Perspectives on Gene Therapy for Diabetes Management
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy Evolution and Diabetes Treatment Goals
Gene therapy has evolved significantly since its conceptual inception in the 1970s, transitioning from theoretical possibility to clinical reality. The field has progressed through distinct phases, beginning with basic vector development and moving toward sophisticated gene editing technologies. Early approaches focused on viral vectors for gene delivery, while contemporary methods leverage advanced tools like CRISPR-Cas9, zinc finger nucleases, and TALENs to achieve precise genetic modifications. This evolution has created unprecedented opportunities for addressing genetic components of complex diseases, including diabetes.
Diabetes management has traditionally relied on insulin therapy, oral medications, and lifestyle modifications. However, these approaches treat symptoms rather than addressing underlying genetic and molecular mechanisms. Gene therapy aims to fundamentally transform diabetes treatment by targeting the root causes of both Type 1 and Type 2 diabetes. For Type 1 diabetes, the primary goal is restoring insulin production through genetic modification of pancreatic cells or other suitable cell types that can be reprogrammed to function as insulin-producing beta cells. For Type 2 diabetes, gene therapy targets include improving insulin sensitivity, enhancing glucose metabolism, and modulating inflammatory pathways that contribute to insulin resistance.
The convergence of gene therapy and diabetes research has established several key technical objectives. First, developing safe and efficient delivery systems capable of targeting specific tissues relevant to diabetes pathophysiology, particularly pancreatic islets and liver cells. Second, achieving durable therapeutic effects that provide long-term glycemic control without requiring repeated interventions. Third, creating responsive gene expression systems that can dynamically adjust insulin production according to fluctuating blood glucose levels, mimicking natural physiological regulation.
Recent technological breakthroughs have accelerated progress toward these goals. The development of tissue-specific promoters has improved targeting precision, while advancements in vector design have enhanced safety profiles and reduced immunogenicity. Innovations in genome editing have opened new avenues for correcting specific genetic mutations associated with monogenic forms of diabetes and for engineering glucose-responsive insulin production systems.
The field is now moving toward integrating gene therapy with complementary technologies such as stem cell therapy, tissue engineering, and artificial intelligence-driven predictive models. This multidisciplinary approach aims to create comprehensive treatment strategies that address the complex, multifactorial nature of diabetes. The ultimate vision is to develop curative interventions that eliminate the need for lifelong medication and monitoring, significantly improving quality of life for millions of diabetes patients worldwide while reducing the enormous healthcare burden associated with diabetes management and complications.
Diabetes management has traditionally relied on insulin therapy, oral medications, and lifestyle modifications. However, these approaches treat symptoms rather than addressing underlying genetic and molecular mechanisms. Gene therapy aims to fundamentally transform diabetes treatment by targeting the root causes of both Type 1 and Type 2 diabetes. For Type 1 diabetes, the primary goal is restoring insulin production through genetic modification of pancreatic cells or other suitable cell types that can be reprogrammed to function as insulin-producing beta cells. For Type 2 diabetes, gene therapy targets include improving insulin sensitivity, enhancing glucose metabolism, and modulating inflammatory pathways that contribute to insulin resistance.
The convergence of gene therapy and diabetes research has established several key technical objectives. First, developing safe and efficient delivery systems capable of targeting specific tissues relevant to diabetes pathophysiology, particularly pancreatic islets and liver cells. Second, achieving durable therapeutic effects that provide long-term glycemic control without requiring repeated interventions. Third, creating responsive gene expression systems that can dynamically adjust insulin production according to fluctuating blood glucose levels, mimicking natural physiological regulation.
Recent technological breakthroughs have accelerated progress toward these goals. The development of tissue-specific promoters has improved targeting precision, while advancements in vector design have enhanced safety profiles and reduced immunogenicity. Innovations in genome editing have opened new avenues for correcting specific genetic mutations associated with monogenic forms of diabetes and for engineering glucose-responsive insulin production systems.
The field is now moving toward integrating gene therapy with complementary technologies such as stem cell therapy, tissue engineering, and artificial intelligence-driven predictive models. This multidisciplinary approach aims to create comprehensive treatment strategies that address the complex, multifactorial nature of diabetes. The ultimate vision is to develop curative interventions that eliminate the need for lifelong medication and monitoring, significantly improving quality of life for millions of diabetes patients worldwide while reducing the enormous healthcare burden associated with diabetes management and complications.
Market Analysis for Diabetes Gene Therapy Solutions
The global market for diabetes gene therapy solutions is experiencing significant growth potential, driven by the increasing prevalence of diabetes worldwide. Currently, approximately 537 million adults are living with diabetes globally, with projections suggesting this number could rise to 783 million by 2045. This expanding patient population creates a substantial market opportunity for innovative treatment approaches such as gene therapy.
The diabetes gene therapy market can be segmented based on therapy type, including ex vivo and in vivo approaches, with each addressing different aspects of diabetes pathophysiology. The market is further divided by diabetes type, with Type 1 diabetes representing the initial focus for most gene therapy developers due to its clear autoimmune etiology and the potential for genetic intervention in beta cell preservation or regeneration.
From a regional perspective, North America currently dominates the diabetes gene therapy research landscape, accounting for the largest share of ongoing clinical trials and research funding. This is followed by Europe and Asia-Pacific regions, with China emerging as a rapidly growing contributor to the field. The concentration of biotechnology infrastructure and venture capital in these regions has created geographic disparities in market development.
Investment in diabetes gene therapy has shown remarkable growth, with venture capital funding for diabetes-focused gene therapy startups increasing by over 300% in the past five years. Major pharmaceutical companies are also entering this space through strategic partnerships and acquisitions, recognizing the long-term commercial potential of curative approaches to diabetes management.
Market adoption barriers remain significant, including high development and manufacturing costs, regulatory complexities, and reimbursement challenges. The average cost of developing a gene therapy through clinical trials exceeds $5 billion, creating substantial entry barriers for smaller companies. Additionally, healthcare systems worldwide are still adapting reimbursement models to accommodate potentially curative but expensive one-time treatments.
Consumer acceptance represents another critical market factor, with patient surveys indicating growing openness to gene therapy approaches, particularly among younger diabetes patients. However, concerns about long-term safety and genetic modification persist among certain demographic groups, necessitating targeted educational initiatives.
The competitive landscape features both established pharmaceutical companies and specialized biotechnology firms. Key market players include Vertex Pharmaceuticals, CRISPR Therapeutics, Sernova, and Novo Nordisk, each pursuing different technological approaches to address the genetic and cellular aspects of diabetes. This diverse competitive environment is driving rapid innovation while creating market fragmentation that may eventually lead to industry consolidation.
The diabetes gene therapy market can be segmented based on therapy type, including ex vivo and in vivo approaches, with each addressing different aspects of diabetes pathophysiology. The market is further divided by diabetes type, with Type 1 diabetes representing the initial focus for most gene therapy developers due to its clear autoimmune etiology and the potential for genetic intervention in beta cell preservation or regeneration.
From a regional perspective, North America currently dominates the diabetes gene therapy research landscape, accounting for the largest share of ongoing clinical trials and research funding. This is followed by Europe and Asia-Pacific regions, with China emerging as a rapidly growing contributor to the field. The concentration of biotechnology infrastructure and venture capital in these regions has created geographic disparities in market development.
Investment in diabetes gene therapy has shown remarkable growth, with venture capital funding for diabetes-focused gene therapy startups increasing by over 300% in the past five years. Major pharmaceutical companies are also entering this space through strategic partnerships and acquisitions, recognizing the long-term commercial potential of curative approaches to diabetes management.
Market adoption barriers remain significant, including high development and manufacturing costs, regulatory complexities, and reimbursement challenges. The average cost of developing a gene therapy through clinical trials exceeds $5 billion, creating substantial entry barriers for smaller companies. Additionally, healthcare systems worldwide are still adapting reimbursement models to accommodate potentially curative but expensive one-time treatments.
Consumer acceptance represents another critical market factor, with patient surveys indicating growing openness to gene therapy approaches, particularly among younger diabetes patients. However, concerns about long-term safety and genetic modification persist among certain demographic groups, necessitating targeted educational initiatives.
The competitive landscape features both established pharmaceutical companies and specialized biotechnology firms. Key market players include Vertex Pharmaceuticals, CRISPR Therapeutics, Sernova, and Novo Nordisk, each pursuing different technological approaches to address the genetic and cellular aspects of diabetes. This diverse competitive environment is driving rapid innovation while creating market fragmentation that may eventually lead to industry consolidation.
Current Landscape and Challenges in Diabetes Gene Therapy
Gene therapy for diabetes management has evolved significantly over the past decade, with research efforts intensifying globally. Currently, the landscape encompasses both Type 1 and Type 2 diabetes approaches, with Type 1 receiving greater attention due to its clear autoimmune etiology and more defined genetic components. Major research centers in North America, Europe, and increasingly in Asia are advancing various gene therapy strategies, though clinical translation remains limited.
The primary technical challenges in diabetes gene therapy center around delivery mechanisms, target specificity, and long-term expression stability. Viral vectors, particularly adeno-associated viruses (AAVs), have emerged as leading delivery vehicles, but issues with immunogenicity and limited payload capacity persist. Non-viral delivery systems using lipid nanoparticles show promise but struggle with efficiency in pancreatic targeting.
For Type 1 diabetes, current approaches focus on three main strategies: beta-cell regeneration through transcription factor delivery (Pdx1, Ngn3, MafA), immune modulation to prevent autoimmune destruction, and insulin gene transfer to non-beta cells. Each pathway faces distinct technical hurdles, with beta-cell regeneration showing encouraging preclinical results but limited durability in larger animal models.
Type 2 diabetes gene therapy approaches target insulin resistance pathways, incretin enhancement, and glucose metabolism regulation. The multifactorial nature of Type 2 diabetes presents additional complexity, requiring combination approaches that simultaneously address multiple pathological mechanisms.
Regulatory challenges compound technical difficulties, with stringent safety requirements for permanent genetic modifications. The FDA and EMA have established specialized pathways for gene therapy products, but diabetes-specific guidance remains limited, creating uncertainty in development pathways.
Manufacturing scalability represents another significant barrier, with current production methods for clinical-grade viral vectors being costly and difficult to scale. This challenge particularly affects diabetes therapies, which would potentially require treatment of millions of patients globally.
Recent technological advancements in gene editing, particularly CRISPR-Cas9 systems, have opened new possibilities for precise genetic modifications in diabetes therapy. However, off-target effects and delivery of these large molecular complexes to pancreatic tissue remain problematic. Several clinical trials using gene therapy approaches for diabetes are currently in Phase I/II, primarily focusing on safety profiles and preliminary efficacy markers.
The geographic distribution of diabetes gene therapy research shows concentration in established biotech hubs, with emerging contributions from China, South Korea, and India. This global research ecosystem has accelerated knowledge sharing but also created competitive challenges in intellectual property protection and commercialization pathways.
The primary technical challenges in diabetes gene therapy center around delivery mechanisms, target specificity, and long-term expression stability. Viral vectors, particularly adeno-associated viruses (AAVs), have emerged as leading delivery vehicles, but issues with immunogenicity and limited payload capacity persist. Non-viral delivery systems using lipid nanoparticles show promise but struggle with efficiency in pancreatic targeting.
For Type 1 diabetes, current approaches focus on three main strategies: beta-cell regeneration through transcription factor delivery (Pdx1, Ngn3, MafA), immune modulation to prevent autoimmune destruction, and insulin gene transfer to non-beta cells. Each pathway faces distinct technical hurdles, with beta-cell regeneration showing encouraging preclinical results but limited durability in larger animal models.
Type 2 diabetes gene therapy approaches target insulin resistance pathways, incretin enhancement, and glucose metabolism regulation. The multifactorial nature of Type 2 diabetes presents additional complexity, requiring combination approaches that simultaneously address multiple pathological mechanisms.
Regulatory challenges compound technical difficulties, with stringent safety requirements for permanent genetic modifications. The FDA and EMA have established specialized pathways for gene therapy products, but diabetes-specific guidance remains limited, creating uncertainty in development pathways.
Manufacturing scalability represents another significant barrier, with current production methods for clinical-grade viral vectors being costly and difficult to scale. This challenge particularly affects diabetes therapies, which would potentially require treatment of millions of patients globally.
Recent technological advancements in gene editing, particularly CRISPR-Cas9 systems, have opened new possibilities for precise genetic modifications in diabetes therapy. However, off-target effects and delivery of these large molecular complexes to pancreatic tissue remain problematic. Several clinical trials using gene therapy approaches for diabetes are currently in Phase I/II, primarily focusing on safety profiles and preliminary efficacy markers.
The geographic distribution of diabetes gene therapy research shows concentration in established biotech hubs, with emerging contributions from China, South Korea, and India. This global research ecosystem has accelerated knowledge sharing but also created competitive challenges in intellectual property protection and commercialization pathways.
Current Gene Therapy Approaches for Diabetes
01 Viral vector delivery systems for gene therapy
Viral vectors are commonly used as delivery systems for gene therapy due to their natural ability to infect cells and deliver genetic material. These vectors include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can be engineered to carry therapeutic genes while minimizing pathogenicity. The selection of appropriate viral vectors is crucial for targeting specific tissues and achieving efficient gene expression with minimal immune response.- Viral vector delivery systems for gene therapy: Viral vectors are commonly used as delivery systems for gene therapy due to their ability to efficiently transfer genetic material into target cells. These vectors include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can be engineered to carry therapeutic genes. The selection of appropriate viral vectors is crucial for successful gene therapy, as different vectors have varying tissue tropisms, packaging capacities, and immunogenicity profiles.
- Non-viral gene delivery methods: Non-viral gene delivery methods offer alternatives to viral vectors for gene therapy applications. These approaches include lipid nanoparticles, polymeric carriers, electroporation, and physical methods such as microinjection. Non-viral delivery systems often have advantages in terms of safety, reduced immunogenicity, and larger packaging capacity, though they may have lower transfection efficiency compared to viral vectors. Recent advances in non-viral delivery technologies have improved their efficacy for clinical applications.
- CRISPR-Cas gene editing technology in therapeutic applications: CRISPR-Cas gene editing technology has revolutionized gene therapy by enabling precise modification of genetic sequences. This technology allows for targeted correction of disease-causing mutations, gene knockout, or insertion of therapeutic genes. CRISPR-based therapies are being developed for various genetic disorders, including blood disorders, blindness, and metabolic diseases. The system's versatility and efficiency make it a powerful tool for developing next-generation gene therapies.
- Ex vivo and in vivo gene therapy approaches: Gene therapy can be administered through ex vivo or in vivo approaches. In ex vivo gene therapy, cells are removed from the patient, genetically modified in the laboratory, and then reintroduced into the patient. This approach is commonly used for hematopoietic stem cell therapies. In contrast, in vivo gene therapy involves direct administration of genetic material into the patient's body, targeting specific tissues or organs. Each approach has distinct advantages and challenges related to delivery efficiency, safety, and clinical applications.
- Immune response management in gene therapy: Managing immune responses is a critical aspect of gene therapy development. Immune reactions against viral vectors or transgene products can reduce therapeutic efficacy and cause adverse effects. Strategies to mitigate immune responses include immunosuppressive regimens, vector engineering to reduce immunogenicity, and the use of tissue-specific promoters. Understanding and controlling immune responses is essential for improving the safety and long-term efficacy of gene therapy treatments.
02 Non-viral gene delivery methods
Non-viral gene delivery methods offer alternatives to viral vectors with potential advantages in safety, manufacturing, and reduced immunogenicity. These approaches include lipid nanoparticles, polymeric carriers, physical methods like electroporation, and chemical methods for DNA/RNA delivery. Recent advances in non-viral delivery systems have improved transfection efficiency and targeting specificity, making them increasingly viable options for clinical gene therapy applications.Expand Specific Solutions03 CRISPR-based gene editing technologies
CRISPR-Cas systems represent a revolutionary approach to gene therapy by enabling precise modification of genetic sequences. These technologies allow for gene knockout, insertion, or correction of mutations associated with genetic disorders. Ongoing research focuses on improving the specificity of CRISPR systems, reducing off-target effects, and developing efficient delivery methods to target cells in vivo, expanding the potential applications in treating genetic diseases.Expand Specific Solutions04 Gene therapy for inherited disorders
Gene therapy approaches for inherited genetic disorders focus on replacing defective genes, supplementing gene function, or correcting mutations. These therapies target conditions such as hemophilia, cystic fibrosis, muscular dystrophy, and various metabolic disorders. Clinical trials have demonstrated promising results in treating previously incurable genetic conditions, with some therapies receiving regulatory approval. Long-term expression of therapeutic genes remains a challenge being addressed through improved vector design and integration strategies.Expand Specific Solutions05 Immunotherapy and cancer gene therapy
Gene therapy approaches for cancer treatment include genetic modification of immune cells to enhance tumor recognition and destruction, delivery of tumor-suppressor genes, and expression of cytokines to stimulate anti-tumor immune responses. CAR-T cell therapy, which involves genetically modifying T cells to express chimeric antigen receptors, has shown remarkable success in treating certain blood cancers. Ongoing research focuses on expanding these approaches to solid tumors and combining gene therapy with other cancer treatment modalities.Expand Specific Solutions
Key Industry Players in Diabetes Gene Therapy Research
Gene therapy for diabetes management is evolving rapidly in a competitive landscape characterized by early-stage clinical development and significant research investment. The market is projected to grow substantially as innovative approaches address both Type 1 and Type 2 diabetes limitations. Leading players include established pharmaceutical companies like Pfizer and Takeda alongside specialized biotechnology firms such as Kriya Therapeutics, Fractyl Health, and Jaguar Gene Therapy. Academic institutions including the University of Washington and Joslin Diabetes Center contribute significant research. Technical challenges remain in delivery systems, gene expression control, and immune response management, with companies like enGene and Matrix Biomed developing novel delivery platforms to overcome these barriers.
enGene, Inc.
Technical Solution: enGene has pioneered a non-viral gene delivery platform called "Gut-Localized Oral-delivery of Genes" (GLObesTM) specifically designed for diabetes management. This innovative approach focuses on delivering therapeutic genes to intestinal cells rather than pancreatic tissue. The technology uses engineered lipid nanoparticles that protect genetic material from degradation in the gastrointestinal tract and facilitate cellular uptake in the intestinal epithelium. For diabetes applications, enGene's platform delivers genes encoding glucagon-like peptide-1 (GLP-1) or insulin directly to intestinal cells, transforming them into "endocrine factories" that can produce these hormones in response to food intake. The oral delivery system eliminates the need for injections, potentially improving patient compliance. Additionally, their technology includes glucose-responsive elements that regulate gene expression based on blood glucose levels, mimicking physiological insulin secretion patterns.
Strengths: Non-invasive oral delivery system improves patient compliance; targeted delivery to intestinal cells reduces systemic side effects; glucose-responsive elements enable physiological hormone production; non-viral vectors may have improved safety profile compared to viral alternatives. Weaknesses: Durability of gene expression in intestinal cells may be limited due to epithelial turnover; potential variability in absorption across patients; may require more frequent administration compared to viral vector approaches.
Joslin Diabetes Center, Inc.
Technical Solution: Joslin Diabetes Center has developed a comprehensive gene therapy platform targeting multiple aspects of diabetes pathophysiology. Their approach combines AAV-mediated gene delivery with CRISPR/Cas9 gene editing technologies to address both insulin production and autoimmune components of diabetes. The center's research has pioneered the use of liver-directed gene therapy to express insulin under the control of glucose-responsive promoters, effectively turning hepatocytes into surrogate beta cells. This approach circumvents the autoimmune destruction of pancreatic beta cells in type 1 diabetes. Additionally, Joslin researchers have developed gene therapy strategies targeting immune tolerance, delivering genes encoding immunomodulatory proteins that can suppress the autoimmune response against beta cells. Their platform includes proprietary vector designs that enhance tissue specificity and reduce immunogenicity, addressing key challenges in gene therapy applications. Recent advances include the development of regulatable gene expression systems that allow for fine-tuning of therapeutic protein levels in response to both glucose fluctuations and external control mechanisms.
Strengths: Comprehensive approach addressing both insulin production and autoimmunity; established expertise in diabetes pathophysiology; innovative liver-directed strategy avoids pancreatic autoimmunity; regulatable expression systems provide safety controls. Weaknesses: Complex multi-component system may face regulatory challenges; potential for off-target effects with gene editing approaches; durability of expression in liver cells remains to be fully established; translation from research to clinical application may be lengthy.
Breakthrough Technologies in Diabetes Gene Therapy
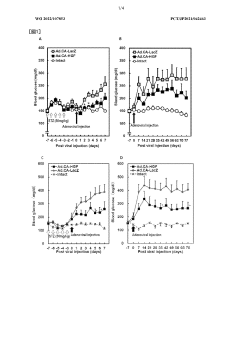
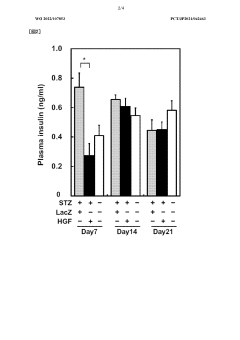
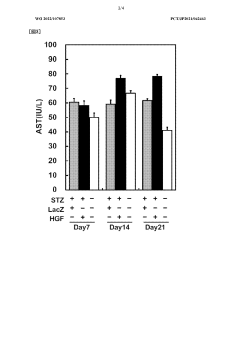
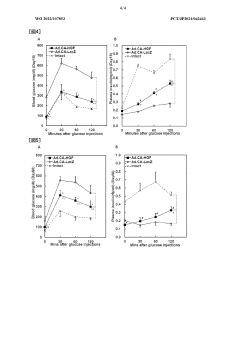
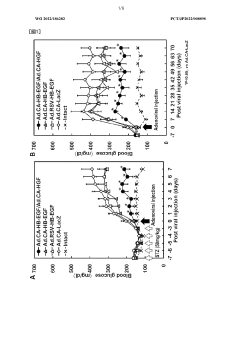
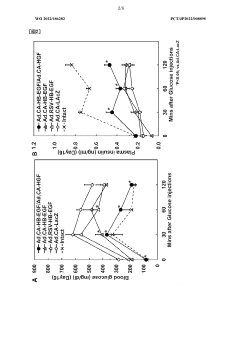
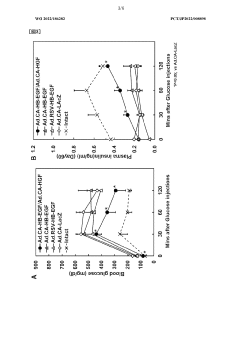
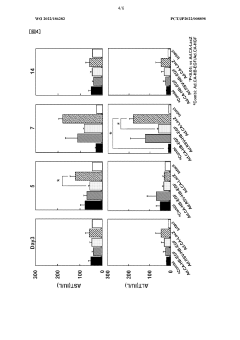
Low-dose hepatocyte growth factor gene therapy for diabetes
PatentWO2022107853A1
Innovation
- A low-dose gene therapy using a recombinant viral vector expressing hepatocyte growth factor (HGF) under the control of a strong promoter like the CA promoter, administered systemically to achieve therapeutic HGF levels, which effectively protects and regenerates pancreatic β cells without causing adverse events.
HB-EGF gene therapy for diabetes
PatentWO2022186282A1
Innovation
- A gene therapy approach using systemic administration of a nucleic acid encoding heparin-binding epidermal growth factor-like growth factor (HB-EGF), potentially combined with hepatocyte growth factor (HGF), delivered via adenovirus or adeno-associated virus vectors to protect and regenerate pancreatic β cells, maintaining glucose-responsive insulin secretion and suppressing hyperglycemia.
Regulatory Framework for Diabetes Gene Therapy
The regulatory landscape for gene therapy in diabetes management represents a complex and evolving framework that significantly impacts research progression, clinical trials, and eventual market approval. Currently, gene therapy for diabetes falls under the oversight of multiple regulatory bodies worldwide, with the FDA in the United States, the EMA in Europe, and the NMPA in China serving as primary gatekeepers. These agencies have established specific pathways for advanced therapy medicinal products (ATMPs), which include gene therapies targeting diabetes.
Regulatory requirements for diabetes gene therapy are particularly stringent due to the chronic nature of the disease and the potential for long-term genetic modifications. Developers must navigate a multi-phase clinical trial process with enhanced safety monitoring requirements, including long-term follow-up studies that may extend 5-15 years post-treatment to assess durability and delayed adverse effects.
The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer accelerated pathways for promising diabetes gene therapies, potentially reducing time to market for treatments demonstrating significant clinical benefits in early trials. However, these expedited routes still maintain rigorous safety standards and require substantial evidence of efficacy.
Regulatory considerations specific to diabetes gene therapy include demonstration of precise glucose regulation, prevention of hypoglycemic episodes, and evidence of reduced long-term complications. Agencies typically require comparative data against standard insulin therapy and other diabetes management approaches, with emphasis on quality-of-life improvements and reduction in disease burden.
Manufacturing and quality control regulations present significant hurdles, as gene therapy products must meet stringent standards for purity, potency, and consistency. Vector production, genetic material integrity, and final product stability all face intensive scrutiny during the approval process.
Recent regulatory developments include the implementation of harmonized international standards through the International Council for Harmonisation (ICH) and increased regulatory flexibility for innovative delivery methods. Several regulatory agencies have also published diabetes-specific guidance documents addressing unique considerations for gene therapy applications in this therapeutic area.
Ethical oversight adds another layer to the regulatory framework, with institutional review boards and ethics committees evaluating patient consent processes, risk-benefit ratios, and long-term monitoring plans. These considerations are particularly important for pediatric diabetes applications, where additional protections and specialized regulatory pathways exist.
The evolving regulatory landscape reflects growing recognition of gene therapy's potential in diabetes management, with agencies increasingly willing to engage in early dialogue with developers through scientific advice meetings and protocol assistance programs to optimize development strategies and address regulatory concerns proactively.
Regulatory requirements for diabetes gene therapy are particularly stringent due to the chronic nature of the disease and the potential for long-term genetic modifications. Developers must navigate a multi-phase clinical trial process with enhanced safety monitoring requirements, including long-term follow-up studies that may extend 5-15 years post-treatment to assess durability and delayed adverse effects.
The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme offer accelerated pathways for promising diabetes gene therapies, potentially reducing time to market for treatments demonstrating significant clinical benefits in early trials. However, these expedited routes still maintain rigorous safety standards and require substantial evidence of efficacy.
Regulatory considerations specific to diabetes gene therapy include demonstration of precise glucose regulation, prevention of hypoglycemic episodes, and evidence of reduced long-term complications. Agencies typically require comparative data against standard insulin therapy and other diabetes management approaches, with emphasis on quality-of-life improvements and reduction in disease burden.
Manufacturing and quality control regulations present significant hurdles, as gene therapy products must meet stringent standards for purity, potency, and consistency. Vector production, genetic material integrity, and final product stability all face intensive scrutiny during the approval process.
Recent regulatory developments include the implementation of harmonized international standards through the International Council for Harmonisation (ICH) and increased regulatory flexibility for innovative delivery methods. Several regulatory agencies have also published diabetes-specific guidance documents addressing unique considerations for gene therapy applications in this therapeutic area.
Ethical oversight adds another layer to the regulatory framework, with institutional review boards and ethics committees evaluating patient consent processes, risk-benefit ratios, and long-term monitoring plans. These considerations are particularly important for pediatric diabetes applications, where additional protections and specialized regulatory pathways exist.
The evolving regulatory landscape reflects growing recognition of gene therapy's potential in diabetes management, with agencies increasingly willing to engage in early dialogue with developers through scientific advice meetings and protocol assistance programs to optimize development strategies and address regulatory concerns proactively.
Safety and Ethical Considerations in Diabetes Gene Therapy
Gene therapy for diabetes management presents significant safety challenges that must be carefully addressed before widespread clinical implementation. The primary concern involves viral vector safety, as adeno-associated viruses (AAVs) and lentiviruses used for gene delivery may trigger immune responses or cause insertional mutagenesis. Recent improvements in vector design have reduced these risks, but long-term safety data remains limited, particularly regarding potential oncogenic effects from genomic integration.
Immune system reactions represent another critical safety consideration. Patients may develop antibodies against viral vectors or transgene products, potentially neutralizing therapeutic effects or causing systemic inflammatory responses. Pre-screening for existing antibodies and immunosuppressive protocols are being developed to mitigate these risks, though optimal approaches remain under investigation.
Off-target effects constitute a significant concern in diabetes gene therapy. CRISPR-Cas9 and other gene-editing technologies may cause unintended modifications at genomic sites similar to the target sequence. These modifications could potentially disrupt essential genes or regulatory elements, leading to unpredictable consequences. Advanced sequencing techniques are now employed to detect and minimize such off-target effects, but complete elimination remains challenging.
The ethical landscape surrounding diabetes gene therapy is equally complex. Questions of equitable access loom large, as advanced gene therapies typically emerge with high costs that may restrict availability to wealthy populations or nations. This raises concerns about exacerbating existing healthcare disparities, particularly relevant for diabetes which disproportionately affects disadvantaged communities globally.
Informed consent presents unique challenges in gene therapy trials. The novelty and complexity of these interventions make it difficult for patients to fully comprehend potential risks and benefits. Additionally, the potentially permanent nature of genetic modifications raises questions about long-term consequences that cannot be fully predicted during the consent process.
Germline modification concerns, though not directly applicable to most current diabetes gene therapy approaches, remain relevant as technology advances. While somatic cell modifications target only the patient's non-reproductive cells, inadvertent germline effects could theoretically occur. International consensus generally prohibits intentional germline modifications, but regulatory frameworks vary globally, creating potential for ethical disparities across jurisdictions.
Regulatory oversight for diabetes gene therapy varies significantly worldwide, with agencies like the FDA and EMA establishing stringent requirements for clinical trials while other regions may have less developed frameworks. This regulatory heterogeneity creates challenges for multinational research efforts and raises questions about appropriate safety standards.
Immune system reactions represent another critical safety consideration. Patients may develop antibodies against viral vectors or transgene products, potentially neutralizing therapeutic effects or causing systemic inflammatory responses. Pre-screening for existing antibodies and immunosuppressive protocols are being developed to mitigate these risks, though optimal approaches remain under investigation.
Off-target effects constitute a significant concern in diabetes gene therapy. CRISPR-Cas9 and other gene-editing technologies may cause unintended modifications at genomic sites similar to the target sequence. These modifications could potentially disrupt essential genes or regulatory elements, leading to unpredictable consequences. Advanced sequencing techniques are now employed to detect and minimize such off-target effects, but complete elimination remains challenging.
The ethical landscape surrounding diabetes gene therapy is equally complex. Questions of equitable access loom large, as advanced gene therapies typically emerge with high costs that may restrict availability to wealthy populations or nations. This raises concerns about exacerbating existing healthcare disparities, particularly relevant for diabetes which disproportionately affects disadvantaged communities globally.
Informed consent presents unique challenges in gene therapy trials. The novelty and complexity of these interventions make it difficult for patients to fully comprehend potential risks and benefits. Additionally, the potentially permanent nature of genetic modifications raises questions about long-term consequences that cannot be fully predicted during the consent process.
Germline modification concerns, though not directly applicable to most current diabetes gene therapy approaches, remain relevant as technology advances. While somatic cell modifications target only the patient's non-reproductive cells, inadvertent germline effects could theoretically occur. International consensus generally prohibits intentional germline modifications, but regulatory frameworks vary globally, creating potential for ethical disparities across jurisdictions.
Regulatory oversight for diabetes gene therapy varies significantly worldwide, with agencies like the FDA and EMA establishing stringent requirements for clinical trials while other regions may have less developed frameworks. This regulatory heterogeneity creates challenges for multinational research efforts and raises questions about appropriate safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!