evolved significantly since their initial development in the 1970s, transforming from basic gene transfer tools to sophisticated delivery systems for therapeutic genetic material. The evolution began with simple retroviral vectors, which were limited by their inability to transduce non-dividing cells and potential for insertional mutagenesis. This led to the development of adenoviral vectors in the 1980s, offering improved transduction efficiency but facing challenges with immunogenicity.
The 1990s witnessed a breakthrough with the emergence of adeno-associated viral (AAV) vectors, which demonstrated superior safety profiles and long-term gene expression capabilities. Lentiviral vectors, derived from HIV-1, followed in the late 1990s, addressing many limitations of earlier retroviral systems by efficiently transducing non-dividing cells while maintaining genomic integration capabilities.
Recent years have seen remarkable advancements in vector engineering techniques, including capsid modification, promoter optimization, and integration of tissue-specific regulatory elements. These innovations have dramatically enhanced targeting precision, reduced immunogenicity, and improved manufacturing scalability—critical factors for clinical application.
The primary objective of viral vector design for gene therapy is to develop delivery systems that can efficiently and selectively transport therapeutic genetic material to target tissues while minimizing off-target effects and immune responses. This involves optimizing vector tropism through rational design of viral capsids or envelopes to interact specifically with receptors on target cells.
Another crucial objective is enhancing the safety profile of viral vectors by minimizing the risk of insertional mutagenesis, reducing immunogenicity, and ensuring predictable gene expression patterns. This includes developing self-inactivating vectors and incorporating safety switches to control transgene expression.
Scalability and manufacturing consistency represent additional key objectives, as the transition from laboratory to clinical application requires robust, reproducible production processes that meet regulatory standards. This encompasses developing serum-free production methods, improving vector purification techniques, and establishing quality control measures.
The field is now moving toward personalized gene therapy approaches, with objectives focused on developing vectors that can be tailored to individual patient needs, considering factors such as pre-existing immunity, genetic background, and specific disease manifestations. This personalization aims to maximize therapeutic efficacy while minimizing adverse effects, representing the next frontier in viral vector evolution for gene therapy applications.
The 1990s witnessed a breakthrough with the emergence of adeno-associated viral (AAV) vectors, which demonstrated superior safety profiles and long-term gene expression capabilities. Lentiviral vectors, derived from HIV-1, followed in the late 1990s, addressing many limitations of earlier retroviral systems by efficiently transducing non-dividing cells while maintaining genomic integration capabilities.
Recent years have seen remarkable advancements in vector engineering techniques, including capsid modification, promoter optimization, and integration of tissue-specific regulatory elements. These innovations have dramatically enhanced targeting precision, reduced immunogenicity, and improved manufacturing scalability—critical factors for clinical application.
The primary objective of viral vector design for gene therapy is to develop delivery systems that can efficiently and selectively transport therapeutic genetic material to target tissues while minimizing off-target effects and immune responses. This involves optimizing vector tropism through rational design of viral capsids or envelopes to interact specifically with receptors on target cells.
Another crucial objective is enhancing the safety profile of viral vectors by minimizing the risk of insertional mutagenesis, reducing immunogenicity, and ensuring predictable gene expression patterns. This includes developing self-inactivating vectors and incorporating safety switches to control transgene expression.
Scalability and manufacturing consistency represent additional key objectives, as the transition from laboratory to clinical application requires robust, reproducible production processes that meet regulatory standards. This encompasses developing serum-free production methods, improving vector purification techniques, and establishing quality control measures.
The field is now moving toward personalized gene therapy approaches, with objectives focused on developing vectors that can be tailored to individual patient needs, considering factors such as pre-existing immunity, genetic background, and specific disease manifestations. This personalization aims to maximize therapeutic efficacy while minimizing adverse effects, representing the next frontier in viral vector evolution for gene therapy applications.
Market Analysis of Gene Therapy Delivery Systems
The gene therapy delivery systems market is experiencing unprecedented growth, with a global valuation reaching $7.6 billion in 2022 and projected to expand at a compound annual growth rate of 18.3% through 2030. This remarkable trajectory is fueled by increasing prevalence of genetic disorders, rising investment in advanced healthcare technologies, and growing acceptance of gene therapy as a viable treatment option for previously untreatable conditions.
Viral vectors dominate the current market landscape, accounting for approximately 70% of delivery systems used in clinical applications. Among these, adeno-associated virus (AAV) vectors hold the largest market share at 38%, followed by lentiviral vectors at 22%, adenoviral vectors at 15%, and other viral vectors comprising the remaining percentage. This distribution reflects the proven efficacy and safety profiles of AAV vectors in particular, which have demonstrated superior targeting capabilities and reduced immunogenicity.
North America currently leads the global market with a 45% share, driven by robust research infrastructure, favorable regulatory frameworks, and substantial investment from both public and private sectors. Europe follows at 30%, with Asia-Pacific emerging as the fastest-growing region at a CAGR of 22.1%, primarily due to increasing healthcare expenditure and expanding research activities in countries like China, Japan, and South Korea.
The competitive landscape features both established pharmaceutical giants and specialized biotech companies. Key market players include Novartis, Spark Therapeutics (Roche), Pfizer, Biogen, and emerging companies like Voyager Therapeutics and 4D Molecular Therapeutics. Strategic collaborations between vector manufacturing specialists and therapeutic developers have become increasingly common, creating an interconnected ecosystem that accelerates innovation.
Pricing remains a significant market challenge, with approved gene therapies commanding premium prices ranging from $375,000 to over $2 million per treatment. This has prompted the development of novel payment models, including outcomes-based agreements and installment plans, to address affordability concerns while maintaining commercial viability.
Looking forward, the market is witnessing a shift toward non-viral delivery systems, including lipid nanoparticles and polymer-based vectors, which are expected to capture an increasing market share due to their improved safety profiles and manufacturing scalability. Additionally, the emergence of targeted delivery technologies that enhance tissue specificity represents a high-growth segment, with potential to address current limitations in systemic administration and reduce off-target effects.
Viral vectors dominate the current market landscape, accounting for approximately 70% of delivery systems used in clinical applications. Among these, adeno-associated virus (AAV) vectors hold the largest market share at 38%, followed by lentiviral vectors at 22%, adenoviral vectors at 15%, and other viral vectors comprising the remaining percentage. This distribution reflects the proven efficacy and safety profiles of AAV vectors in particular, which have demonstrated superior targeting capabilities and reduced immunogenicity.
North America currently leads the global market with a 45% share, driven by robust research infrastructure, favorable regulatory frameworks, and substantial investment from both public and private sectors. Europe follows at 30%, with Asia-Pacific emerging as the fastest-growing region at a CAGR of 22.1%, primarily due to increasing healthcare expenditure and expanding research activities in countries like China, Japan, and South Korea.
The competitive landscape features both established pharmaceutical giants and specialized biotech companies. Key market players include Novartis, Spark Therapeutics (Roche), Pfizer, Biogen, and emerging companies like Voyager Therapeutics and 4D Molecular Therapeutics. Strategic collaborations between vector manufacturing specialists and therapeutic developers have become increasingly common, creating an interconnected ecosystem that accelerates innovation.
Pricing remains a significant market challenge, with approved gene therapies commanding premium prices ranging from $375,000 to over $2 million per treatment. This has prompted the development of novel payment models, including outcomes-based agreements and installment plans, to address affordability concerns while maintaining commercial viability.
Looking forward, the market is witnessing a shift toward non-viral delivery systems, including lipid nanoparticles and polymer-based vectors, which are expected to capture an increasing market share due to their improved safety profiles and manufacturing scalability. Additionally, the emergence of targeted delivery technologies that enhance tissue specificity represents a high-growth segment, with potential to address current limitations in systemic administration and reduce off-target effects.
Current Viral Vector Technologies and Barriers
Viral vectors remain the predominant delivery vehicles for gene therapy applications, with several established platforms demonstrating clinical efficacy. Adeno-associated viruses (AAVs) have emerged as frontrunners due to their favorable safety profile, minimal immunogenicity, and ability to provide long-term gene expression without genome integration. Currently, AAV serotypes 1-9 are widely utilized, each exhibiting distinct tissue tropism characteristics that enable targeted delivery to specific organs.
Lentiviral vectors, derived from HIV-1, offer the advantage of stable gene integration into dividing and non-dividing cells, making them particularly valuable for ex vivo cell therapies. Third-generation lentiviral systems have significantly improved safety profiles through the removal of virulence genes and self-inactivating designs.
Adenoviral vectors provide high transduction efficiency and accommodate larger genetic payloads (up to 36kb) compared to AAVs (limited to 4.7kb). However, their clinical application remains constrained by pronounced immunogenicity and transient expression profiles.
Despite these advances, significant barriers impede broader implementation of viral vector technologies. Limited packaging capacity represents a fundamental constraint, particularly for AAVs, restricting their utility for delivering large therapeutic genes or complex regulatory elements. This limitation has necessitated the development of dual-vector approaches and transgene miniaturization strategies.
Pre-existing immunity poses another substantial challenge, with neutralizing antibodies against common viral capsids present in large segments of the human population. This immune recognition can dramatically reduce transduction efficiency and prevent repeated dosing, significantly limiting therapeutic efficacy in seropositive patients.
Manufacturing scalability remains problematic across all vector platforms. Current production systems struggle to meet clinical demand, with inconsistent yields and product quality. The complex biomanufacturing processes require specialized facilities and expertise, contributing to prohibitive costs that can exceed $500,000 per treatment.
Off-target effects and unpredictable biodistribution profiles continue to raise safety concerns. Despite engineering efforts, achieving precise tissue specificity remains elusive, with vectors often accumulating in non-target tissues, particularly the liver, potentially causing toxicity and reducing therapeutic efficacy at intended sites.
Insertional mutagenesis risks associated with integrating vectors (primarily lentiviral and retroviral) necessitate careful monitoring for oncogenic potential, despite significant safety improvements in modern vector designs. This concern has limited their application primarily to ex vivo approaches or life-threatening conditions where benefit-risk calculations are more favorable.
Lentiviral vectors, derived from HIV-1, offer the advantage of stable gene integration into dividing and non-dividing cells, making them particularly valuable for ex vivo cell therapies. Third-generation lentiviral systems have significantly improved safety profiles through the removal of virulence genes and self-inactivating designs.
Adenoviral vectors provide high transduction efficiency and accommodate larger genetic payloads (up to 36kb) compared to AAVs (limited to 4.7kb). However, their clinical application remains constrained by pronounced immunogenicity and transient expression profiles.
Despite these advances, significant barriers impede broader implementation of viral vector technologies. Limited packaging capacity represents a fundamental constraint, particularly for AAVs, restricting their utility for delivering large therapeutic genes or complex regulatory elements. This limitation has necessitated the development of dual-vector approaches and transgene miniaturization strategies.
Pre-existing immunity poses another substantial challenge, with neutralizing antibodies against common viral capsids present in large segments of the human population. This immune recognition can dramatically reduce transduction efficiency and prevent repeated dosing, significantly limiting therapeutic efficacy in seropositive patients.
Manufacturing scalability remains problematic across all vector platforms. Current production systems struggle to meet clinical demand, with inconsistent yields and product quality. The complex biomanufacturing processes require specialized facilities and expertise, contributing to prohibitive costs that can exceed $500,000 per treatment.
Off-target effects and unpredictable biodistribution profiles continue to raise safety concerns. Despite engineering efforts, achieving precise tissue specificity remains elusive, with vectors often accumulating in non-target tissues, particularly the liver, potentially causing toxicity and reducing therapeutic efficacy at intended sites.
Insertional mutagenesis risks associated with integrating vectors (primarily lentiviral and retroviral) necessitate careful monitoring for oncogenic potential, despite significant safety improvements in modern vector designs. This concern has limited their application primarily to ex vivo approaches or life-threatening conditions where benefit-risk calculations are more favorable.
Contemporary Approaches to Viral Vector Design
01 Viral vector design for enhanced targeting efficiency
Modifications to viral vector structures can significantly enhance their targeting efficiency. These modifications include alterations to viral capsid proteins, incorporation of tissue-specific promoters, and engineering of viral envelope proteins. Such design improvements allow for more precise delivery of genetic material to target cells while reducing off-target effects, ultimately increasing the therapeutic index of viral vector-based treatments.- Viral vector design for enhanced targeting efficiency: Modifications to viral vector structures can significantly enhance their targeting efficiency. These modifications include alterations to viral capsid proteins, incorporation of tissue-specific promoters, and engineering of viral envelope proteins. Such design improvements allow for more precise delivery of genetic material to target cells while reducing off-target effects. Advanced vector engineering techniques enable researchers to develop vectors with improved tropism for specific cell types or tissues.
- Cell-specific targeting strategies: Various strategies have been developed to enhance the cell-specific targeting efficiency of viral vectors. These include the use of cell-specific ligands, antibodies, or peptides that recognize receptors on target cells. By incorporating these targeting moieties into viral vectors, researchers can increase the specificity of gene delivery to particular cell types. This approach reduces off-target effects and improves the therapeutic index of viral vector-based treatments.
- Immune evasion techniques for improved vector delivery: Immune responses against viral vectors can significantly reduce their targeting efficiency. Various techniques have been developed to help viral vectors evade the host immune system, including the use of immunosuppressive agents, vector pseudotyping, and engineering vectors with reduced immunogenicity. These approaches help to prevent neutralization of viral vectors by antibodies and clearance by immune cells, thereby improving their ability to reach target tissues and cells.
- Tissue-specific delivery methods: Enhancing the tissue-specific delivery of viral vectors is crucial for improving their targeting efficiency. Methods include local administration, use of tissue-specific promoters, and exploitation of natural viral tropism. Additionally, physical methods such as ultrasound, electroporation, or hydrodynamic injection can be employed to enhance vector delivery to specific tissues. These approaches help to concentrate the vectors in the target tissue while minimizing exposure to non-target tissues.
- Novel viral vector platforms with enhanced targeting capabilities: Research has led to the development of novel viral vector platforms with inherently improved targeting capabilities. These include engineered adeno-associated viruses (AAVs), lentiviruses, and hybrid vectors that combine elements from different viral types. These novel platforms often feature modifications that enhance their ability to transduce specific cell types or tissues, resist neutralization by antibodies, and achieve sustained gene expression in target cells.
02 Cell-specific targeting strategies
Various strategies have been developed to enhance the cell-specific targeting efficiency of viral vectors. These include the use of cell-specific ligands, antibody-directed targeting, and receptor-mediated endocytosis mechanisms. By incorporating cell recognition elements into viral vectors, researchers can achieve more selective delivery to target tissues, reducing systemic toxicity and improving therapeutic outcomes in gene therapy applications.Expand Specific Solutions03 Immune evasion techniques for improved vector delivery
Immune responses against viral vectors can significantly reduce targeting efficiency. Techniques to evade or modulate the immune system include vector pseudotyping, use of immunosuppressive agents, and engineering vectors with reduced immunogenicity. These approaches help prevent neutralization of viral vectors by pre-existing antibodies and reduce inflammatory responses, allowing for more effective delivery to target tissues and enabling repeat administration when necessary.Expand Specific Solutions04 Tissue penetration and biodistribution optimization
Enhancing the ability of viral vectors to penetrate tissues and achieve optimal biodistribution is crucial for targeting efficiency. Methods include modification of vector size, surface charge, and incorporation of elements that facilitate crossing biological barriers such as the blood-brain barrier. Advanced delivery techniques and formulations can also improve vector stability and circulation time, leading to more efficient targeting of difficult-to-reach tissues.Expand Specific Solutions05 Computational and high-throughput screening approaches
Advanced computational methods and high-throughput screening techniques are increasingly used to optimize viral vector targeting efficiency. These approaches include machine learning algorithms to predict vector tropism, directed evolution strategies to select for vectors with enhanced targeting properties, and systematic screening of vector libraries. Such methods accelerate the development of viral vectors with improved targeting capabilities by identifying optimal vector designs and targeting elements.Expand Specific Solutions
Leading Companies in Viral Vector Engineering
Viral vector design for targeted gene therapy delivery is currently in a growth phase, with the market expected to reach significant expansion due to increasing applications in treating genetic disorders. The technology is advancing from early-stage development toward clinical implementation, with market size projected to exceed $10 billion by 2025. Technical maturity varies across vector types, with companies demonstrating different specialization levels. Established pharmaceutical leaders like Novartis AG and GlaxoSmithKline are investing heavily in platform technologies, while specialized players such as Oxford Biomedica and Evox Therapeutics are driving innovation in vector design and manufacturing. Academic institutions including Johns Hopkins University and Boston University collaborate extensively with industry partners, creating a competitive ecosystem balancing commercial development with fundamental research advances in delivery efficiency and targeting specificity.
Novartis AG
Technical Solution: Novartis has developed an advanced viral vector platform focusing on adeno-associated virus (AAV) and lentiviral vectors for targeted gene therapy delivery. Their approach incorporates tissue-specific promoters and engineered capsid proteins to enhance targeting specificity. Novartis utilizes directed evolution techniques to create novel AAV variants with improved tissue tropism, particularly for neurological and ocular applications. Their platform includes proprietary manufacturing processes that yield high-titer, high-purity viral vectors with reduced immunogenicity. Notably, their AAV9 variants demonstrate enhanced blood-brain barrier penetration for CNS disorders. Novartis has implemented computational modeling to predict vector behavior in vivo, allowing for rational design modifications that optimize transduction efficiency while minimizing off-target effects. Their vectors incorporate detargeting strategies using microRNA binding sites to prevent expression in non-target tissues.
Strengths: Extensive clinical experience with approved gene therapies (Zolgensma), robust manufacturing capabilities at commercial scale, and strong intellectual property portfolio. Weaknesses: Higher production costs compared to academic vectors, potential pre-existing immunity issues with some AAV serotypes, and limited payload capacity requiring optimization for larger transgenes.
Regeneron Pharmaceuticals, Inc.
Technical Solution: Regeneron has pioneered a viral vector design platform centered on engineered adenovirus vectors with enhanced targeting capabilities. Their technology incorporates synthetic biology approaches to modify viral capsids and envelope proteins, creating vectors with improved tissue specificity. Regeneron's vectors feature proprietary genetic elements that enable regulated transgene expression through inducible promoter systems, allowing for temporal control of therapeutic gene delivery. Their platform includes novel pseudotyping strategies that combine the structural components of one virus with the envelope proteins of another to optimize cellular entry mechanisms. Regeneron has developed vectors with reduced immunogenicity through the deletion of viral genes and incorporation of immune-evading elements. Their manufacturing process utilizes suspension-adapted cell lines and serum-free media to produce clinical-grade vectors with consistent quality attributes and high functional titers.
Strengths: Proprietary VelociGene technology for rapid vector engineering, integration with antibody expertise for targeted delivery, and established large-scale production capabilities. Weaknesses: More limited clinical data compared to some competitors in the gene therapy space, and potential challenges with vector immunogenicity requiring patient pre-screening.
Key Innovations in Targeting and Transduction Efficiency
Composition for viral preservation
PatentInactiveEP1453537A1
Innovation
- Incorporating a lipid into the viral composition to enhance preservation, maintaining viral activity across a range of storage temperatures and conditions, including agitation and mechanical stress, for both viable and attenuated virus particles.
Vector production
PatentPendingAU2024203415A1
Innovation
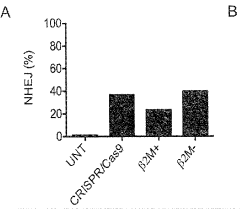
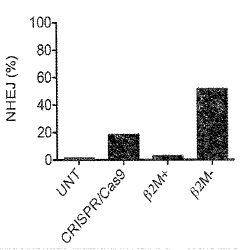
- Genetically engineered cells with decreased surface expression of MHC-I molecules are used to produce enveloped viral particles, minimizing immune recognition and maintaining viral particle infectivity and production yields.
Safety and Immunogenicity Considerations
Safety and immunogenicity considerations represent critical challenges in viral vector design for targeted gene therapy delivery. The immune response to viral vectors can significantly impact both the safety profile and therapeutic efficacy of gene therapy treatments. Pre-existing immunity against common viral vectors, such as adeno-associated viruses (AAVs) and adenoviruses, remains a substantial barrier, with studies indicating that 30-60% of the human population possesses neutralizing antibodies against various AAV serotypes.
Capsid modifications have emerged as a promising approach to mitigate immunogenicity. Recent advancements include the development of synthetic capsids with reduced recognition by neutralizing antibodies while maintaining transduction efficiency. For instance, the AAV-Anc80 ancestral vector demonstrates reduced immunogenicity compared to naturally occurring serotypes, with preclinical studies showing up to 70% reduction in neutralizing antibody production.
Vector-related toxicity manifests through multiple mechanisms, including direct cellular damage, insertional mutagenesis, and off-target effects. Lentiviral vectors, while efficient for gene delivery, carry risks of insertional oncogenesis, as evidenced in early clinical trials for X-linked severe combined immunodeficiency. Third-generation self-inactivating lentiviral vectors have significantly improved safety profiles, reducing the risk of insertional mutagenesis by approximately 80% compared to first-generation vectors.
Dose-dependent toxicity represents another significant concern, particularly with AAV vectors. High vector doses can trigger severe immune responses, including thrombotic microangiopathy and hepatotoxicity. Clinical data indicates that doses exceeding 2×10^14 vector genomes per kilogram significantly increase the risk of severe adverse events, necessitating careful dose optimization strategies.
Transient immunosuppression protocols have shown promise in managing immune responses to viral vectors. Combination regimens utilizing corticosteroids, tacrolimus, and mycophenolate mofetil have demonstrated efficacy in preventing vector neutralization and enhancing transgene expression. Studies indicate that properly timed immunosuppression can increase transgene expression by 2-5 fold compared to non-immunosuppressed controls.
Manufacturing considerations also impact safety profiles, with contaminants from production processes potentially triggering immune responses. Advanced purification techniques, including affinity chromatography and tangential flow filtration, have reduced host cell protein contamination to below 50 parts per million, significantly decreasing immunogenicity risks.
Regulatory frameworks continue to evolve, with the FDA and EMA implementing risk-based approaches to evaluate vector safety. Current guidelines mandate comprehensive immunological profiling during preclinical development, including assessment of cellular and humoral immune responses against both the vector and transgene product.
Capsid modifications have emerged as a promising approach to mitigate immunogenicity. Recent advancements include the development of synthetic capsids with reduced recognition by neutralizing antibodies while maintaining transduction efficiency. For instance, the AAV-Anc80 ancestral vector demonstrates reduced immunogenicity compared to naturally occurring serotypes, with preclinical studies showing up to 70% reduction in neutralizing antibody production.
Vector-related toxicity manifests through multiple mechanisms, including direct cellular damage, insertional mutagenesis, and off-target effects. Lentiviral vectors, while efficient for gene delivery, carry risks of insertional oncogenesis, as evidenced in early clinical trials for X-linked severe combined immunodeficiency. Third-generation self-inactivating lentiviral vectors have significantly improved safety profiles, reducing the risk of insertional mutagenesis by approximately 80% compared to first-generation vectors.
Dose-dependent toxicity represents another significant concern, particularly with AAV vectors. High vector doses can trigger severe immune responses, including thrombotic microangiopathy and hepatotoxicity. Clinical data indicates that doses exceeding 2×10^14 vector genomes per kilogram significantly increase the risk of severe adverse events, necessitating careful dose optimization strategies.
Transient immunosuppression protocols have shown promise in managing immune responses to viral vectors. Combination regimens utilizing corticosteroids, tacrolimus, and mycophenolate mofetil have demonstrated efficacy in preventing vector neutralization and enhancing transgene expression. Studies indicate that properly timed immunosuppression can increase transgene expression by 2-5 fold compared to non-immunosuppressed controls.
Manufacturing considerations also impact safety profiles, with contaminants from production processes potentially triggering immune responses. Advanced purification techniques, including affinity chromatography and tangential flow filtration, have reduced host cell protein contamination to below 50 parts per million, significantly decreasing immunogenicity risks.
Regulatory frameworks continue to evolve, with the FDA and EMA implementing risk-based approaches to evaluate vector safety. Current guidelines mandate comprehensive immunological profiling during preclinical development, including assessment of cellular and humoral immune responses against both the vector and transgene product.
Manufacturing Scalability and Regulatory Pathways
The scalability of viral vector manufacturing represents a critical bottleneck in the widespread implementation of gene therapy treatments. Current production methods typically rely on transient transfection or baculovirus expression systems, which face significant challenges when scaled beyond small-batch production. These limitations manifest in reduced vector quality, inconsistent titers, and substantially increased costs at commercial scales. Recent innovations in suspension cell culture systems and the development of stable producer cell lines show promise for addressing these constraints, potentially enabling 10-100 fold increases in production capacity.
Regulatory frameworks for viral vector-based gene therapies continue to evolve as the field matures. The FDA's expedited approval pathways, including Breakthrough Therapy and Regenerative Medicine Advanced Therapy designations, have facilitated faster market entry for several gene therapy products. However, manufacturers must navigate complex requirements spanning chemistry, manufacturing, and controls (CMC) documentation, comprehensive characterization of vector purity, potency assessment protocols, and rigorous stability testing.
International regulatory harmonization remains incomplete, with significant differences between FDA, EMA, and PMDA approaches creating challenges for global development programs. The FDA typically requires more extensive characterization of residual host cell proteins and DNA, while the EMA places greater emphasis on viral clearance validation. These divergent requirements often necessitate parallel manufacturing strategies tailored to specific markets.
Quality control represents another manufacturing challenge, with current analytical methods struggling to provide adequate sensitivity for detecting empty capsids, aggregates, and other impurities at the levels required by regulatory agencies. Advanced analytical techniques such as digital PCR, next-generation sequencing, and multi-angle light scattering are increasingly being incorporated into quality control workflows to address these limitations.
Cost considerations remain paramount, with current manufacturing expenses ranging from $50,000 to $200,000 per dose for AAV-based therapies. Economic viability demands significant improvements in production efficiency, with industry leaders targeting at least a 50-fold reduction in cost-per-dose over the next decade. Contract development and manufacturing organizations (CDMOs) have emerged as key players in this ecosystem, offering specialized expertise and infrastructure that may accelerate both manufacturing optimization and regulatory approval timelines.
Regulatory frameworks for viral vector-based gene therapies continue to evolve as the field matures. The FDA's expedited approval pathways, including Breakthrough Therapy and Regenerative Medicine Advanced Therapy designations, have facilitated faster market entry for several gene therapy products. However, manufacturers must navigate complex requirements spanning chemistry, manufacturing, and controls (CMC) documentation, comprehensive characterization of vector purity, potency assessment protocols, and rigorous stability testing.
International regulatory harmonization remains incomplete, with significant differences between FDA, EMA, and PMDA approaches creating challenges for global development programs. The FDA typically requires more extensive characterization of residual host cell proteins and DNA, while the EMA places greater emphasis on viral clearance validation. These divergent requirements often necessitate parallel manufacturing strategies tailored to specific markets.
Quality control represents another manufacturing challenge, with current analytical methods struggling to provide adequate sensitivity for detecting empty capsids, aggregates, and other impurities at the levels required by regulatory agencies. Advanced analytical techniques such as digital PCR, next-generation sequencing, and multi-angle light scattering are increasingly being incorporated into quality control workflows to address these limitations.
Cost considerations remain paramount, with current manufacturing expenses ranging from $50,000 to $200,000 per dose for AAV-based therapies. Economic viability demands significant improvements in production efficiency, with industry leaders targeting at least a 50-fold reduction in cost-per-dose over the next decade. Contract development and manufacturing organizations (CDMOs) have emerged as key players in this ecosystem, offering specialized expertise and infrastructure that may accelerate both manufacturing optimization and regulatory approval timelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!