How Do Recent Advances in Genome Editing Tools Enhance Gene Therapy?
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Genome Editing Evolution and Therapeutic Goals
Genome editing has evolved dramatically over the past three decades, transforming from rudimentary techniques with limited precision to sophisticated tools capable of making targeted modifications to DNA with remarkable accuracy. The journey began in the 1990s with zinc finger nucleases (ZFNs), which represented the first generation of programmable nucleases but faced challenges in design complexity and off-target effects. The subsequent development of transcription activator-like effector nucleases (TALENs) in the early 2010s offered improved specificity but still presented significant engineering hurdles.
The field experienced a revolutionary breakthrough with the discovery and adaptation of CRISPR-Cas systems around 2012. This technology dramatically simplified genome editing, offering unprecedented accessibility, versatility, and cost-effectiveness. Since then, CRISPR technology has undergone rapid refinement, with variants like CRISPR-Cas9, CRISPR-Cas12a, and base editors expanding the toolkit's capabilities and precision.
The therapeutic goals of genome editing have evolved in parallel with these technological advances. Initially, applications focused on ex vivo modification of cells for conditions like severe combined immunodeficiency (SCID) and hemoglobinopathies. As editing technologies matured, therapeutic ambitions expanded to include in vivo editing for a broader range of genetic disorders, including inherited retinal diseases, neurodegenerative conditions, and metabolic disorders.
Current therapeutic objectives center on achieving several critical outcomes: maximizing editing efficiency while minimizing off-target effects; developing delivery systems capable of targeting specific tissues; ensuring long-term expression and stability of edits; and addressing immune responses to editing components. The ultimate goal is to transition from treating symptoms to providing curative interventions for previously untreatable genetic conditions.
Recent advances aim to overcome persistent challenges in gene therapy implementation. These include developing more precise editing tools with reduced off-target effects, creating improved delivery vectors that can target specific tissues, and enhancing the efficiency of homology-directed repair for precise gene correction rather than disruption. Additionally, there is growing focus on making these therapies more accessible through reduced manufacturing costs and simplified regulatory pathways.
The convergence of genome editing evolution and therapeutic goals represents a paradigm shift in medicine, potentially enabling personalized genetic medicine that addresses the root causes of thousands of genetic disorders affecting millions worldwide. This technological trajectory suggests we are approaching an inflection point where genome editing may become a mainstream therapeutic modality rather than an experimental approach.
The field experienced a revolutionary breakthrough with the discovery and adaptation of CRISPR-Cas systems around 2012. This technology dramatically simplified genome editing, offering unprecedented accessibility, versatility, and cost-effectiveness. Since then, CRISPR technology has undergone rapid refinement, with variants like CRISPR-Cas9, CRISPR-Cas12a, and base editors expanding the toolkit's capabilities and precision.
The therapeutic goals of genome editing have evolved in parallel with these technological advances. Initially, applications focused on ex vivo modification of cells for conditions like severe combined immunodeficiency (SCID) and hemoglobinopathies. As editing technologies matured, therapeutic ambitions expanded to include in vivo editing for a broader range of genetic disorders, including inherited retinal diseases, neurodegenerative conditions, and metabolic disorders.
Current therapeutic objectives center on achieving several critical outcomes: maximizing editing efficiency while minimizing off-target effects; developing delivery systems capable of targeting specific tissues; ensuring long-term expression and stability of edits; and addressing immune responses to editing components. The ultimate goal is to transition from treating symptoms to providing curative interventions for previously untreatable genetic conditions.
Recent advances aim to overcome persistent challenges in gene therapy implementation. These include developing more precise editing tools with reduced off-target effects, creating improved delivery vectors that can target specific tissues, and enhancing the efficiency of homology-directed repair for precise gene correction rather than disruption. Additionally, there is growing focus on making these therapies more accessible through reduced manufacturing costs and simplified regulatory pathways.
The convergence of genome editing evolution and therapeutic goals represents a paradigm shift in medicine, potentially enabling personalized genetic medicine that addresses the root causes of thousands of genetic disorders affecting millions worldwide. This technological trajectory suggests we are approaching an inflection point where genome editing may become a mainstream therapeutic modality rather than an experimental approach.
Gene Therapy Market Landscape and Patient Needs
The gene therapy market has experienced remarkable growth in recent years, expanding from approximately $1 billion in 2018 to over $5 billion by 2022. Industry analysts project this trajectory to continue, with the market potentially reaching $20 billion by 2027, representing a compound annual growth rate of around 30%. This explosive growth reflects both increasing investment in the sector and the gradual maturation of gene therapy technologies from experimental treatments to approved therapeutic options.
Patient needs driving this market expansion are primarily concentrated around rare genetic disorders, many of which previously had few or no treatment options. Currently, over 7,000 rare diseases affect approximately 400 million people worldwide, with genetic factors playing a significant role in about 80% of these conditions. For these patients, gene therapy represents not merely an incremental improvement but potentially a transformative or curative approach to previously untreatable conditions.
The oncology segment represents another substantial market driver, with CAR-T cell therapies and other gene-modified approaches showing remarkable efficacy in certain blood cancers. Patient response rates exceeding 80% in some refractory leukemia populations have created significant demand, despite high treatment costs often exceeding $400,000 per patient.
Accessibility remains a critical challenge in meeting patient needs. Current approved gene therapies frequently carry price tags between $375,000 and $2.1 million per treatment, creating significant barriers to access. Insurance coverage remains inconsistent, with many patients facing substantial out-of-pocket costs or complete inability to access treatments.
Geographic disparities in access are equally concerning. While the United States, Europe, and Japan have approved multiple gene therapies, access in developing regions remains severely limited. This disparity is particularly troubling given that genetic diseases affect populations worldwide, with some conditions showing higher prevalence in underserved regions.
Manufacturing capacity represents another constraint in meeting patient needs. Current production methods for viral vectors and modified cell therapies face scalability challenges, creating treatment delays even for approved therapies. Waiting periods of 3-6 months for manufacturing slots are not uncommon, creating significant hardship for patients with progressive conditions.
The regulatory landscape continues to evolve to address these market and patient needs. Accelerated approval pathways have been established in major markets, with the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's PRIME program providing mechanisms to expedite promising therapies to patients with serious conditions and limited treatment options.
Patient needs driving this market expansion are primarily concentrated around rare genetic disorders, many of which previously had few or no treatment options. Currently, over 7,000 rare diseases affect approximately 400 million people worldwide, with genetic factors playing a significant role in about 80% of these conditions. For these patients, gene therapy represents not merely an incremental improvement but potentially a transformative or curative approach to previously untreatable conditions.
The oncology segment represents another substantial market driver, with CAR-T cell therapies and other gene-modified approaches showing remarkable efficacy in certain blood cancers. Patient response rates exceeding 80% in some refractory leukemia populations have created significant demand, despite high treatment costs often exceeding $400,000 per patient.
Accessibility remains a critical challenge in meeting patient needs. Current approved gene therapies frequently carry price tags between $375,000 and $2.1 million per treatment, creating significant barriers to access. Insurance coverage remains inconsistent, with many patients facing substantial out-of-pocket costs or complete inability to access treatments.
Geographic disparities in access are equally concerning. While the United States, Europe, and Japan have approved multiple gene therapies, access in developing regions remains severely limited. This disparity is particularly troubling given that genetic diseases affect populations worldwide, with some conditions showing higher prevalence in underserved regions.
Manufacturing capacity represents another constraint in meeting patient needs. Current production methods for viral vectors and modified cell therapies face scalability challenges, creating treatment delays even for approved therapies. Waiting periods of 3-6 months for manufacturing slots are not uncommon, creating significant hardship for patients with progressive conditions.
The regulatory landscape continues to evolve to address these market and patient needs. Accelerated approval pathways have been established in major markets, with the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's PRIME program providing mechanisms to expedite promising therapies to patients with serious conditions and limited treatment options.
Current Genome Editing Technologies and Limitations
Genome editing technologies have evolved significantly over the past two decades, revolutionizing the field of gene therapy. Currently, several major genome editing platforms dominate the landscape, each with distinct mechanisms and applications. CRISPR-Cas9 has emerged as the most widely adopted system due to its versatility, efficiency, and relative ease of use. This RNA-guided nuclease system can be programmed to target specific DNA sequences with high precision, making it suitable for a broad range of therapeutic applications.
Zinc Finger Nucleases (ZFNs) represent one of the earliest programmable genome editing tools, utilizing engineered zinc finger proteins fused to FokI nuclease domains. While highly specific, ZFNs require complex protein engineering for each target site, limiting their scalability and widespread adoption in clinical settings. Similarly, Transcription Activator-Like Effector Nucleases (TALENs) offer improved targeting specificity but face challenges in delivery and manufacturing due to their large size and repetitive sequences.
Base editors, a more recent innovation, enable direct conversion of one nucleotide to another without introducing double-strand breaks. These systems combine a catalytically impaired Cas9 with deaminase enzymes to achieve precise point mutations. Prime editing, an even newer technology, offers the capability to perform targeted insertions, deletions, and all possible base-to-base conversions without requiring donor DNA templates or double-strand breaks.
Despite these advances, significant limitations persist across all current genome editing platforms. Off-target effects remain a primary concern, as unintended edits can potentially lead to oncogenesis or other adverse outcomes. The efficiency of editing varies considerably across different cell types and genomic loci, with some regions proving particularly recalcitrant to modification. This variability creates challenges for achieving therapeutic thresholds in certain disease contexts.
Delivery systems represent another major bottleneck in translating genome editing technologies to clinical applications. Viral vectors, while effective for certain tissues, face limitations in packaging capacity, potential immunogenicity, and manufacturing scalability. Non-viral delivery methods show promise but currently lack the efficiency needed for many therapeutic applications, particularly for in vivo treatments.
Size constraints of editing machinery pose additional challenges, particularly for AAV-based delivery systems commonly used in gene therapy. The large size of Cas9 proteins from certain species exceeds the packaging capacity of these vectors, necessitating alternative delivery strategies or the development of smaller editing systems.
Immune responses to both delivery vehicles and editing components themselves present further complications. Pre-existing immunity to Cas proteins derived from common bacterial species can reduce efficacy and potentially trigger adverse immune reactions in patients, limiting the repeated administration of certain genome editing therapies.
Zinc Finger Nucleases (ZFNs) represent one of the earliest programmable genome editing tools, utilizing engineered zinc finger proteins fused to FokI nuclease domains. While highly specific, ZFNs require complex protein engineering for each target site, limiting their scalability and widespread adoption in clinical settings. Similarly, Transcription Activator-Like Effector Nucleases (TALENs) offer improved targeting specificity but face challenges in delivery and manufacturing due to their large size and repetitive sequences.
Base editors, a more recent innovation, enable direct conversion of one nucleotide to another without introducing double-strand breaks. These systems combine a catalytically impaired Cas9 with deaminase enzymes to achieve precise point mutations. Prime editing, an even newer technology, offers the capability to perform targeted insertions, deletions, and all possible base-to-base conversions without requiring donor DNA templates or double-strand breaks.
Despite these advances, significant limitations persist across all current genome editing platforms. Off-target effects remain a primary concern, as unintended edits can potentially lead to oncogenesis or other adverse outcomes. The efficiency of editing varies considerably across different cell types and genomic loci, with some regions proving particularly recalcitrant to modification. This variability creates challenges for achieving therapeutic thresholds in certain disease contexts.
Delivery systems represent another major bottleneck in translating genome editing technologies to clinical applications. Viral vectors, while effective for certain tissues, face limitations in packaging capacity, potential immunogenicity, and manufacturing scalability. Non-viral delivery methods show promise but currently lack the efficiency needed for many therapeutic applications, particularly for in vivo treatments.
Size constraints of editing machinery pose additional challenges, particularly for AAV-based delivery systems commonly used in gene therapy. The large size of Cas9 proteins from certain species exceeds the packaging capacity of these vectors, necessitating alternative delivery strategies or the development of smaller editing systems.
Immune responses to both delivery vehicles and editing components themselves present further complications. Pre-existing immunity to Cas proteins derived from common bacterial species can reduce efficacy and potentially trigger adverse immune reactions in patients, limiting the repeated administration of certain genome editing therapies.
Current Gene Therapy Delivery Systems and Methods
01 CRISPR-Cas system enhancements
Various enhancements to CRISPR-Cas genome editing systems have been developed to improve efficiency, specificity, and versatility. These include engineered Cas proteins with reduced off-target effects, improved on-target activity, and expanded targeting capabilities. Modified guide RNAs and delivery methods have also been developed to enhance the performance of CRISPR-based genome editing tools in various applications including therapeutic gene editing.- CRISPR-Cas system improvements: Enhancements to CRISPR-Cas genome editing systems focus on improving specificity, efficiency, and reducing off-target effects. These improvements include engineered Cas proteins with higher fidelity, optimized guide RNA designs, and novel delivery methods. Advanced CRISPR systems enable more precise genetic modifications in various cell types and organisms, expanding their therapeutic potential for treating genetic disorders.
- Base and prime editing technologies: Base and prime editing represent next-generation genome editing approaches that enable precise nucleotide changes without double-strand breaks. These technologies use modified Cas proteins fused with deaminases or reverse transcriptases to make specific edits. They offer advantages in precision, reduced off-target effects, and the ability to make targeted modifications without requiring donor DNA templates, advancing applications in both research and therapeutic contexts.
- Delivery systems for genome editing tools: Advanced delivery systems for genome editing tools include viral vectors (AAV, lentivirus), lipid nanoparticles, and cell-penetrating peptides. These systems enhance the efficiency of delivering editing components to target cells and tissues while minimizing immune responses. Innovations in delivery technologies address key challenges in therapeutic applications by improving targeting specificity, payload capacity, and in vivo stability of genome editing components.
- Alternative genome editing nucleases: Beyond CRISPR, alternative genome editing nucleases include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and meganucleases. These systems offer complementary capabilities for specific applications where CRISPR may have limitations. Recent enhancements focus on improving their specificity, efficiency, and reducing cytotoxicity, providing researchers with a diverse toolkit for different genome editing requirements.
- Computational tools and AI for genome editing: Computational tools and artificial intelligence enhance genome editing by optimizing guide RNA design, predicting off-target effects, and analyzing editing outcomes. Machine learning algorithms improve target site selection and editing efficiency while minimizing unintended modifications. These computational approaches accelerate the development of genome editing applications by providing data-driven insights and automating complex design processes for more successful editing outcomes.
02 Base editing and prime editing technologies
Advanced genome editing tools that enable precise nucleotide changes without double-strand breaks have been developed. Base editors can convert specific base pairs (e.g., C•G to T•A or A•T to G•C) with minimal off-target effects. Prime editing systems allow for targeted insertions, deletions, and all possible base-to-base conversions without requiring donor DNA templates. These technologies represent significant enhancements over traditional genome editing methods by offering greater precision and reduced cellular toxicity.Expand Specific Solutions03 Delivery systems for genome editing tools
Enhanced delivery methods have been developed to improve the efficiency of genome editing tools reaching target cells and tissues. These include viral vectors (AAV, lentivirus), lipid nanoparticles, cell-penetrating peptides, and exosome-based delivery systems. Advancements in delivery technologies have focused on reducing immunogenicity, improving targeting specificity to particular tissues, and enhancing cellular uptake, which are critical factors for successful therapeutic applications of genome editing.Expand Specific Solutions04 Computational tools and AI for genome editing
Computational approaches and artificial intelligence have been developed to enhance genome editing tools. These include algorithms for guide RNA design with improved specificity and efficiency, prediction of off-target effects, and optimization of editing outcomes. Machine learning models trained on large datasets help identify optimal target sites and editing strategies. These computational enhancements significantly improve the precision and success rate of genome editing experiments while reducing unintended modifications.Expand Specific Solutions05 Novel nucleases and editing systems
Beyond CRISPR-Cas, alternative genome editing nucleases and systems have been developed with unique properties and advantages. These include engineered meganucleases, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and novel RNA-guided nucleases discovered from diverse microbial sources. These alternative systems offer different PAM requirements, size advantages, or reduced immunogenicity compared to conventional CRISPR systems, expanding the toolkit available for different genome editing applications.Expand Specific Solutions
Leading Companies and Research Institutions in Gene Therapy
Recent advances in genome editing tools have significantly enhanced gene therapy, with the field currently in a rapid growth phase. The market size for gene therapy is expanding exponentially, projected to reach billions of dollars by 2025. Technologically, CRISPR-Cas9 systems have reached moderate maturity, while newer technologies are still emerging. Key players shaping the competitive landscape include academic powerhouses like The Broad Institute, MIT, and Harvard, alongside commercial entities such as CRISPR Therapeutics, Editas Medicine, and Novartis. Biotechnology companies like Inari Agriculture and Specific Biologics are developing specialized applications, while pharmaceutical giants including Bayer and GlaxoSmithKline are investing heavily in the space. This creates a dynamic ecosystem where academic innovation rapidly translates into commercial therapeutics.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has been at the forefront of developing CRISPR-Cas systems for genome editing, with foundational contributions to the field. Their technology platform includes the optimization of Cas9 variants with enhanced specificity and reduced off-target effects, such as the engineered SpCas9-HF1 and eSpCas9 variants that show substantially reduced off-target activity while maintaining on-target efficiency. The Institute has pioneered base editing technology, which enables precise C•G to T•A or A•T to G•C conversions without double-strand breaks, reducing unwanted indels and chromosomal rearrangements. Their researchers have developed REPAIR (RNA Editing for Programmable A to I Replacement), a technology that uses Cas13 to edit RNA rather than DNA, offering temporary and reversible editing options. The Broad has also advanced prime editing, a versatile editing approach capable of making all possible base-to-base conversions, small insertions, and small deletions without requiring donor DNA templates or double-strand breaks.
Strengths: World-leading research institution with pioneering contributions to multiple gene editing platforms; extensive intellectual property portfolio; continuous innovation in editing technologies. Weaknesses: As a research institution, relies on commercial partners for clinical development; complex IP landscape with ongoing litigation; less direct involvement in therapeutic development compared to biotech companies.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed several groundbreaking genome editing technologies that significantly enhance gene therapy approaches. Their scientists pioneered base editing technology, which enables direct conversion of one DNA base to another without requiring double-strand breaks, reducing unwanted insertions and deletions. This technology uses a catalytically impaired Cas9 fused to a deaminase enzyme that can convert cytosine to uracil (which pairs like thymine) or adenine to inosine (which pairs like guanine). Harvard researchers have also developed prime editing, a "search-and-replace" genome editing technology that uses a Cas9 nickase fused to an engineered reverse transcriptase and a prime editing guide RNA (pegRNA) that both specifies the target site and encodes the desired edit. This approach can perform all possible base-to-base conversions, small insertions, and small deletions with minimal byproducts. Additionally, Harvard scientists have advanced RNA editing technologies using ADAR enzymes that can make temporary changes to gene expression without permanent DNA modifications.
Strengths: World-leading research institution with pioneering contributions to precise editing technologies; strong basic science capabilities driving innovation; extensive collaborative network. Weaknesses: As an academic institution, requires commercial partners for clinical translation; complex IP arrangements; less direct involvement in therapeutic product development.
Breakthrough CRISPR and Base Editing Technologies
Anti-CD45-based conditioning methods and uses thereof in conjunction with gene-edited cell-based therapies
PatentWO2019084258A1
Innovation
- Administration of a radiolabeled anti-CD45 antibody to effectively deplete hematopoietic stem cells, minimizing adverse effects and enhancing the outcome of subsequent gene-edited cell-based therapies.
Programmable transposases and uses thereof
PatentPendingUS20240052371A1
Innovation
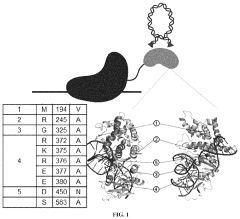
- A composition comprising a site-specific DNA binding protein and a modified hyperactive PiggyBac transposase, with specific amino acid mutations, is used to facilitate targeted gene delivery and integration of both small and large nucleic acid fragments into mammalian cells, achieving high efficiency and precision through site-directed integration.
Regulatory Framework for Gene Therapy Applications
The regulatory landscape for gene therapy applications has evolved significantly in response to rapid advancements in genome editing technologies. Regulatory bodies worldwide have established frameworks to ensure the safety, efficacy, and ethical implementation of these powerful therapeutic approaches. The U.S. Food and Drug Administration (FDA) has developed a comprehensive regulatory pathway for gene therapy products, requiring rigorous preclinical testing, phased clinical trials, and post-market surveillance. Similarly, the European Medicines Agency (EMA) has established the Committee for Advanced Therapies (CAT) specifically to evaluate gene and cell therapy products.
These regulatory frameworks typically address several critical aspects of gene therapy applications. Risk assessment protocols have been established to evaluate potential off-target effects of genome editing tools like CRISPR-Cas9, TALENs, and zinc finger nucleases. Long-term safety monitoring requirements have been implemented to track potential delayed adverse effects that may not be apparent during initial clinical trials. Additionally, manufacturing standards have been developed to ensure consistency, purity, and potency of gene therapy products.
Regulatory challenges remain significant in this rapidly evolving field. The novelty of genome editing technologies has created regulatory gaps that agencies are working to address. International harmonization efforts are underway to standardize regulatory approaches across different jurisdictions, facilitating global development and access to gene therapies. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been instrumental in these efforts.
Ethical considerations are deeply embedded in regulatory frameworks for gene therapy. Distinctions between somatic and germline editing are particularly important, with most jurisdictions prohibiting clinical applications of germline editing while allowing somatic cell therapies under appropriate oversight. Informed consent requirements are especially stringent for gene therapy trials, reflecting the novel nature and potential long-term implications of these interventions.
Recent regulatory adaptations have included accelerated approval pathways for gene therapies targeting rare diseases with high unmet medical needs. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme exemplify these approaches. These pathways aim to balance the need for thorough safety evaluation with the urgency of bringing potentially life-saving therapies to patients with limited treatment options.
These regulatory frameworks typically address several critical aspects of gene therapy applications. Risk assessment protocols have been established to evaluate potential off-target effects of genome editing tools like CRISPR-Cas9, TALENs, and zinc finger nucleases. Long-term safety monitoring requirements have been implemented to track potential delayed adverse effects that may not be apparent during initial clinical trials. Additionally, manufacturing standards have been developed to ensure consistency, purity, and potency of gene therapy products.
Regulatory challenges remain significant in this rapidly evolving field. The novelty of genome editing technologies has created regulatory gaps that agencies are working to address. International harmonization efforts are underway to standardize regulatory approaches across different jurisdictions, facilitating global development and access to gene therapies. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been instrumental in these efforts.
Ethical considerations are deeply embedded in regulatory frameworks for gene therapy. Distinctions between somatic and germline editing are particularly important, with most jurisdictions prohibiting clinical applications of germline editing while allowing somatic cell therapies under appropriate oversight. Informed consent requirements are especially stringent for gene therapy trials, reflecting the novel nature and potential long-term implications of these interventions.
Recent regulatory adaptations have included accelerated approval pathways for gene therapies targeting rare diseases with high unmet medical needs. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme exemplify these approaches. These pathways aim to balance the need for thorough safety evaluation with the urgency of bringing potentially life-saving therapies to patients with limited treatment options.
Ethical Implications of Human Genome Modification
The rapid advancement of genome editing technologies, particularly CRISPR-Cas9, has revolutionized gene therapy approaches but simultaneously raised profound ethical questions about human genome modification. These ethical implications extend beyond technical considerations into fundamental questions about human identity, social justice, and the future of humanity.
The ability to precisely edit the human genome presents unprecedented moral dilemmas regarding the distinction between therapeutic applications and enhancement purposes. While correcting disease-causing mutations to alleviate suffering garners broad ethical support, modifications aimed at enhancing non-medical traits such as intelligence, physical attributes, or longevity generate significant controversy. This blurry boundary between treatment and enhancement represents one of the central ethical tensions in the field.
Concerns about germline editing—modifications that would be inherited by future generations—are particularly acute. Unlike somatic cell editing that affects only the treated individual, germline modifications permanently alter the human gene pool with unpredictable long-term consequences. The 2018 case of CRISPR-edited babies in China sparked international outrage precisely because it crossed this critical ethical line without adequate scientific consensus or regulatory oversight.
Equity and access issues further complicate the ethical landscape. Advanced gene therapies remain prohibitively expensive, raising concerns that genome editing technologies could exacerbate existing healthcare disparities. The potential emergence of genetic privilege—where only wealthy individuals can access genetic enhancements—threatens to create new forms of discrimination and social stratification based on genetic status.
Informed consent presents another significant ethical challenge, especially for applications involving embryos or children who cannot personally consent to genetic modifications. Questions about who should have decision-making authority over genetic modifications and what constitutes adequate information for truly informed consent remain unresolved.
Cultural and religious perspectives on genome editing vary widely, with some traditions viewing genetic intervention as interfering with divine will or natural processes. These diverse viewpoints necessitate inclusive ethical frameworks that respect pluralistic values while establishing reasonable boundaries for research and clinical applications.
The governance of human genome editing technologies requires balancing scientific progress with ethical safeguards. International harmonization of regulatory approaches remains challenging but essential, as uneven regulations could lead to "regulatory havens" where controversial research proceeds with minimal oversight. Multidisciplinary engagement involving scientists, ethicists, policymakers, and diverse public stakeholders is crucial for developing responsible governance frameworks that maximize benefits while minimizing risks and respecting human dignity.
The ability to precisely edit the human genome presents unprecedented moral dilemmas regarding the distinction between therapeutic applications and enhancement purposes. While correcting disease-causing mutations to alleviate suffering garners broad ethical support, modifications aimed at enhancing non-medical traits such as intelligence, physical attributes, or longevity generate significant controversy. This blurry boundary between treatment and enhancement represents one of the central ethical tensions in the field.
Concerns about germline editing—modifications that would be inherited by future generations—are particularly acute. Unlike somatic cell editing that affects only the treated individual, germline modifications permanently alter the human gene pool with unpredictable long-term consequences. The 2018 case of CRISPR-edited babies in China sparked international outrage precisely because it crossed this critical ethical line without adequate scientific consensus or regulatory oversight.
Equity and access issues further complicate the ethical landscape. Advanced gene therapies remain prohibitively expensive, raising concerns that genome editing technologies could exacerbate existing healthcare disparities. The potential emergence of genetic privilege—where only wealthy individuals can access genetic enhancements—threatens to create new forms of discrimination and social stratification based on genetic status.
Informed consent presents another significant ethical challenge, especially for applications involving embryos or children who cannot personally consent to genetic modifications. Questions about who should have decision-making authority over genetic modifications and what constitutes adequate information for truly informed consent remain unresolved.
Cultural and religious perspectives on genome editing vary widely, with some traditions viewing genetic intervention as interfering with divine will or natural processes. These diverse viewpoints necessitate inclusive ethical frameworks that respect pluralistic values while establishing reasonable boundaries for research and clinical applications.
The governance of human genome editing technologies requires balancing scientific progress with ethical safeguards. International harmonization of regulatory approaches remains challenging but essential, as uneven regulations could lead to "regulatory havens" where controversial research proceeds with minimal oversight. Multidisciplinary engagement involving scientists, ethicists, policymakers, and diverse public stakeholders is crucial for developing responsible governance frameworks that maximize benefits while minimizing risks and respecting human dignity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!