The Influence of Advances in Nanotechnology on Gene Therapy Methods
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanotechnology in Gene Therapy: Background and Objectives
Nanotechnology has emerged as a transformative force in the field of gene therapy over the past two decades, representing a convergence of molecular engineering and genetic medicine. The evolution of nanotechnology began in the 1980s with theoretical concepts, but practical applications in gene therapy only materialized in the early 2000s when researchers successfully demonstrated the first nano-carriers for genetic material. This technological progression has been characterized by increasingly sophisticated delivery systems that address the fundamental challenges of gene therapy: efficient cellular targeting, protection of genetic cargo, and controlled release mechanisms.
The current trajectory of nanotechnology in gene therapy is moving toward more precise, biocompatible, and multifunctional nanoplatforms. Recent advances in materials science have enabled the development of stimuli-responsive nanoparticles that can navigate biological barriers and release genetic payloads under specific conditions. Concurrently, progress in surface modification techniques has improved the targeting capabilities of these nano-vehicles, enhancing their therapeutic efficacy while minimizing off-target effects.
The primary technical objective in this field is to develop nanotechnology-based delivery systems that overcome the biological barriers impeding effective gene therapy. These barriers include nuclease degradation, endosomal entrapment, and inefficient nuclear localization of therapeutic genes. Additionally, researchers aim to create nanoplatforms that can simultaneously deliver multiple therapeutic agents, enabling combination therapies that address complex genetic disorders through synergistic mechanisms.
Another critical objective is to enhance the safety profile of gene therapy by designing nanocarriers that minimize immunogenicity and cytotoxicity. This involves engineering biodegradable nanomaterials with predictable clearance pathways and developing surface chemistries that evade immune surveillance. The field is also focused on improving the scalability and reproducibility of nanomanufacturing processes to facilitate clinical translation and regulatory approval.
Looking forward, the integration of nanotechnology with emerging technologies such as CRISPR-Cas9 gene editing represents a promising frontier. Researchers are exploring nano-delivery systems that can efficiently transport gene-editing components to specific tissues and cell types, potentially revolutionizing the treatment of monogenic disorders. Additionally, the development of "smart" nanoparticles capable of responding to the physiological microenvironment could enable spatiotemporally controlled gene expression, opening new avenues for personalized medicine.
The ultimate goal of this technological convergence is to establish gene therapy as a mainstream therapeutic modality for a broad spectrum of diseases, from rare genetic disorders to cancer and infectious diseases. This requires not only technical innovations but also addressing regulatory challenges and ensuring cost-effectiveness to maximize patient access to these potentially curative treatments.
The current trajectory of nanotechnology in gene therapy is moving toward more precise, biocompatible, and multifunctional nanoplatforms. Recent advances in materials science have enabled the development of stimuli-responsive nanoparticles that can navigate biological barriers and release genetic payloads under specific conditions. Concurrently, progress in surface modification techniques has improved the targeting capabilities of these nano-vehicles, enhancing their therapeutic efficacy while minimizing off-target effects.
The primary technical objective in this field is to develop nanotechnology-based delivery systems that overcome the biological barriers impeding effective gene therapy. These barriers include nuclease degradation, endosomal entrapment, and inefficient nuclear localization of therapeutic genes. Additionally, researchers aim to create nanoplatforms that can simultaneously deliver multiple therapeutic agents, enabling combination therapies that address complex genetic disorders through synergistic mechanisms.
Another critical objective is to enhance the safety profile of gene therapy by designing nanocarriers that minimize immunogenicity and cytotoxicity. This involves engineering biodegradable nanomaterials with predictable clearance pathways and developing surface chemistries that evade immune surveillance. The field is also focused on improving the scalability and reproducibility of nanomanufacturing processes to facilitate clinical translation and regulatory approval.
Looking forward, the integration of nanotechnology with emerging technologies such as CRISPR-Cas9 gene editing represents a promising frontier. Researchers are exploring nano-delivery systems that can efficiently transport gene-editing components to specific tissues and cell types, potentially revolutionizing the treatment of monogenic disorders. Additionally, the development of "smart" nanoparticles capable of responding to the physiological microenvironment could enable spatiotemporally controlled gene expression, opening new avenues for personalized medicine.
The ultimate goal of this technological convergence is to establish gene therapy as a mainstream therapeutic modality for a broad spectrum of diseases, from rare genetic disorders to cancer and infectious diseases. This requires not only technical innovations but also addressing regulatory challenges and ensuring cost-effectiveness to maximize patient access to these potentially curative treatments.
Market Analysis of Nano-enabled Gene Therapy Solutions
The global market for nano-enabled gene therapy solutions has experienced remarkable growth in recent years, driven by significant technological advancements and increasing investment in research and development. The market was valued at approximately $2.5 billion in 2022 and is projected to reach $7.8 billion by 2028, representing a compound annual growth rate (CAGR) of 20.9%. This substantial growth trajectory reflects the expanding applications of nanotechnology in addressing previously untreatable genetic disorders.
North America currently dominates the market landscape, accounting for roughly 42% of the global market share, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. This regional distribution correlates strongly with healthcare expenditure, regulatory frameworks favorable to advanced therapies, and concentration of biotechnology research institutions.
From a therapeutic application perspective, oncology represents the largest segment, comprising approximately 35% of the market. This is followed by rare genetic disorders (25%), cardiovascular diseases (15%), neurological disorders (12%), and other applications (13%). The oncology segment's dominance can be attributed to the high prevalence of cancer globally and the significant funding directed toward cancer research.
Key market drivers include increasing prevalence of genetic disorders, growing adoption of precision medicine approaches, advancements in nanoparticle engineering, and favorable regulatory pathways for gene therapy products. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation has particularly accelerated the development timeline for promising gene therapy candidates, including those utilizing nanotechnology delivery systems.
Market restraints primarily revolve around high treatment costs, manufacturing challenges, safety concerns related to nanoparticle toxicity, and stringent regulatory requirements. The average cost of nano-enabled gene therapy treatments ranges from $250,000 to over $2 million per patient, creating significant reimbursement challenges and limiting market penetration in developing economies.
Emerging trends in the market include the development of non-viral nanocarriers as alternatives to viral vectors, integration of CRISPR-Cas9 technology with nanoparticle delivery systems, and increasing focus on targeted delivery to reduce off-target effects. Additionally, there is growing interest in developing nano-enabled gene therapy solutions for in vivo applications, which could significantly expand the addressable market by eliminating the need for ex vivo cell manipulation.
The competitive landscape is characterized by a mix of established pharmaceutical companies, specialized biotech firms, and academic research institutions. Strategic collaborations between nanotechnology experts and gene therapy developers have become increasingly common, driving innovation and accelerating commercialization timelines.
North America currently dominates the market landscape, accounting for roughly 42% of the global market share, followed by Europe at 28% and Asia-Pacific at 22%. The remaining 8% is distributed across other regions. This regional distribution correlates strongly with healthcare expenditure, regulatory frameworks favorable to advanced therapies, and concentration of biotechnology research institutions.
From a therapeutic application perspective, oncology represents the largest segment, comprising approximately 35% of the market. This is followed by rare genetic disorders (25%), cardiovascular diseases (15%), neurological disorders (12%), and other applications (13%). The oncology segment's dominance can be attributed to the high prevalence of cancer globally and the significant funding directed toward cancer research.
Key market drivers include increasing prevalence of genetic disorders, growing adoption of precision medicine approaches, advancements in nanoparticle engineering, and favorable regulatory pathways for gene therapy products. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation has particularly accelerated the development timeline for promising gene therapy candidates, including those utilizing nanotechnology delivery systems.
Market restraints primarily revolve around high treatment costs, manufacturing challenges, safety concerns related to nanoparticle toxicity, and stringent regulatory requirements. The average cost of nano-enabled gene therapy treatments ranges from $250,000 to over $2 million per patient, creating significant reimbursement challenges and limiting market penetration in developing economies.
Emerging trends in the market include the development of non-viral nanocarriers as alternatives to viral vectors, integration of CRISPR-Cas9 technology with nanoparticle delivery systems, and increasing focus on targeted delivery to reduce off-target effects. Additionally, there is growing interest in developing nano-enabled gene therapy solutions for in vivo applications, which could significantly expand the addressable market by eliminating the need for ex vivo cell manipulation.
The competitive landscape is characterized by a mix of established pharmaceutical companies, specialized biotech firms, and academic research institutions. Strategic collaborations between nanotechnology experts and gene therapy developers have become increasingly common, driving innovation and accelerating commercialization timelines.
Current Landscape and Technical Barriers in Nanomedicine
Nanomedicine represents the convergence of nanotechnology with medical science, offering unprecedented opportunities for targeted drug delivery, diagnostics, and therapeutic interventions. Currently, the global nanomedicine market is experiencing robust growth, valued at approximately $160 billion in 2023, with projections suggesting it could reach $350 billion by 2030. This growth is primarily driven by applications in oncology, neurology, cardiovascular diseases, and infectious diseases.
The current landscape of nanomedicine is characterized by diverse nanocarrier systems including liposomes, polymeric nanoparticles, dendrimers, carbon nanotubes, and metallic nanoparticles. Among these, lipid nanoparticles (LNPs) have gained significant attention following their successful implementation in mRNA COVID-19 vaccines, demonstrating their potential for nucleic acid delivery in gene therapy applications.
Despite promising advancements, nanomedicine faces substantial technical barriers that impede its widespread clinical adoption. The foremost challenge is achieving consistent and scalable manufacturing processes. Current production methods often result in batch-to-batch variability, affecting the physicochemical properties of nanoparticles and consequently their biological performance. This inconsistency poses significant regulatory hurdles and complicates clinical translation.
Another critical challenge is the biological barriers that nanoparticles must overcome. The reticuloendothelial system rapidly clears foreign particles from circulation, while the blood-brain barrier restricts access to central nervous system targets. Additionally, the tumor microenvironment presents complex obstacles including heterogeneous vasculature and elevated interstitial fluid pressure, limiting nanoparticle penetration and distribution.
Toxicity concerns also persist as a significant barrier. The long-term effects of nanomaterials in the human body remain incompletely understood. Potential issues include immunogenicity, complement activation, and accumulation in non-target tissues. These safety considerations necessitate extensive preclinical testing, further extending development timelines.
From a regulatory perspective, nanomedicines face a complex approval pathway. Regulatory agencies worldwide are still developing standardized frameworks for evaluating these novel therapeutics. The lack of harmonized guidelines creates uncertainty for developers and investors, potentially slowing innovation and market entry.
Lastly, the translation of nanomedicine from laboratory to clinic faces economic barriers. The high costs associated with development, manufacturing, and clinical trials can be prohibitive, particularly for startups and academic institutions. This economic reality often necessitates partnerships with pharmaceutical companies, potentially limiting the diversity of approaches that reach clinical testing.
These technical and practical challenges collectively represent the current bottlenecks in nanomedicine development, particularly as they relate to gene therapy applications. Addressing these barriers requires interdisciplinary collaboration among materials scientists, pharmaceutical technologists, clinicians, and regulatory experts.
The current landscape of nanomedicine is characterized by diverse nanocarrier systems including liposomes, polymeric nanoparticles, dendrimers, carbon nanotubes, and metallic nanoparticles. Among these, lipid nanoparticles (LNPs) have gained significant attention following their successful implementation in mRNA COVID-19 vaccines, demonstrating their potential for nucleic acid delivery in gene therapy applications.
Despite promising advancements, nanomedicine faces substantial technical barriers that impede its widespread clinical adoption. The foremost challenge is achieving consistent and scalable manufacturing processes. Current production methods often result in batch-to-batch variability, affecting the physicochemical properties of nanoparticles and consequently their biological performance. This inconsistency poses significant regulatory hurdles and complicates clinical translation.
Another critical challenge is the biological barriers that nanoparticles must overcome. The reticuloendothelial system rapidly clears foreign particles from circulation, while the blood-brain barrier restricts access to central nervous system targets. Additionally, the tumor microenvironment presents complex obstacles including heterogeneous vasculature and elevated interstitial fluid pressure, limiting nanoparticle penetration and distribution.
Toxicity concerns also persist as a significant barrier. The long-term effects of nanomaterials in the human body remain incompletely understood. Potential issues include immunogenicity, complement activation, and accumulation in non-target tissues. These safety considerations necessitate extensive preclinical testing, further extending development timelines.
From a regulatory perspective, nanomedicines face a complex approval pathway. Regulatory agencies worldwide are still developing standardized frameworks for evaluating these novel therapeutics. The lack of harmonized guidelines creates uncertainty for developers and investors, potentially slowing innovation and market entry.
Lastly, the translation of nanomedicine from laboratory to clinic faces economic barriers. The high costs associated with development, manufacturing, and clinical trials can be prohibitive, particularly for startups and academic institutions. This economic reality often necessitates partnerships with pharmaceutical companies, potentially limiting the diversity of approaches that reach clinical testing.
These technical and practical challenges collectively represent the current bottlenecks in nanomedicine development, particularly as they relate to gene therapy applications. Addressing these barriers requires interdisciplinary collaboration among materials scientists, pharmaceutical technologists, clinicians, and regulatory experts.
Existing Nanoparticle Platforms for Gene Delivery
01 Nanoparticle delivery systems for gene therapy
Nanoparticles serve as effective delivery vehicles for genetic material in gene therapy applications. These systems protect nucleic acids from degradation and facilitate cellular uptake. Various types of nanoparticles, including lipid nanoparticles, polymeric nanoparticles, and inorganic nanoparticles, have been developed to enhance transfection efficiency and target specific tissues. These delivery systems overcome biological barriers and improve the stability of genetic material during transport to target cells.- Nanoparticle delivery systems for gene therapy: Nanoparticles serve as effective delivery vehicles for genetic material in gene therapy applications. These systems protect nucleic acids from degradation and facilitate cellular uptake. Various types of nanoparticles, including lipid nanoparticles, polymeric nanoparticles, and inorganic nanoparticles, have been developed to enhance transfection efficiency and targeting specificity. These delivery systems overcome biological barriers and improve the therapeutic efficacy of gene therapy treatments.
- CRISPR-Cas9 delivery using nanotechnology: Nanotechnology has significantly advanced the delivery of CRISPR-Cas9 gene editing tools. Nanocarriers designed specifically for CRISPR components protect the large ribonucleoprotein complexes from degradation and immune recognition while facilitating their transport across cellular membranes. These nanotechnology-based delivery systems improve editing efficiency, reduce off-target effects, and enhance the safety profile of CRISPR-based gene therapies, making precise genetic modifications more achievable in clinical settings.
- Targeted gene delivery using functionalized nanomaterials: Functionalized nanomaterials with targeting ligands enable site-specific delivery of genetic material to diseased tissues or specific cell types. Surface modifications of nanoparticles with antibodies, peptides, aptamers, or small molecules enhance binding to target cells while minimizing off-target effects. This targeted approach increases therapeutic efficacy, reduces systemic toxicity, and allows for lower dosing requirements in gene therapy applications, representing a significant advancement in precision medicine.
- Stimuli-responsive nanocarriers for controlled gene release: Stimuli-responsive nanocarriers release genetic payloads in response to specific triggers such as pH changes, temperature variations, enzymatic activity, or external stimuli like light or ultrasound. These smart delivery systems ensure that therapeutic genes are released precisely at the target site and at the optimal time. The controlled release mechanisms improve transfection efficiency, reduce side effects, and allow for temporal control over gene expression, representing a significant advancement in gene therapy technology.
- Non-viral gene delivery nanosystems: Non-viral nanosystems offer safer alternatives to viral vectors for gene delivery. These include lipid nanoparticles, polymeric nanocarriers, dendrimers, and inorganic nanoparticles engineered to efficiently deliver genetic material without the immunogenicity and insertional mutagenesis risks associated with viral vectors. Recent advances in non-viral nanosystems have significantly improved transfection efficiency, reduced cytotoxicity, and enhanced stability, making them increasingly viable options for clinical gene therapy applications.
02 Targeted gene delivery using nanotechnology
Nanotechnology enables precise targeting of gene therapies to specific cells or tissues, improving therapeutic efficacy while reducing off-target effects. Nanoparticles can be functionalized with targeting ligands, antibodies, or peptides that recognize receptors on target cells. This targeted approach enhances the specificity of gene delivery, allowing for lower doses and minimizing potential side effects. Advanced targeting strategies include stimuli-responsive nanoparticles that release their genetic payload under specific physiological conditions.Expand Specific Solutions03 CRISPR-Cas delivery using nanomaterials
Nanomaterials have revolutionized the delivery of CRISPR-Cas gene editing systems, addressing key challenges in this emerging therapeutic approach. Nanoparticle formulations protect the CRISPR components from degradation and immune recognition while facilitating their entry into target cells. Various nanomaterial platforms, including lipid nanoparticles, gold nanoparticles, and polymeric nanocarriers, have been developed specifically for CRISPR delivery. These systems improve editing efficiency and reduce off-target effects in gene therapy applications.Expand Specific Solutions04 Non-viral gene delivery nanosystems
Non-viral nanosystems offer safer alternatives to viral vectors for gene therapy, with reduced immunogenicity and improved loading capacity. These systems include lipid nanoparticles, polymeric nanocarriers, dendrimers, and inorganic nanoparticles engineered to efficiently deliver genetic material. Advances in non-viral delivery include the development of biodegradable materials, stimuli-responsive release mechanisms, and surface modifications that enhance cellular uptake. These nanosystems address safety concerns associated with viral vectors while maintaining efficient gene transfer.Expand Specific Solutions05 Nanomaterial-based RNA therapeutics delivery
Nanomaterials have enabled significant advances in the delivery of RNA therapeutics, including mRNA vaccines and siRNA-based gene silencing therapies. These delivery systems protect RNA molecules from degradation by nucleases and facilitate their cellular uptake and endosomal escape. Lipid nanoparticles, polymeric nanocarriers, and hybrid nanomaterials have been developed specifically for RNA delivery, with optimized formulations that enhance stability and transfection efficiency. These nanotechnology platforms have been crucial for the clinical translation of RNA-based gene therapies.Expand Specific Solutions
Leading Organizations in Nanomedicine and Gene Therapy
The nanotechnology-gene therapy landscape is evolving rapidly, currently in a growth phase characterized by increasing clinical applications and expanding market potential, projected to reach $18-20 billion by 2025. Academic institutions like Yale University, Harvard College, and Johns Hopkins University are driving fundamental research, while pharmaceutical companies including Otsuka Pharmaceutical and Arrowhead Pharmaceuticals are advancing clinical translation. Research centers such as the National Center for Nanoscience & Technology and specialized biotech firms like Sapreme Technologies and Yoltech Therapeutics are developing novel delivery systems. The field is transitioning from experimental to clinical implementation, with increasing collaboration between academia and industry accelerating the development of targeted gene delivery platforms using nanoscale carriers.
President & Fellows of Harvard College
Technical Solution: Harvard University researchers have developed several groundbreaking nanotechnology platforms that significantly advance gene therapy approaches. Their work includes the development of lipid-derived nanoparticles with precisely engineered structures that enhance cellular uptake and endosomal escape of nucleic acid therapeutics. Harvard's technology incorporates ionizable lipids with optimized pKa values that facilitate efficient complexation with nucleic acids at acidic pH while promoting release in the neutral cytoplasmic environment. Their researchers have pioneered combinatorial approaches to nanoparticle design, utilizing high-throughput screening methods to identify optimal formulations for specific tissue targeting and gene delivery applications. Harvard has also developed DNA-origami nanostructures that can carry multiple therapeutic payloads simultaneously, enabling combination gene therapy approaches. Additionally, their teams have created stimuli-responsive nanoparticles that can respond to tumor microenvironment conditions, such as matrix metalloproteinases or reduced pH, to trigger the release of therapeutic genes specifically at disease sites. These technologies have shown promising results in treating genetic disorders, cancer, and infectious diseases in preclinical models.
Strengths: Sophisticated design approaches incorporating computational modeling and high-throughput screening; versatility across multiple therapeutic applications; advanced control over biodistribution and targeting. Weaknesses: Some platforms involve complex manufacturing processes that present scalability challenges; potential regulatory hurdles for novel nanomaterial compositions; variable performance across different genetic cargoes and target tissues.
Sapreme Technologies BV
Technical Solution: Sapreme Technologies has developed an innovative nanotechnology platform called ENDOSCAPE™ that specifically addresses the endosomal escape bottleneck in gene therapy delivery. Their approach utilizes proprietary compounds (SPT compounds) that selectively permeabilize endosomal membranes, significantly enhancing the cytosolic delivery of therapeutic payloads. The technology can be applied either through direct conjugation to antibodies or other targeting moieties, or by co-administration with existing delivery systems such as lipid nanoparticles (LNPs) or antibody-drug conjugates. Sapreme's nanoparticles incorporate specialized peptides that interact with endosomal membranes under acidic conditions, creating temporary pores that allow therapeutic nucleic acids to escape into the cytoplasm before lysosomal degradation occurs. This platform has demonstrated up to 20-fold improvement in functional delivery efficiency compared to conventional methods in preclinical studies. The company has successfully applied this technology to enhance the delivery of various genetic cargoes including siRNA, mRNA, and antisense oligonucleotides across multiple cell types and tissues.
Strengths: Exceptional endosomal escape efficiency that addresses a major bottleneck in gene therapy; compatibility with existing delivery technologies; versatility across different therapeutic modalities. Weaknesses: Relatively early-stage technology with limited clinical validation; potential cytotoxicity concerns with membrane-disrupting mechanisms; challenges in achieving consistent performance across different tissue types and disease states.
Key Patents and Breakthroughs in Nano-Gene Therapy
Compositions and methods for delivery of nucleic acids to cells
PatentActiveUS11850284B2
Innovation
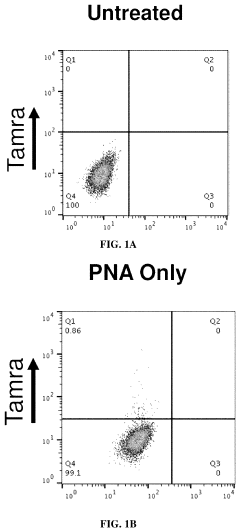
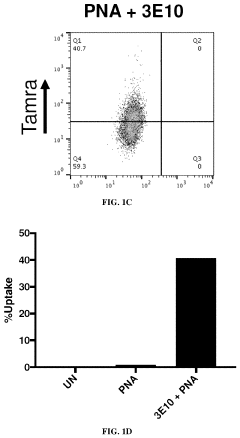
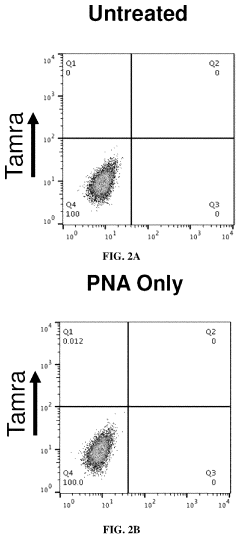
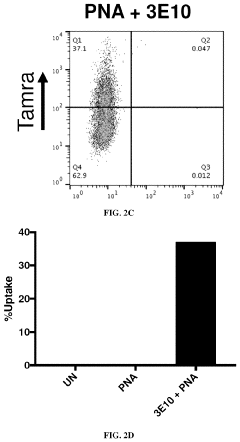
- The use of 3E10 monoclonal antibodies or their fragments, which form non-covalent complexes with nucleic acid cargo, facilitating their delivery into cells by binding to DNA, RNA, and other nucleic acids, enabling efficient intracellular uptake without the need for carrier lipids.
Nanoparticle delivery vehicle
PatentInactiveEP1450751A2
Innovation
- A nanoparticle delivery vehicle comprising a nanoparticle, an active agent, and sequences like nuclear localization signals (NLS) or cell surface recognition sequences, which can be designed to target specific cellular locations, including the nucleus or cytoplasm, and are formulated with protective coatings and biocompatibility enhancements.
Regulatory Framework for Nanomedicine-Based Therapeutics
The regulatory landscape for nanomedicine-based therapeutics, particularly in the context of gene therapy applications, presents a complex and evolving framework that significantly impacts research, development, and commercialization pathways. Current regulatory approaches vary considerably across different jurisdictions, creating challenges for global development strategies.
In the United States, the FDA has established a multi-tiered regulatory framework for nanomedicine products through collaborative efforts between the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and the Nanotechnology Task Force. Gene therapy products incorporating nanotechnology typically undergo review through the Investigational New Drug (IND) pathway, with additional considerations for nanoscale components.
The European Medicines Agency (EMA) has developed specific guidelines for nanomedicines through its Innovation Task Force, emphasizing characterization requirements for nanoparticle-based delivery systems. The Committee for Advanced Therapies (CAT) provides specialized oversight for gene therapy products, with additional protocols when nanotechnology elements are incorporated.
Safety assessment frameworks for nanomedicine-based gene therapies require specialized considerations beyond conventional therapeutics. Regulatory bodies increasingly demand comprehensive characterization of nanoparticle size distribution, surface properties, stability, and biodistribution profiles. The potential for unique toxicity mechanisms, including immunogenicity concerns and long-term accumulation effects, necessitates expanded preclinical testing requirements.
Harmonization efforts are underway through international collaborations such as the International Council for Harmonisation (ICH) and the International Pharmaceutical Regulators Programme (IPRP) Nanomedicines Working Group. These initiatives aim to standardize testing methodologies and establish consistent regulatory expectations across markets, though significant regional differences persist.
Accelerated approval pathways are increasingly available for nanomedicine-based gene therapies targeting serious conditions with unmet medical needs. The FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme offer opportunities for expedited development and review, though qualifying products must still demonstrate compelling preliminary clinical evidence.
Emerging regulatory considerations include the development of reference standards for nanomaterials, post-market surveillance requirements specific to nanomedicine products, and evolving guidelines for combination products that incorporate both nanotechnology and genetic components. Regulatory agencies are also addressing manufacturing challenges unique to nanomedicine-based gene therapies, including scale-up consistency and quality control parameters.
As the field advances, regulatory frameworks will likely continue to evolve, balancing the need to ensure patient safety while enabling innovation in this promising therapeutic area. Companies developing nanomedicine-based gene therapies must maintain active engagement with regulatory authorities throughout the development process to navigate this complex landscape effectively.
In the United States, the FDA has established a multi-tiered regulatory framework for nanomedicine products through collaborative efforts between the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and the Nanotechnology Task Force. Gene therapy products incorporating nanotechnology typically undergo review through the Investigational New Drug (IND) pathway, with additional considerations for nanoscale components.
The European Medicines Agency (EMA) has developed specific guidelines for nanomedicines through its Innovation Task Force, emphasizing characterization requirements for nanoparticle-based delivery systems. The Committee for Advanced Therapies (CAT) provides specialized oversight for gene therapy products, with additional protocols when nanotechnology elements are incorporated.
Safety assessment frameworks for nanomedicine-based gene therapies require specialized considerations beyond conventional therapeutics. Regulatory bodies increasingly demand comprehensive characterization of nanoparticle size distribution, surface properties, stability, and biodistribution profiles. The potential for unique toxicity mechanisms, including immunogenicity concerns and long-term accumulation effects, necessitates expanded preclinical testing requirements.
Harmonization efforts are underway through international collaborations such as the International Council for Harmonisation (ICH) and the International Pharmaceutical Regulators Programme (IPRP) Nanomedicines Working Group. These initiatives aim to standardize testing methodologies and establish consistent regulatory expectations across markets, though significant regional differences persist.
Accelerated approval pathways are increasingly available for nanomedicine-based gene therapies targeting serious conditions with unmet medical needs. The FDA's Breakthrough Therapy designation and the EMA's PRIME (PRIority MEdicines) scheme offer opportunities for expedited development and review, though qualifying products must still demonstrate compelling preliminary clinical evidence.
Emerging regulatory considerations include the development of reference standards for nanomaterials, post-market surveillance requirements specific to nanomedicine products, and evolving guidelines for combination products that incorporate both nanotechnology and genetic components. Regulatory agencies are also addressing manufacturing challenges unique to nanomedicine-based gene therapies, including scale-up consistency and quality control parameters.
As the field advances, regulatory frameworks will likely continue to evolve, balancing the need to ensure patient safety while enabling innovation in this promising therapeutic area. Companies developing nanomedicine-based gene therapies must maintain active engagement with regulatory authorities throughout the development process to navigate this complex landscape effectively.
Safety and Biocompatibility Considerations of Nanocarriers
The safety and biocompatibility of nanocarriers represent critical considerations in the advancement of nanotechnology-based gene therapy methods. As these novel delivery systems interact directly with biological systems, their potential toxicity profiles must be thoroughly evaluated before clinical implementation. Current research indicates that nanoparticle size, shape, surface charge, and chemical composition significantly influence their biocompatibility profiles, with smaller particles generally demonstrating greater cellular penetration but potentially higher toxicity risks.
Immune system recognition presents a substantial challenge, as many nanocarriers trigger inflammatory responses or complement activation upon administration. This immunogenicity can lead to rapid clearance of therapeutic agents, reduced efficacy, and potentially severe adverse reactions in patients. Recent advances have focused on developing "stealth" nanoparticles with surface modifications such as PEGylation to reduce immune recognition, though even these approaches face challenges with complement activation and anti-PEG antibody development after repeated administration.
Biodegradability and elimination pathways constitute another critical safety dimension. Ideal nanocarriers should degrade into non-toxic byproducts that can be readily eliminated through normal physiological processes. Non-biodegradable nanoparticles risk accumulation in tissues, particularly the liver and spleen, potentially leading to long-term toxicity concerns. The field has consequently witnessed increased development of biodegradable polymeric nanoparticles and lipid-based systems with predictable degradation profiles.
Regulatory frameworks for evaluating nanocarrier safety continue to evolve as understanding of nanotoxicology advances. Current assessment protocols include in vitro cytotoxicity screening, hemolysis assays, complement activation tests, and comprehensive in vivo toxicology studies examining biodistribution, organ accumulation, and long-term effects. The FDA and EMA have developed specialized guidance documents for nanomedicine evaluation, though harmonization of international standards remains an ongoing challenge.
Emerging research directions include the development of predictive in silico models to forecast nanocarrier-biological interactions, reducing reliance on animal testing while improving safety prediction accuracy. Additionally, researchers are exploring biomimetic approaches, designing nanocarriers that mimic endogenous cellular components to enhance biocompatibility. Cell membrane-coated nanoparticles represent one promising strategy, leveraging natural cellular components to improve safety profiles while maintaining therapeutic efficacy.
The balance between therapeutic efficacy and safety remains the central challenge in nanocarrier development for gene therapy applications. Future advances will likely focus on personalized approaches that consider individual patient characteristics in nanocarrier design, potentially reducing adverse effects while maximizing therapeutic outcomes.
Immune system recognition presents a substantial challenge, as many nanocarriers trigger inflammatory responses or complement activation upon administration. This immunogenicity can lead to rapid clearance of therapeutic agents, reduced efficacy, and potentially severe adverse reactions in patients. Recent advances have focused on developing "stealth" nanoparticles with surface modifications such as PEGylation to reduce immune recognition, though even these approaches face challenges with complement activation and anti-PEG antibody development after repeated administration.
Biodegradability and elimination pathways constitute another critical safety dimension. Ideal nanocarriers should degrade into non-toxic byproducts that can be readily eliminated through normal physiological processes. Non-biodegradable nanoparticles risk accumulation in tissues, particularly the liver and spleen, potentially leading to long-term toxicity concerns. The field has consequently witnessed increased development of biodegradable polymeric nanoparticles and lipid-based systems with predictable degradation profiles.
Regulatory frameworks for evaluating nanocarrier safety continue to evolve as understanding of nanotoxicology advances. Current assessment protocols include in vitro cytotoxicity screening, hemolysis assays, complement activation tests, and comprehensive in vivo toxicology studies examining biodistribution, organ accumulation, and long-term effects. The FDA and EMA have developed specialized guidance documents for nanomedicine evaluation, though harmonization of international standards remains an ongoing challenge.
Emerging research directions include the development of predictive in silico models to forecast nanocarrier-biological interactions, reducing reliance on animal testing while improving safety prediction accuracy. Additionally, researchers are exploring biomimetic approaches, designing nanocarriers that mimic endogenous cellular components to enhance biocompatibility. Cell membrane-coated nanoparticles represent one promising strategy, leveraging natural cellular components to improve safety profiles while maintaining therapeutic efficacy.
The balance between therapeutic efficacy and safety remains the central challenge in nanocarrier development for gene therapy applications. Future advances will likely focus on personalized approaches that consider individual patient characteristics in nanocarrier design, potentially reducing adverse effects while maximizing therapeutic outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!