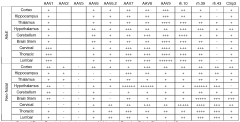
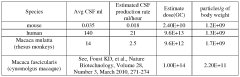
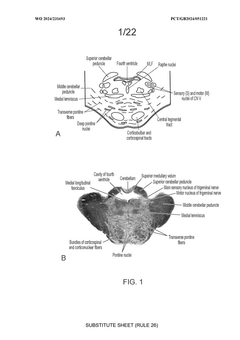
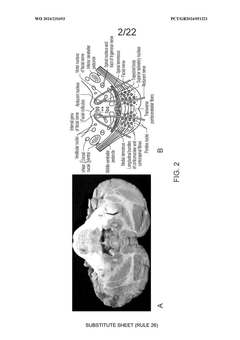
Challenges in Delivering Gene Therapy to the Central Nervous System
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CNS Gene Therapy Background and Objectives
Gene therapy for central nervous system (CNS) disorders represents one of the most promising yet challenging frontiers in modern medicine. The evolution of this field traces back to the early 1990s when the first gene therapy clinical trials began, though CNS-specific applications emerged later due to the unique challenges presented by the blood-brain barrier (BBB). Over the past three decades, significant advancements in viral vector design, delivery methods, and understanding of neurological disease mechanisms have propelled this field forward.
The trajectory of CNS gene therapy development has been marked by critical milestones, including the development of adeno-associated virus (AAV) vectors with enhanced neurotropism, the discovery of AAV serotypes capable of crossing the BBB, and the implementation of novel delivery strategies such as intrathecal and intraventricular administration. Recent breakthroughs in genome editing technologies, particularly CRISPR-Cas systems, have further expanded the potential applications of gene therapy for neurological disorders.
Current technological trends in this field include the development of engineered AAV capsids with improved CNS targeting capabilities, non-viral delivery systems utilizing nanoparticles and exosomes, and the exploration of regulatory RNA-based therapeutic approaches. Additionally, there is growing interest in combining gene therapy with cell therapy approaches, particularly for neurodegenerative conditions.
The primary objectives of CNS gene therapy research encompass several dimensions. First, enhancing delivery efficiency across the BBB remains a paramount goal, as current methods achieve limited brain penetration or require invasive procedures. Second, improving the specificity of neuronal targeting to minimize off-target effects and optimize therapeutic outcomes represents another critical objective. Third, extending the duration of therapeutic gene expression to reduce the need for repeated administrations is essential for treating chronic neurological conditions.
Additional technical objectives include developing scalable manufacturing processes for clinical-grade vectors, establishing reliable safety profiles through comprehensive preclinical studies, and creating versatile delivery platforms adaptable to various CNS disorders. The ultimate aim is to translate these technologies into clinically viable treatments for previously untreatable neurological diseases, including neurodegenerative disorders like Alzheimer's and Parkinson's disease, genetic conditions such as spinal muscular atrophy and Huntington's disease, and acquired CNS injuries.
The convergence of advances in neuroscience, molecular biology, and bioengineering has created unprecedented opportunities for addressing these challenges, potentially revolutionizing treatment paradigms for millions of patients worldwide suffering from debilitating CNS disorders.
The trajectory of CNS gene therapy development has been marked by critical milestones, including the development of adeno-associated virus (AAV) vectors with enhanced neurotropism, the discovery of AAV serotypes capable of crossing the BBB, and the implementation of novel delivery strategies such as intrathecal and intraventricular administration. Recent breakthroughs in genome editing technologies, particularly CRISPR-Cas systems, have further expanded the potential applications of gene therapy for neurological disorders.
Current technological trends in this field include the development of engineered AAV capsids with improved CNS targeting capabilities, non-viral delivery systems utilizing nanoparticles and exosomes, and the exploration of regulatory RNA-based therapeutic approaches. Additionally, there is growing interest in combining gene therapy with cell therapy approaches, particularly for neurodegenerative conditions.
The primary objectives of CNS gene therapy research encompass several dimensions. First, enhancing delivery efficiency across the BBB remains a paramount goal, as current methods achieve limited brain penetration or require invasive procedures. Second, improving the specificity of neuronal targeting to minimize off-target effects and optimize therapeutic outcomes represents another critical objective. Third, extending the duration of therapeutic gene expression to reduce the need for repeated administrations is essential for treating chronic neurological conditions.
Additional technical objectives include developing scalable manufacturing processes for clinical-grade vectors, establishing reliable safety profiles through comprehensive preclinical studies, and creating versatile delivery platforms adaptable to various CNS disorders. The ultimate aim is to translate these technologies into clinically viable treatments for previously untreatable neurological diseases, including neurodegenerative disorders like Alzheimer's and Parkinson's disease, genetic conditions such as spinal muscular atrophy and Huntington's disease, and acquired CNS injuries.
The convergence of advances in neuroscience, molecular biology, and bioengineering has created unprecedented opportunities for addressing these challenges, potentially revolutionizing treatment paradigms for millions of patients worldwide suffering from debilitating CNS disorders.
Market Analysis for CNS Gene Therapeutics
The global market for gene therapies targeting the central nervous system (CNS) is experiencing significant growth, projected to reach $3.2 billion by 2026 with a compound annual growth rate of 20.5%. This expansion is primarily driven by the increasing prevalence of neurological disorders such as Alzheimer's disease, Parkinson's disease, and various rare genetic conditions affecting the CNS. With approximately 600 million people worldwide suffering from neurological disorders, the demand for effective treatments continues to rise.
The CNS gene therapy market is segmented by delivery method, with viral vectors dominating at 75% market share due to their higher efficiency in neuronal transduction. Among these, adeno-associated virus (AAV) vectors represent the largest subsegment at 60% of viral vector approaches, followed by lentiviral vectors at 25%. Non-viral delivery methods, while currently holding a smaller market share, are growing at a faster rate of 25% annually due to improved safety profiles and reduced immunogenicity concerns.
Geographically, North America leads the market with 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The United States specifically accounts for 38% of the global market, benefiting from favorable regulatory frameworks and substantial research funding. China represents the fastest-growing market with 28% annual growth, driven by increasing investment in biotechnology and healthcare infrastructure.
From a therapeutic application perspective, treatments for neurodegenerative diseases constitute 40% of the market, with rare genetic disorders accounting for 35% and neurological cancers representing 15%. The remaining 10% encompasses various other CNS conditions. Notably, therapies targeting single-gene disorders show the highest commercial success rate at 22%, compared to 8% for complex polygenic conditions.
The payer landscape presents significant challenges, with the average cost of CNS gene therapies ranging from $850,000 to $2.1 million per treatment. This has prompted the development of novel payment models, with 65% of approved therapies now utilizing outcome-based agreements or installment payment structures. Insurance coverage remains limited, with only 40% of private insurers providing comprehensive coverage for CNS gene therapies.
Patient accessibility represents another critical market factor, with current treatments reaching only 12% of eligible patients globally. This gap is more pronounced in developing regions, where access rates fall below 5%. The market is responding with increased focus on scalable manufacturing processes, with production costs decreasing by approximately 18% annually as technologies mature and economies of scale are realized.
The CNS gene therapy market is segmented by delivery method, with viral vectors dominating at 75% market share due to their higher efficiency in neuronal transduction. Among these, adeno-associated virus (AAV) vectors represent the largest subsegment at 60% of viral vector approaches, followed by lentiviral vectors at 25%. Non-viral delivery methods, while currently holding a smaller market share, are growing at a faster rate of 25% annually due to improved safety profiles and reduced immunogenicity concerns.
Geographically, North America leads the market with 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The United States specifically accounts for 38% of the global market, benefiting from favorable regulatory frameworks and substantial research funding. China represents the fastest-growing market with 28% annual growth, driven by increasing investment in biotechnology and healthcare infrastructure.
From a therapeutic application perspective, treatments for neurodegenerative diseases constitute 40% of the market, with rare genetic disorders accounting for 35% and neurological cancers representing 15%. The remaining 10% encompasses various other CNS conditions. Notably, therapies targeting single-gene disorders show the highest commercial success rate at 22%, compared to 8% for complex polygenic conditions.
The payer landscape presents significant challenges, with the average cost of CNS gene therapies ranging from $850,000 to $2.1 million per treatment. This has prompted the development of novel payment models, with 65% of approved therapies now utilizing outcome-based agreements or installment payment structures. Insurance coverage remains limited, with only 40% of private insurers providing comprehensive coverage for CNS gene therapies.
Patient accessibility represents another critical market factor, with current treatments reaching only 12% of eligible patients globally. This gap is more pronounced in developing regions, where access rates fall below 5%. The market is responding with increased focus on scalable manufacturing processes, with production costs decreasing by approximately 18% annually as technologies mature and economies of scale are realized.
Blood-Brain Barrier Challenges and Current Solutions
The blood-brain barrier (BBB) represents one of the most significant obstacles in delivering gene therapies to the central nervous system (CNS). This highly selective semipermeable border separates the circulating blood from the brain parenchyma, preventing approximately 98% of small molecule drugs and nearly all large molecule therapeutics from reaching the brain tissue. The BBB consists of specialized endothelial cells connected by tight junctions, surrounded by pericytes, astrocytic end-feet, and extracellular matrix components, collectively forming the neurovascular unit.
The physical properties of the BBB pose substantial challenges for gene therapy delivery. The tight junctions between endothelial cells restrict paracellular transport, while the limited presence of fenestrations and pinocytotic vesicles minimizes transcellular passage. Additionally, efflux transporters like P-glycoprotein actively pump many substances back into the bloodstream, further complicating therapeutic delivery.
Current solutions for overcoming the BBB can be categorized into invasive and non-invasive approaches. Invasive methods include direct intraparenchymal injection, which bypasses the BBB entirely but is limited by poor distribution beyond the injection site. Intrathecal and intraventricular administrations deliver therapeutics into the cerebrospinal fluid, offering wider distribution but still facing challenges with penetration into deep brain tissues.
Non-invasive approaches have gained significant attention in recent years. Chemical modifications of viral vectors, such as mannosylation or incorporation of BBB-targeting peptides, enhance their ability to cross the barrier through receptor-mediated transcytosis. Adeno-associated virus (AAV) serotypes, particularly AAV9 and AAVrh.10, have demonstrated natural tropism for CNS tissues and ability to cross the BBB following systemic administration.
Physical disruption techniques represent another promising approach. Focused ultrasound combined with microbubbles can temporarily open the BBB in specific regions without permanent damage. This technique has shown efficacy in preclinical models and is advancing to clinical trials. Similarly, hyperosmotic agents like mannitol can temporarily shrink endothelial cells, widening tight junctions for enhanced therapeutic delivery.
Nanoparticle-based delivery systems offer versatile platforms for BBB penetration. Lipid nanoparticles, polymeric nanoparticles, and exosomes can be engineered with surface ligands targeting specific transporters or receptors on brain endothelial cells. These systems protect genetic cargo from degradation while facilitating BBB crossing through receptor-mediated transcytosis pathways.
Despite these advances, significant challenges remain. The efficiency of BBB crossing remains suboptimal for most approaches, and concerns about off-target effects and safety profiles persist. The heterogeneity of the BBB across different brain regions and disease states further complicates the development of universally effective delivery strategies for CNS gene therapies.
The physical properties of the BBB pose substantial challenges for gene therapy delivery. The tight junctions between endothelial cells restrict paracellular transport, while the limited presence of fenestrations and pinocytotic vesicles minimizes transcellular passage. Additionally, efflux transporters like P-glycoprotein actively pump many substances back into the bloodstream, further complicating therapeutic delivery.
Current solutions for overcoming the BBB can be categorized into invasive and non-invasive approaches. Invasive methods include direct intraparenchymal injection, which bypasses the BBB entirely but is limited by poor distribution beyond the injection site. Intrathecal and intraventricular administrations deliver therapeutics into the cerebrospinal fluid, offering wider distribution but still facing challenges with penetration into deep brain tissues.
Non-invasive approaches have gained significant attention in recent years. Chemical modifications of viral vectors, such as mannosylation or incorporation of BBB-targeting peptides, enhance their ability to cross the barrier through receptor-mediated transcytosis. Adeno-associated virus (AAV) serotypes, particularly AAV9 and AAVrh.10, have demonstrated natural tropism for CNS tissues and ability to cross the BBB following systemic administration.
Physical disruption techniques represent another promising approach. Focused ultrasound combined with microbubbles can temporarily open the BBB in specific regions without permanent damage. This technique has shown efficacy in preclinical models and is advancing to clinical trials. Similarly, hyperosmotic agents like mannitol can temporarily shrink endothelial cells, widening tight junctions for enhanced therapeutic delivery.
Nanoparticle-based delivery systems offer versatile platforms for BBB penetration. Lipid nanoparticles, polymeric nanoparticles, and exosomes can be engineered with surface ligands targeting specific transporters or receptors on brain endothelial cells. These systems protect genetic cargo from degradation while facilitating BBB crossing through receptor-mediated transcytosis pathways.
Despite these advances, significant challenges remain. The efficiency of BBB crossing remains suboptimal for most approaches, and concerns about off-target effects and safety profiles persist. The heterogeneity of the BBB across different brain regions and disease states further complicates the development of universally effective delivery strategies for CNS gene therapies.
Current Viral and Non-Viral Vector Approaches
01 Viral vector delivery systems
Viral vectors are commonly used for gene therapy delivery due to their natural ability to infect cells and deliver genetic material. These systems include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can be engineered to carry therapeutic genes while minimizing immunogenicity. The efficiency of viral vectors can be enhanced through modifications to their capsid proteins or envelope structures, allowing for improved targeting of specific tissues and increased transduction rates.- Viral vector delivery systems: Viral vectors are widely used for gene therapy delivery due to their natural ability to infect cells and deliver genetic material efficiently. These systems include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), which can be engineered to carry therapeutic genes while minimizing immunogenicity. The efficiency of viral vectors can be enhanced through modifications to their capsid proteins or envelope structures to improve target cell specificity and transduction rates.
- Non-viral delivery methods: Non-viral gene delivery systems offer advantages in terms of safety, reduced immunogenicity, and larger payload capacity compared to viral vectors. These methods include lipid nanoparticles, polymeric carriers, and physical techniques such as electroporation or sonoporation. Recent advances in lipid formulations and polymer chemistry have significantly improved transfection efficiency while reducing cytotoxicity, making non-viral approaches increasingly viable for clinical applications.
- Targeted delivery strategies: Enhancing gene therapy delivery efficiency through targeting specific cell types or tissues is a key focus area. This includes the use of cell-specific promoters, receptor-mediated endocytosis, and tissue-specific targeting ligands. By directing therapeutic genes to intended target cells while minimizing off-target effects, these approaches improve both efficacy and safety profiles of gene therapy. Advanced targeting strategies incorporate responsive elements that activate gene expression only under specific physiological conditions.
- Physical and mechanical delivery methods: Physical and mechanical approaches to gene delivery include techniques such as microinjection, ballistic DNA delivery (gene gun), ultrasound-mediated delivery, and hydrodynamic injection. These methods physically force genetic material into target cells by creating temporary pores in cell membranes or applying pressure. Recent innovations in device design and application protocols have improved the precision and efficiency of these techniques, particularly for ex vivo applications and accessible tissues.
- Enhancers and adjuvants for gene delivery: Various enhancers and adjuvants can significantly improve gene therapy delivery efficiency. These include endosomal escape peptides, nuclear localization signals, and immune modulators that reduce barriers to gene transfer. Chemical enhancers that improve cell membrane permeability or protect nucleic acids from degradation are also employed. Combination approaches that address multiple delivery barriers simultaneously have shown particular promise in overcoming efficiency limitations in gene therapy applications.
02 Non-viral delivery methods
Non-viral gene delivery systems offer advantages in terms of safety, reduced immunogenicity, and larger payload capacity compared to viral vectors. These methods include lipid nanoparticles, polymeric carriers, and physical methods such as electroporation or sonoporation. Recent advancements in non-viral delivery systems have focused on improving cellular uptake, endosomal escape, and nuclear localization to enhance transfection efficiency while maintaining cell viability.Expand Specific Solutions03 Targeted delivery strategies
Targeted delivery approaches aim to increase gene therapy efficiency by directing therapeutic agents to specific cell types or tissues while minimizing off-target effects. These strategies include the use of cell-specific promoters, ligand-receptor interactions, and tissue-specific targeting moieties. By enhancing the specificity of gene delivery, these methods can improve therapeutic outcomes while reducing the required dose and potential side effects.Expand Specific Solutions04 Physical and mechanical delivery techniques
Physical and mechanical methods for gene therapy delivery involve the use of external forces or devices to facilitate the entry of genetic material into target cells. These techniques include ultrasound-mediated delivery, microinjection, gene guns, and hydrodynamic delivery. These approaches can bypass some of the biological barriers that limit conventional delivery methods, potentially increasing transfection efficiency in difficult-to-transduce cell types or tissues.Expand Specific Solutions05 Enhancing cellular uptake and expression
Various strategies have been developed to enhance the cellular uptake of gene therapy vectors and improve the expression of therapeutic genes. These include the use of cell-penetrating peptides, nuclear localization signals, and modifications to overcome intracellular barriers. Additionally, optimization of vector design, promoter selection, and codon usage can significantly impact transgene expression levels and duration, ultimately improving the therapeutic efficacy of gene therapy approaches.Expand Specific Solutions
Leading Companies in CNS Gene Therapy Development
The gene therapy delivery to the central nervous system (CNS) market is in an early growth phase, characterized by significant research activity but limited commercial products. The market size is expanding rapidly, projected to reach several billion dollars by 2030, driven by increasing prevalence of neurological disorders. Technical challenges remain substantial, particularly regarding blood-brain barrier penetration and targeted delivery. Companies like Voyager Therapeutics, REGENXBIO, and Encoded Therapeutics are leading innovation with proprietary AAV vector platforms, while academic institutions including Massachusetts Institute of Technology, Johns Hopkins University, and University of Pennsylvania contribute foundational research. Genzyme and Medtronic bring established pharmaceutical and medical device expertise to address delivery challenges, creating a competitive landscape balanced between specialized biotechs and larger healthcare players.
Trustees of the University of Pennsylvania
Technical Solution: The University of Pennsylvania has developed groundbreaking approaches for CNS gene therapy delivery through their Gene Therapy Program led by Dr. James Wilson. Their platform includes engineered AAV vectors with enhanced BBB penetration capabilities, particularly novel AAV9 variants with up to 5-fold improved CNS transduction efficiency compared to standard AAV9. Penn researchers have pioneered intrathecal delivery techniques that achieve widespread CNS distribution while using significantly lower vector doses than systemic administration. Their technology incorporates cell-type-specific promoters and microRNA-regulated expression cassettes to restrict transgene expression to target CNS cell populations. Penn has also developed innovative manufacturing processes that produce highly pure AAV vectors with improved CNS tropism and reduced peripheral tissue targeting, addressing key challenges in CNS gene therapy delivery.
Strengths: World-leading expertise in AAV vector engineering; validated delivery approaches with extensive preclinical data; strong translational pipeline. Weaknesses: Technology often licensed to commercial partners for clinical development; complex intellectual property landscape; competition from dedicated gene therapy companies.
The Johns Hopkins University
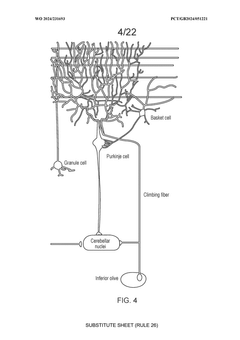
Technical Solution: Johns Hopkins University has developed pioneering approaches for CNS gene therapy delivery, focusing on both vector engineering and innovative administration routes. Their platform includes engineered AAV vectors with enhanced neuronal tropism through capsid modifications that incorporate specific peptide sequences targeting neuronal receptors. Johns Hopkins researchers have developed convection-enhanced delivery (CED) techniques that use pressure gradients to improve vector distribution throughout brain tissue, achieving up to 10-fold greater volume of distribution compared to standard injection methods. Their technology incorporates advanced imaging guidance systems for precise vector delivery to specific CNS structures. Additionally, Johns Hopkins has pioneered the use of stem cell-mediated gene delivery, where genetically modified neural stem cells serve as "factories" for continuous production of therapeutic proteins within the CNS, addressing the challenge of sustained therapeutic effect.
Strengths: Advanced surgical delivery techniques with precise targeting; innovative stem cell-based approaches; strong translational research pipeline. Weaknesses: Some approaches require invasive neurosurgical procedures; limited scalability for certain techniques; complex regulatory pathway for combined cell and gene therapies.
Key Innovations in BBB Penetration Technologies
CNS targeting AAV vectors and methods of use thereof
PatentWO2011133890A1
Innovation
- Recombinant AAV vectors are developed with specific capsid proteins and administration routes, including intrathecal and intracerebral injections, to achieve widespread distribution across CNS tissue, and are engineered to carry transgenes that inhibit harmful protein expression, such as SOD1 in ALS and ASPA in Canavan disease.
method
PatentWO2024231693A1
Innovation
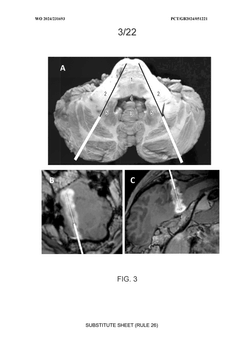
- Convection-enhanced delivery (CED) of viral vectors, specifically targeting the pons and middle cerebellar peduncle, utilizing anterogradely and retrogradely transported vectors to achieve widespread transgene expression in motor and sensory pathways through a trans-cerebellar trajectory, facilitating effective distribution to key neural circuits.
Safety and Immunogenicity Considerations
Gene therapy targeting the central nervous system (CNS) presents unique safety challenges that must be carefully addressed before clinical application. The blood-brain barrier (BBB), while protecting the CNS from harmful substances, also creates significant obstacles for therapeutic delivery. When viral vectors breach this barrier, they can trigger severe immune responses within the delicate neural environment, potentially causing neuroinflammation and tissue damage that may lead to long-term neurological consequences.
Immunogenicity remains a primary concern in CNS gene therapy. Pre-existing neutralizing antibodies against viral vectors, particularly adeno-associated viruses (AAVs), can significantly reduce therapeutic efficacy and increase safety risks. Studies indicate that 30-70% of the human population carries antibodies against various AAV serotypes, necessitating comprehensive screening protocols before treatment. Furthermore, even in antibody-negative patients, the introduction of viral vectors can stimulate adaptive immune responses that may eliminate transduced cells and reduce therapeutic durability.
The risk of insertional mutagenesis presents another critical safety consideration, particularly with integrating vectors like lentiviruses. While AAVs predominantly remain episomal, integration events do occur at low frequencies and could potentially disrupt tumor suppressor genes or activate oncogenes in neural progenitor cells. Long-term safety monitoring protocols are essential as CNS gene therapy effects may manifest years after administration.
Dose-dependent toxicity represents a significant challenge in CNS gene therapy development. High vector doses, often necessary to achieve therapeutic transgene expression across sufficient neural tissue, can trigger severe adverse events including hepatotoxicity and thrombotic microangiopathy. Recent clinical trials have documented fatal cases associated with high-dose systemic AAV administration, highlighting the critical importance of dose optimization strategies.
Off-target expression in non-CNS tissues remains problematic despite advances in vector engineering. Even with CNS-directed delivery methods, vector leakage into peripheral circulation can lead to transgene expression in unintended tissues, potentially causing systemic complications. This risk is particularly pronounced with intrathecal or intraventricular administration approaches, where cerebrospinal fluid dynamics can facilitate vector redistribution.
Overexpression toxicity presents unique challenges in the CNS environment, where precise regulation of transgene expression is crucial for maintaining neural circuit homeostasis. Excessive production of therapeutic proteins can disrupt delicate neuronal signaling pathways and potentially trigger excitotoxicity or other forms of cellular stress. Development of regulatable expression systems with CNS compatibility represents an important frontier in addressing these safety concerns.
Immunogenicity remains a primary concern in CNS gene therapy. Pre-existing neutralizing antibodies against viral vectors, particularly adeno-associated viruses (AAVs), can significantly reduce therapeutic efficacy and increase safety risks. Studies indicate that 30-70% of the human population carries antibodies against various AAV serotypes, necessitating comprehensive screening protocols before treatment. Furthermore, even in antibody-negative patients, the introduction of viral vectors can stimulate adaptive immune responses that may eliminate transduced cells and reduce therapeutic durability.
The risk of insertional mutagenesis presents another critical safety consideration, particularly with integrating vectors like lentiviruses. While AAVs predominantly remain episomal, integration events do occur at low frequencies and could potentially disrupt tumor suppressor genes or activate oncogenes in neural progenitor cells. Long-term safety monitoring protocols are essential as CNS gene therapy effects may manifest years after administration.
Dose-dependent toxicity represents a significant challenge in CNS gene therapy development. High vector doses, often necessary to achieve therapeutic transgene expression across sufficient neural tissue, can trigger severe adverse events including hepatotoxicity and thrombotic microangiopathy. Recent clinical trials have documented fatal cases associated with high-dose systemic AAV administration, highlighting the critical importance of dose optimization strategies.
Off-target expression in non-CNS tissues remains problematic despite advances in vector engineering. Even with CNS-directed delivery methods, vector leakage into peripheral circulation can lead to transgene expression in unintended tissues, potentially causing systemic complications. This risk is particularly pronounced with intrathecal or intraventricular administration approaches, where cerebrospinal fluid dynamics can facilitate vector redistribution.
Overexpression toxicity presents unique challenges in the CNS environment, where precise regulation of transgene expression is crucial for maintaining neural circuit homeostasis. Excessive production of therapeutic proteins can disrupt delicate neuronal signaling pathways and potentially trigger excitotoxicity or other forms of cellular stress. Development of regulatable expression systems with CNS compatibility represents an important frontier in addressing these safety concerns.
Regulatory Pathway for CNS Gene Therapies
The regulatory landscape for gene therapies targeting the central nervous system (CNS) presents a complex and evolving framework that developers must navigate carefully. The U.S. Food and Drug Administration (FDA) has established specific pathways for these advanced therapeutic products under the Center for Biologics Evaluation and Research (CBER), with particular emphasis on the Office of Tissues and Advanced Therapies (OTAT). These regulatory bodies have developed specialized guidance documents addressing the unique challenges of CNS-targeted gene therapies.
For CNS gene therapies, the regulatory pathway typically begins with extensive preclinical testing that must specifically address blood-brain barrier penetration, neuronal tropism, and long-term expression in neural tissues. Unlike conventional therapeutics, regulators require comprehensive biodistribution studies with particular focus on off-target effects in the CNS and potential for germline transmission.
The Investigational New Drug (IND) application for CNS gene therapies demands robust chemistry, manufacturing, and controls (CMC) documentation, with heightened scrutiny on viral vector characterization and stability in neural environments. Regulatory agencies typically require enhanced safety monitoring protocols due to the irreversible nature of gene therapy and the sensitive target tissue.
Accelerated approval pathways have become increasingly important for CNS gene therapies, particularly for rare neurological disorders. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the European Medicines Agency's Priority Medicines (PRIME) scheme offer expedited review processes for promising CNS gene therapies addressing unmet medical needs. These pathways provide opportunities for earlier and more frequent interactions with regulators.
Post-approval requirements present unique challenges, with regulatory authorities mandating long-term follow-up studies ranging from 5 to 15 years depending on the vector system and integration potential. This extended monitoring period reflects concerns about delayed adverse events in neural tissues and the limited understanding of long-term gene expression patterns in the CNS.
International regulatory harmonization remains incomplete, creating challenges for global development programs. While the International Council for Harmonisation (ICH) has made progress in standardizing certain aspects of gene therapy regulation, significant differences persist in requirements for CNS-targeted therapies across major markets including the US, EU, Japan, and China.
Recent regulatory trends indicate movement toward more adaptive approaches for CNS gene therapies, including the acceptance of novel endpoints specific to neurological disorders and increased flexibility in clinical trial designs. Regulatory agencies are increasingly incorporating patient experience data and real-world evidence into their assessment frameworks, recognizing the unique challenges in demonstrating efficacy for rare neurological conditions.
For CNS gene therapies, the regulatory pathway typically begins with extensive preclinical testing that must specifically address blood-brain barrier penetration, neuronal tropism, and long-term expression in neural tissues. Unlike conventional therapeutics, regulators require comprehensive biodistribution studies with particular focus on off-target effects in the CNS and potential for germline transmission.
The Investigational New Drug (IND) application for CNS gene therapies demands robust chemistry, manufacturing, and controls (CMC) documentation, with heightened scrutiny on viral vector characterization and stability in neural environments. Regulatory agencies typically require enhanced safety monitoring protocols due to the irreversible nature of gene therapy and the sensitive target tissue.
Accelerated approval pathways have become increasingly important for CNS gene therapies, particularly for rare neurological disorders. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the European Medicines Agency's Priority Medicines (PRIME) scheme offer expedited review processes for promising CNS gene therapies addressing unmet medical needs. These pathways provide opportunities for earlier and more frequent interactions with regulators.
Post-approval requirements present unique challenges, with regulatory authorities mandating long-term follow-up studies ranging from 5 to 15 years depending on the vector system and integration potential. This extended monitoring period reflects concerns about delayed adverse events in neural tissues and the limited understanding of long-term gene expression patterns in the CNS.
International regulatory harmonization remains incomplete, creating challenges for global development programs. While the International Council for Harmonisation (ICH) has made progress in standardizing certain aspects of gene therapy regulation, significant differences persist in requirements for CNS-targeted therapies across major markets including the US, EU, Japan, and China.
Recent regulatory trends indicate movement toward more adaptive approaches for CNS gene therapies, including the acceptance of novel endpoints specific to neurological disorders and increased flexibility in clinical trial designs. Regulatory agencies are increasingly incorporating patient experience data and real-world evidence into their assessment frameworks, recognizing the unique challenges in demonstrating efficacy for rare neurological conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!