The Influence of Recent Patents on CRISPR-Based Gene Therapy Tools
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CRISPR Gene Therapy Evolution and Objectives
CRISPR technology has undergone remarkable evolution since its discovery as a bacterial adaptive immune system in the early 1990s. The pivotal moment came in 2012 when Jennifer Doudna and Emmanuelle Charpentier demonstrated that CRISPR-Cas9 could be programmed to cut specific DNA sequences, revolutionizing genetic engineering capabilities. This breakthrough earned them the 2020 Nobel Prize in Chemistry and catalyzed an explosion of research and development in gene therapy applications.
The technological trajectory of CRISPR has been characterized by continuous refinement and expansion. From the original Cas9 system, researchers have discovered and engineered numerous Cas variants with specialized functions, including Cas12, Cas13, and Cas14, each offering unique capabilities for different therapeutic applications. Base editors and prime editors represent significant advancements, enabling precise nucleotide changes without double-strand breaks, thus reducing off-target effects that plagued earlier iterations.
Recent patent developments have substantially shaped the direction of CRISPR gene therapy tools. The contentious patent dispute between the Broad Institute and UC Berkeley has influenced licensing landscapes and commercial development pathways. These intellectual property considerations have driven innovation toward novel CRISPR systems that circumvent existing patents, resulting in diversification of available tools and approaches.
The primary objectives in CRISPR gene therapy development currently focus on enhancing specificity, delivery efficiency, and safety profiles. Reducing off-target effects remains paramount, as unintended genomic modifications could lead to serious adverse effects including carcinogenesis. Delivery systems represent another critical objective, with significant research directed toward developing improved viral vectors, lipid nanoparticles, and cell-penetrating peptides capable of efficiently delivering CRISPR components to target tissues.
Expanding the therapeutic window constitutes another key goal, with researchers working to develop CRISPR systems that can address a broader range of genetic disorders, including those requiring multiple gene modifications or regulatory adjustments rather than simple gene knockouts or replacements. The development of controllable or inducible CRISPR systems represents an emerging objective, allowing temporal regulation of gene editing activity.
Looking forward, the field is trending toward more sophisticated applications, including multiplexed editing (modifying multiple genes simultaneously), epigenetic modifications that alter gene expression without changing DNA sequence, and in vivo editing that can be performed directly in patients rather than on extracted cells. These advancements aim to address increasingly complex genetic disorders and expand therapeutic applications beyond rare monogenic diseases to more common conditions with polygenic components.
The technological trajectory of CRISPR has been characterized by continuous refinement and expansion. From the original Cas9 system, researchers have discovered and engineered numerous Cas variants with specialized functions, including Cas12, Cas13, and Cas14, each offering unique capabilities for different therapeutic applications. Base editors and prime editors represent significant advancements, enabling precise nucleotide changes without double-strand breaks, thus reducing off-target effects that plagued earlier iterations.
Recent patent developments have substantially shaped the direction of CRISPR gene therapy tools. The contentious patent dispute between the Broad Institute and UC Berkeley has influenced licensing landscapes and commercial development pathways. These intellectual property considerations have driven innovation toward novel CRISPR systems that circumvent existing patents, resulting in diversification of available tools and approaches.
The primary objectives in CRISPR gene therapy development currently focus on enhancing specificity, delivery efficiency, and safety profiles. Reducing off-target effects remains paramount, as unintended genomic modifications could lead to serious adverse effects including carcinogenesis. Delivery systems represent another critical objective, with significant research directed toward developing improved viral vectors, lipid nanoparticles, and cell-penetrating peptides capable of efficiently delivering CRISPR components to target tissues.
Expanding the therapeutic window constitutes another key goal, with researchers working to develop CRISPR systems that can address a broader range of genetic disorders, including those requiring multiple gene modifications or regulatory adjustments rather than simple gene knockouts or replacements. The development of controllable or inducible CRISPR systems represents an emerging objective, allowing temporal regulation of gene editing activity.
Looking forward, the field is trending toward more sophisticated applications, including multiplexed editing (modifying multiple genes simultaneously), epigenetic modifications that alter gene expression without changing DNA sequence, and in vivo editing that can be performed directly in patients rather than on extracted cells. These advancements aim to address increasingly complex genetic disorders and expand therapeutic applications beyond rare monogenic diseases to more common conditions with polygenic components.
Market Analysis of CRISPR-Based Therapeutics
The CRISPR-based therapeutics market has experienced exponential growth since the discovery of CRISPR-Cas9 gene editing technology in 2012. Current market valuations place the global CRISPR therapeutics sector at approximately $1.65 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 29.8% through 2030, potentially reaching $7.8 billion.
This remarkable growth trajectory is driven by several key factors. First, the increasing prevalence of genetic disorders worldwide has created substantial demand for innovative treatment approaches. According to the World Health Organization, over 10,000 human diseases are caused by single-gene defects, affecting millions of patients globally who currently have limited therapeutic options.
Investment in CRISPR therapeutics has surged dramatically, with venture capital funding exceeding $5.2 billion between 2020 and 2023. Major pharmaceutical companies have established strategic partnerships with CRISPR-focused biotechnology firms, evidenced by deals such as Vertex Pharmaceuticals' $900 million collaboration with CRISPR Therapeutics for developing CTX001 (now exa-cel) for sickle cell disease and beta-thalassemia.
The clinical pipeline for CRISPR therapeutics has expanded significantly, with over 75 active clinical trials worldwide as of early 2023. Oncology represents the largest application segment (38% of trials), followed by genetic blood disorders (27%) and ophthalmological conditions (12%). The recent FDA approval of Casgevy (exa-cel) for sickle cell disease in December 2023 marks a historic milestone as the first CRISPR-based therapy to receive regulatory approval in the United States.
Regional market analysis reveals North America dominates with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). China has emerged as a particularly aggressive competitor, with Chinese institutions filing more CRISPR-related patents than any other country since 2020.
Patent landscape analysis indicates a complex competitive environment with over 12,000 CRISPR-related patents filed globally. Key patent holders include the Broad Institute, University of California, and various biotechnology companies like Editas Medicine, CRISPR Therapeutics, and Intellia Therapeutics. Recent patent disputes have created market uncertainties but have not significantly impeded therapeutic development.
Market challenges include high treatment costs, with current gene therapy prices ranging from $375,000 to $2.1 million per patient, raising significant reimbursement concerns. Additionally, regulatory frameworks continue to evolve, with different approaches across jurisdictions creating compliance complexities for developers seeking global market access.
This remarkable growth trajectory is driven by several key factors. First, the increasing prevalence of genetic disorders worldwide has created substantial demand for innovative treatment approaches. According to the World Health Organization, over 10,000 human diseases are caused by single-gene defects, affecting millions of patients globally who currently have limited therapeutic options.
Investment in CRISPR therapeutics has surged dramatically, with venture capital funding exceeding $5.2 billion between 2020 and 2023. Major pharmaceutical companies have established strategic partnerships with CRISPR-focused biotechnology firms, evidenced by deals such as Vertex Pharmaceuticals' $900 million collaboration with CRISPR Therapeutics for developing CTX001 (now exa-cel) for sickle cell disease and beta-thalassemia.
The clinical pipeline for CRISPR therapeutics has expanded significantly, with over 75 active clinical trials worldwide as of early 2023. Oncology represents the largest application segment (38% of trials), followed by genetic blood disorders (27%) and ophthalmological conditions (12%). The recent FDA approval of Casgevy (exa-cel) for sickle cell disease in December 2023 marks a historic milestone as the first CRISPR-based therapy to receive regulatory approval in the United States.
Regional market analysis reveals North America dominates with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). China has emerged as a particularly aggressive competitor, with Chinese institutions filing more CRISPR-related patents than any other country since 2020.
Patent landscape analysis indicates a complex competitive environment with over 12,000 CRISPR-related patents filed globally. Key patent holders include the Broad Institute, University of California, and various biotechnology companies like Editas Medicine, CRISPR Therapeutics, and Intellia Therapeutics. Recent patent disputes have created market uncertainties but have not significantly impeded therapeutic development.
Market challenges include high treatment costs, with current gene therapy prices ranging from $375,000 to $2.1 million per patient, raising significant reimbursement concerns. Additionally, regulatory frameworks continue to evolve, with different approaches across jurisdictions creating compliance complexities for developers seeking global market access.
Current CRISPR Technology Landscape and Barriers
The CRISPR-Cas9 gene editing technology has revolutionized genetic engineering since its discovery, but the current landscape reveals both remarkable achievements and significant challenges. Globally, CRISPR technology has advanced rapidly with various systems beyond the original Cas9, including Cas12, Cas13, and base editors that offer improved precision and reduced off-target effects. These developments have expanded the toolkit available to researchers and clinicians working on gene therapy applications.
Despite these advancements, several critical barriers impede the full clinical implementation of CRISPR-based gene therapies. Delivery mechanisms remain a primary challenge, with viral vectors like AAV showing limitations in payload capacity and potential immunogenicity. Non-viral delivery systems, while promising, still struggle with efficiency in vivo. The development of lipid nanoparticles and other novel delivery vehicles represents an active area of research but has yet to yield optimal solutions for all therapeutic applications.
Off-target effects continue to pose significant safety concerns, as unintended edits can potentially lead to oncogenesis or other adverse outcomes. While improved Cas variants with enhanced specificity have been developed, complete elimination of off-target activity remains elusive, particularly in complex genomic environments. This challenge is compounded by limitations in current methods for comprehensively detecting and quantifying off-target effects in clinical settings.
Immune responses to both the delivery vehicles and the Cas proteins themselves represent another substantial barrier. Pre-existing immunity to common viral vectors can reduce efficacy, while the bacterial origin of Cas proteins can trigger immune reactions that limit repeated dosing options. Strategies to mitigate these responses, such as immunomodulation or engineered "stealth" Cas variants, are under development but not yet fully realized.
The regulatory landscape presents additional complexity, with evolving frameworks for evaluating the safety and efficacy of gene editing therapies. Regulatory agencies worldwide are still developing standardized approaches to assess these novel therapeutics, creating uncertainty for developers and potentially slowing clinical translation.
Patent disputes further complicate the technology landscape, with major academic institutions and biotechnology companies engaged in ongoing litigation over intellectual property rights. These disputes create uncertainty regarding freedom to operate and may restrict access to certain CRISPR tools or applications, potentially limiting innovation and clinical development.
Scale-up and manufacturing challenges also persist, particularly for personalized autologous therapies that require patient-specific cell modification. Current production methods often lack the efficiency and consistency needed for widespread clinical implementation, contributing to the high costs associated with CRISPR-based treatments.
Despite these advancements, several critical barriers impede the full clinical implementation of CRISPR-based gene therapies. Delivery mechanisms remain a primary challenge, with viral vectors like AAV showing limitations in payload capacity and potential immunogenicity. Non-viral delivery systems, while promising, still struggle with efficiency in vivo. The development of lipid nanoparticles and other novel delivery vehicles represents an active area of research but has yet to yield optimal solutions for all therapeutic applications.
Off-target effects continue to pose significant safety concerns, as unintended edits can potentially lead to oncogenesis or other adverse outcomes. While improved Cas variants with enhanced specificity have been developed, complete elimination of off-target activity remains elusive, particularly in complex genomic environments. This challenge is compounded by limitations in current methods for comprehensively detecting and quantifying off-target effects in clinical settings.
Immune responses to both the delivery vehicles and the Cas proteins themselves represent another substantial barrier. Pre-existing immunity to common viral vectors can reduce efficacy, while the bacterial origin of Cas proteins can trigger immune reactions that limit repeated dosing options. Strategies to mitigate these responses, such as immunomodulation or engineered "stealth" Cas variants, are under development but not yet fully realized.
The regulatory landscape presents additional complexity, with evolving frameworks for evaluating the safety and efficacy of gene editing therapies. Regulatory agencies worldwide are still developing standardized approaches to assess these novel therapeutics, creating uncertainty for developers and potentially slowing clinical translation.
Patent disputes further complicate the technology landscape, with major academic institutions and biotechnology companies engaged in ongoing litigation over intellectual property rights. These disputes create uncertainty regarding freedom to operate and may restrict access to certain CRISPR tools or applications, potentially limiting innovation and clinical development.
Scale-up and manufacturing challenges also persist, particularly for personalized autologous therapies that require patient-specific cell modification. Current production methods often lack the efficiency and consistency needed for widespread clinical implementation, contributing to the high costs associated with CRISPR-based treatments.
Contemporary CRISPR Gene Editing Approaches
01 CRISPR delivery systems for gene therapy
Various delivery systems have been developed to efficiently transport CRISPR-Cas components into target cells for therapeutic applications. These include viral vectors (such as AAV, lentivirus), lipid nanoparticles, and cell-penetrating peptides. The delivery systems are designed to overcome barriers such as cellular uptake, endosomal escape, and nuclear localization to ensure effective gene editing in target tissues while minimizing off-target effects.- CRISPR-Cas9 delivery systems for gene therapy: Various delivery systems have been developed to efficiently transport CRISPR-Cas9 components into target cells for therapeutic applications. These systems include viral vectors (such as AAV, lentivirus), lipid nanoparticles, and cell-penetrating peptides. The delivery methods are designed to overcome challenges like immune responses, off-target effects, and ensuring the components reach the intended tissues for effective gene editing in treatment of genetic disorders.
- CRISPR base and prime editing technologies: Advanced CRISPR technologies like base editing and prime editing have been developed to make precise changes to DNA without causing double-strand breaks. Base editors can convert one nucleotide to another, while prime editing can perform targeted insertions, deletions, and all possible base-to-base conversions. These technologies offer improved precision and reduced off-target effects compared to traditional CRISPR-Cas9 systems, making them valuable tools for correcting disease-causing mutations.
- CRISPR systems for targeting specific genetic disorders: CRISPR-based gene therapy tools have been developed to target specific genetic disorders such as cystic fibrosis, sickle cell disease, muscular dystrophy, and various inherited blood disorders. These systems are designed to correct or modify disease-causing mutations in the patient's genome. The therapeutic approaches include ex vivo modification of patient cells followed by reinfusion, or direct in vivo delivery of CRISPR components to affected tissues.
- Modified CRISPR enzymes with enhanced specificity and efficiency: Engineered variants of CRISPR enzymes have been developed with improved properties for gene therapy applications. These modifications include enhanced target specificity to reduce off-target effects, increased editing efficiency, altered PAM requirements to expand targetable sequences, and reduced size for improved delivery. Some variants also feature reduced immunogenicity or conditional activation mechanisms to improve safety profiles for clinical applications.
- CRISPR-based gene regulation and epigenetic editing: CRISPR systems have been adapted for applications beyond DNA cutting, including gene regulation and epigenetic modifications. These tools use catalytically inactive Cas proteins (dCas) fused to effector domains that can activate or repress gene expression, modify histones, or alter DNA methylation patterns. Such systems enable modulation of gene expression without permanent DNA changes, offering therapeutic potential for diseases where gene regulation, rather than editing, is desired.
02 Modified CRISPR-Cas nucleases for enhanced specificity
Engineered CRISPR-Cas nucleases with improved specificity and reduced off-target effects have been developed for gene therapy applications. These modifications include structural alterations to the Cas protein, incorporation of novel domains, and optimization of guide RNA design. Such enhanced specificity is crucial for clinical applications to minimize unintended genomic modifications that could lead to adverse effects in patients undergoing gene therapy.Expand Specific Solutions03 CRISPR-based therapeutic approaches for genetic diseases
CRISPR technology has been adapted for treating various genetic disorders through different mechanisms including gene correction, gene knockout, or gene regulation. These therapeutic approaches target specific disease-causing mutations in conditions such as cystic fibrosis, sickle cell disease, muscular dystrophy, and various inherited metabolic disorders. The technology allows for precise modification of disease-relevant genes in affected tissues or in stem cells that can be reintroduced into patients.Expand Specific Solutions04 Base and prime editing technologies
Advanced CRISPR derivatives such as base editors and prime editors enable precise nucleotide changes without requiring double-strand breaks. Base editors can convert one nucleotide to another (e.g., C to T or A to G), while prime editors can perform targeted insertions, deletions, and all possible base-to-base conversions. These technologies offer greater precision for correcting point mutations that cause many genetic diseases, with potentially fewer side effects than traditional CRISPR-Cas systems.Expand Specific Solutions05 Regulatory and safety elements for CRISPR gene therapy
Various regulatory and safety elements have been incorporated into CRISPR gene therapy tools to control their activity and enhance their safety profile. These include inducible systems that allow temporal control of gene editing, self-limiting mechanisms that restrict editing duration, tissue-specific promoters for targeted expression, and safety switches to terminate activity if adverse events occur. These elements are crucial for translating CRISPR technology into clinical applications with acceptable risk profiles.Expand Specific Solutions
Leading Organizations in CRISPR Patent Ecosystem
The CRISPR-based gene therapy landscape is currently in a growth phase, with market size expanding rapidly as technologies mature from experimental to clinical applications. Recent patents have created a complex competitive ecosystem dominated by academic institutions (MIT, Broad Institute, Harvard) and specialized biotech companies (CRISPR Therapeutics, Caribou Biosciences, Synthego). The technology is approaching commercial maturity in specific applications, with companies like Cellectis and Sangamo Therapeutics advancing clinical trials. International players from China (Chia Tai Tianqing, Hangzhou Shutong) and Europe (CureVac) are increasingly filing patents, creating a globally competitive environment where intellectual property rights significantly influence market access and therapeutic development pathways.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has pioneered significant advancements in CRISPR-based gene therapy tools through their patented CRISPR-Cas9 technology. Their approach focuses on optimizing gene editing precision and efficiency in human cells. Recent patents cover improved delivery methods using lipid nanoparticles that enhance cellular uptake and reduce off-target effects[1]. The Institute has also developed novel Cas variants with increased specificity, particularly the engineered Cas12a (Cpf1) system that recognizes different PAM sequences than traditional Cas9, expanding the genomic targeting range[2]. Their patented base editing technology allows for precise single nucleotide changes without creating double-strand breaks, significantly reducing unintended mutations. Additionally, they've patented CRISPR screening platforms that enable high-throughput functional genomics studies to identify therapeutic targets[3].
Strengths: Exceptional precision in gene editing with reduced off-target effects; diverse portfolio of Cas variants allowing targeting of previously inaccessible genomic regions; strong intellectual property position in the CRISPR field. Weaknesses: Complex licensing requirements may limit accessibility; some delivery methods still face challenges for in vivo applications; potential for immune responses to Cas proteins remains a concern.
The Regents of the University of California
Technical Solution: The University of California has developed groundbreaking CRISPR-based gene therapy tools centered around their patented CRISPR-Cas9 technology, with particular focus on therapeutic applications. Their recent patents cover novel RNA-guided endonucleases with enhanced specificity and reduced off-target effects[1]. UC researchers have pioneered CasX (Cas12e), a smaller Cas protein that offers advantages for delivery via viral vectors due to its compact size while maintaining editing efficiency[2]. Their patent portfolio includes innovative delivery systems using engineered viral vectors specifically designed for in vivo gene therapy applications. Additionally, they've developed and patented CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) systems that enable precise regulation of gene expression without modifying the underlying DNA sequence, expanding therapeutic possibilities beyond traditional gene editing[3]. Their recent patents also cover methods for multiplex genome editing, allowing simultaneous modification of multiple genetic targets in a single treatment.
Strengths: Diverse CRISPR toolbox including novel Cas variants with unique properties; advanced delivery systems optimized for clinical applications; strong position in gene regulation technologies beyond editing. Weaknesses: Ongoing patent disputes create uncertainty for commercial applications; some delivery methods still show tissue-specific limitations; potential immunogenicity concerns with certain Cas variants remain unresolved.
Key Patent Analysis in CRISPR Gene Therapy
Therapeutic uses of genome editing with crispr/cas systems
PatentWO2014165825A2
Innovation
- A multiple guide strategy using two or more ribonucleic acids to guide the Cas protein to target polynucleotide sequences, achieving up to 80% efficiency in deleting specific sequences like B2M, HPRT, CCR5, and CXCR4 in primary somatic cells, such as human blood cells, compared to single guide strategies which were ineffective in these cells.
RNA-guided gene editing and gene regulation
PatentPendingAU2025213563A1
Innovation
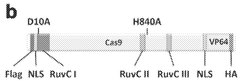
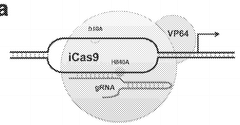
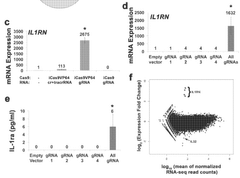
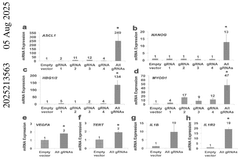
- A fusion protein comprising a Cas9 protein with altered nuclease activity and a transcription activation domain, combined with guide RNA, targets specific genomic regions for precise gene modulation, including dystrophin gene correction, using systems like CRISPR/Cas9 and viral delivery.
Regulatory Framework for CRISPR-Based Therapies
The regulatory landscape for CRISPR-based therapies represents a complex and evolving framework that significantly impacts the development, approval, and commercialization of gene therapy tools. Currently, regulatory bodies worldwide are adapting their approaches to address the unique challenges posed by this revolutionary technology. The U.S. Food and Drug Administration (FDA) has established a specific pathway for gene therapies under its Regenerative Medicine Advanced Therapy (RMAT) designation, which provides expedited review for promising treatments addressing serious conditions.
In Europe, the European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which includes specific provisions for gene therapy medicinal products. These frameworks require comprehensive preclinical and clinical data demonstrating safety and efficacy, with particular emphasis on long-term follow-up studies to monitor potential delayed adverse effects.
China's National Medical Products Administration (NMPA) has also developed guidelines specifically addressing gene therapy products, reflecting the country's growing investment in this field. The regulatory requirements focus heavily on quality control, manufacturing consistency, and rigorous safety assessments.
A critical aspect of the regulatory framework involves intellectual property considerations. The patent landscape for CRISPR technologies has created significant regulatory complexities, with ongoing disputes between major patent holders potentially affecting licensing requirements for therapy developers. Regulatory bodies must navigate these patent issues when evaluating new therapies, often requiring clear documentation of freedom to operate.
Ethical considerations form another crucial component of the regulatory framework. Many jurisdictions have established specialized ethics committees to evaluate CRISPR-based therapies, particularly those involving germline modifications. The 2018 WHO guidelines on human genome editing have influenced regulatory approaches globally, emphasizing the need for transparent governance and international harmonization.
Risk assessment methodologies for CRISPR therapies have evolved significantly, with regulatory agencies now requiring comprehensive off-target analysis using advanced sequencing technologies. The FDA's 2020 guidance specifically addresses the evaluation of off-target effects in gene therapy products, establishing standardized protocols for safety assessment.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to develop consistent regulatory standards for gene therapies across different regions, though significant variations in national approaches persist. These differences create challenges for multinational clinical trials and global market access strategies.
In Europe, the European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which includes specific provisions for gene therapy medicinal products. These frameworks require comprehensive preclinical and clinical data demonstrating safety and efficacy, with particular emphasis on long-term follow-up studies to monitor potential delayed adverse effects.
China's National Medical Products Administration (NMPA) has also developed guidelines specifically addressing gene therapy products, reflecting the country's growing investment in this field. The regulatory requirements focus heavily on quality control, manufacturing consistency, and rigorous safety assessments.
A critical aspect of the regulatory framework involves intellectual property considerations. The patent landscape for CRISPR technologies has created significant regulatory complexities, with ongoing disputes between major patent holders potentially affecting licensing requirements for therapy developers. Regulatory bodies must navigate these patent issues when evaluating new therapies, often requiring clear documentation of freedom to operate.
Ethical considerations form another crucial component of the regulatory framework. Many jurisdictions have established specialized ethics committees to evaluate CRISPR-based therapies, particularly those involving germline modifications. The 2018 WHO guidelines on human genome editing have influenced regulatory approaches globally, emphasizing the need for transparent governance and international harmonization.
Risk assessment methodologies for CRISPR therapies have evolved significantly, with regulatory agencies now requiring comprehensive off-target analysis using advanced sequencing technologies. The FDA's 2020 guidance specifically addresses the evaluation of off-target effects in gene therapy products, establishing standardized protocols for safety assessment.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), are working to develop consistent regulatory standards for gene therapies across different regions, though significant variations in national approaches persist. These differences create challenges for multinational clinical trials and global market access strategies.
Ethical Implications of CRISPR Patent Ownership
The ownership of CRISPR patents has created a complex ethical landscape that extends far beyond traditional intellectual property concerns. The concentration of patent rights among a few key institutions—primarily the Broad Institute, UC Berkeley, and their commercial partners—raises significant questions about equitable access to gene therapy technologies. This patent monopolization potentially restricts research and development activities, particularly for smaller organizations and researchers in developing nations who cannot afford licensing fees or navigate complex patent landscapes.
The ethical implications become more pronounced when considering that CRISPR technology has the potential to address numerous genetic diseases affecting populations worldwide. Patent restrictions may create disparities in healthcare access, where only wealthy nations or individuals can benefit from breakthrough therapies. This situation contradicts the fundamental bioethical principle of justice, which emphasizes fair distribution of healthcare benefits and burdens across populations.
Furthermore, the aggressive patent litigation between major institutions has diverted substantial resources from actual research and development. The University of California and Broad Institute's protracted legal battles exemplify how patent disputes can impede scientific progress rather than promote innovation—the original purpose of patent systems. These legal conflicts have created uncertainty in the biotechnology sector, potentially slowing investment in CRISPR applications.
The commercialization of CRISPR technology through exclusive licensing agreements raises additional ethical concerns about the privatization of knowledge derived from publicly funded research. Many foundational CRISPR discoveries emerged from academic institutions supported by taxpayer money, yet the resulting intellectual property has been licensed to private companies with profit-maximizing incentives that may not align with public health priorities.
Patient advocacy groups have increasingly voiced concerns about how patent ownership affects therapy development timelines and accessibility. The monopolistic control over CRISPR tools may limit the exploration of alternative approaches that could prove more effective or affordable for certain conditions, effectively narrowing the therapeutic pipeline based on commercial rather than medical considerations.
International perspectives on CRISPR patent ownership vary significantly, creating a fragmented global landscape for research and clinical applications. This inconsistency threatens to exacerbate global health inequities, as different regulatory and patent environments may lead to "genetic therapy havens" where oversight is minimal but access is restricted to the privileged few.
The ethical implications become more pronounced when considering that CRISPR technology has the potential to address numerous genetic diseases affecting populations worldwide. Patent restrictions may create disparities in healthcare access, where only wealthy nations or individuals can benefit from breakthrough therapies. This situation contradicts the fundamental bioethical principle of justice, which emphasizes fair distribution of healthcare benefits and burdens across populations.
Furthermore, the aggressive patent litigation between major institutions has diverted substantial resources from actual research and development. The University of California and Broad Institute's protracted legal battles exemplify how patent disputes can impede scientific progress rather than promote innovation—the original purpose of patent systems. These legal conflicts have created uncertainty in the biotechnology sector, potentially slowing investment in CRISPR applications.
The commercialization of CRISPR technology through exclusive licensing agreements raises additional ethical concerns about the privatization of knowledge derived from publicly funded research. Many foundational CRISPR discoveries emerged from academic institutions supported by taxpayer money, yet the resulting intellectual property has been licensed to private companies with profit-maximizing incentives that may not align with public health priorities.
Patient advocacy groups have increasingly voiced concerns about how patent ownership affects therapy development timelines and accessibility. The monopolistic control over CRISPR tools may limit the exploration of alternative approaches that could prove more effective or affordable for certain conditions, effectively narrowing the therapeutic pipeline based on commercial rather than medical considerations.
International perspectives on CRISPR patent ownership vary significantly, creating a fragmented global landscape for research and clinical applications. This inconsistency threatens to exacerbate global health inequities, as different regulatory and patent environments may lead to "genetic therapy havens" where oversight is minimal but access is restricted to the privileged few.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!