Viral Vectors Versus Nanoparticles in Gene Therapy Efficiency
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy Evolution and Objectives
Gene therapy has evolved significantly since its conceptual inception in the 1970s, transforming from theoretical possibility to clinical reality. The field's development can be traced through several distinct phases, beginning with early proof-of-concept studies in the 1990s that demonstrated the potential for genetic material to treat inherited disorders. These initial efforts faced significant setbacks, including the tragic death of Jesse Gelsinger in 1999 due to an immune reaction to a viral vector, which temporarily slowed progress but ultimately led to enhanced safety protocols.
The evolution accelerated in the early 2000s with improved vector designs and delivery systems, culminating in the first gene therapy approval in Europe in 2012 (Glybera) and the first FDA approval in the United States in 2017 (Luxturna). This progression reflects the field's maturation from experimental treatments to validated therapeutic approaches for previously untreatable conditions.
Central to this evolution has been the parallel development of delivery systems, primarily viral vectors and nanoparticles, each with distinct advantages and limitations. Viral vectors, leveraging natural viral infection mechanisms, have dominated early successes but face challenges related to immunogenicity and manufacturing complexity. Nanoparticles represent a newer approach offering potentially improved safety profiles and manufacturing scalability, though often with reduced efficiency compared to viral counterparts.
The current objectives in gene therapy delivery systems focus on several key areas: enhancing transduction efficiency to maximize therapeutic effect with minimal dosing; reducing immunogenicity to enable repeat dosing and minimize adverse reactions; improving tissue specificity to target therapeutic genes precisely to affected cells; expanding payload capacity to accommodate larger therapeutic genes; and developing scalable, cost-effective manufacturing processes to improve accessibility.
Specifically regarding viral vectors versus nanoparticles, research objectives include determining optimal delivery systems for different therapeutic applications, understanding the fundamental mechanisms affecting delivery efficiency, and developing hybrid approaches that combine the strengths of both platforms. The field aims to establish predictive models that can guide rational design of delivery systems tailored to specific genetic disorders and patient populations.
The ultimate goal remains developing safe, efficient, and accessible gene therapy approaches that can address a broader range of diseases beyond the rare monogenic disorders that have been the primary focus to date, potentially expanding to more common conditions with complex genetic components.
The evolution accelerated in the early 2000s with improved vector designs and delivery systems, culminating in the first gene therapy approval in Europe in 2012 (Glybera) and the first FDA approval in the United States in 2017 (Luxturna). This progression reflects the field's maturation from experimental treatments to validated therapeutic approaches for previously untreatable conditions.
Central to this evolution has been the parallel development of delivery systems, primarily viral vectors and nanoparticles, each with distinct advantages and limitations. Viral vectors, leveraging natural viral infection mechanisms, have dominated early successes but face challenges related to immunogenicity and manufacturing complexity. Nanoparticles represent a newer approach offering potentially improved safety profiles and manufacturing scalability, though often with reduced efficiency compared to viral counterparts.
The current objectives in gene therapy delivery systems focus on several key areas: enhancing transduction efficiency to maximize therapeutic effect with minimal dosing; reducing immunogenicity to enable repeat dosing and minimize adverse reactions; improving tissue specificity to target therapeutic genes precisely to affected cells; expanding payload capacity to accommodate larger therapeutic genes; and developing scalable, cost-effective manufacturing processes to improve accessibility.
Specifically regarding viral vectors versus nanoparticles, research objectives include determining optimal delivery systems for different therapeutic applications, understanding the fundamental mechanisms affecting delivery efficiency, and developing hybrid approaches that combine the strengths of both platforms. The field aims to establish predictive models that can guide rational design of delivery systems tailored to specific genetic disorders and patient populations.
The ultimate goal remains developing safe, efficient, and accessible gene therapy approaches that can address a broader range of diseases beyond the rare monogenic disorders that have been the primary focus to date, potentially expanding to more common conditions with complex genetic components.
Market Analysis of Gene Therapy Delivery Systems
The gene therapy delivery systems market is experiencing robust growth, valued at approximately $7.2 billion in 2023 and projected to reach $25.3 billion by 2030, with a compound annual growth rate (CAGR) of 19.6%. This expansion is primarily driven by increasing prevalence of genetic disorders, growing investment in research and development, and advancements in delivery technologies.
Viral vectors currently dominate the market, accounting for roughly 68% of the total market share. Among viral vectors, adeno-associated virus (AAV) vectors lead with 41% of the viral vector segment, followed by lentiviral vectors at 28% and adenoviral vectors at 19%. The preference for viral vectors stems from their high transduction efficiency and established clinical track record, with over 70% of approved gene therapies utilizing viral delivery systems.
Non-viral delivery systems, particularly nanoparticles, are gaining significant traction, growing at a faster CAGR of 22.3% compared to viral vectors' 18.1%. Lipid nanoparticles (LNPs) represent the largest non-viral segment at 45%, followed by polymer-based nanoparticles at 30%. The success of mRNA vaccines using LNPs has accelerated interest in this technology for gene therapy applications.
Regional analysis reveals North America as the dominant market with 42% share, followed by Europe at 31% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the fastest growth at 24.2% CAGR due to increasing healthcare expenditure, growing research infrastructure, and favorable regulatory environments in countries like China and South Korea.
By therapeutic application, oncology represents the largest segment (34%), followed by rare genetic disorders (28%) and neurological diseases (17%). The oncology segment is projected to maintain its leading position due to the high prevalence of cancer and substantial research funding in this area.
Key market drivers include decreasing manufacturing costs for viral vectors (15-20% reduction over the past five years), increasing regulatory approvals (tripled in the last decade), and expanding reimbursement policies for gene therapies. However, high treatment costs remain a significant market restraint, with the average cost of gene therapy treatments exceeding $1 million per patient.
Emerging trends include the development of hybrid delivery systems combining viral and non-viral elements, increased focus on in vivo gene editing applications, and growing interest in targeted delivery technologies to enhance tissue specificity and reduce off-target effects.
Viral vectors currently dominate the market, accounting for roughly 68% of the total market share. Among viral vectors, adeno-associated virus (AAV) vectors lead with 41% of the viral vector segment, followed by lentiviral vectors at 28% and adenoviral vectors at 19%. The preference for viral vectors stems from their high transduction efficiency and established clinical track record, with over 70% of approved gene therapies utilizing viral delivery systems.
Non-viral delivery systems, particularly nanoparticles, are gaining significant traction, growing at a faster CAGR of 22.3% compared to viral vectors' 18.1%. Lipid nanoparticles (LNPs) represent the largest non-viral segment at 45%, followed by polymer-based nanoparticles at 30%. The success of mRNA vaccines using LNPs has accelerated interest in this technology for gene therapy applications.
Regional analysis reveals North America as the dominant market with 42% share, followed by Europe at 31% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the fastest growth at 24.2% CAGR due to increasing healthcare expenditure, growing research infrastructure, and favorable regulatory environments in countries like China and South Korea.
By therapeutic application, oncology represents the largest segment (34%), followed by rare genetic disorders (28%) and neurological diseases (17%). The oncology segment is projected to maintain its leading position due to the high prevalence of cancer and substantial research funding in this area.
Key market drivers include decreasing manufacturing costs for viral vectors (15-20% reduction over the past five years), increasing regulatory approvals (tripled in the last decade), and expanding reimbursement policies for gene therapies. However, high treatment costs remain a significant market restraint, with the average cost of gene therapy treatments exceeding $1 million per patient.
Emerging trends include the development of hybrid delivery systems combining viral and non-viral elements, increased focus on in vivo gene editing applications, and growing interest in targeted delivery technologies to enhance tissue specificity and reduce off-target effects.
Current Challenges in Vector and Nanoparticle Technologies
Despite significant advancements in gene therapy technologies, both viral vectors and nanoparticles face substantial challenges that limit their widespread clinical application. Viral vectors, while offering high transduction efficiency, continue to struggle with immunogenicity issues. Pre-existing immunity against common viral serotypes such as adeno-associated virus (AAV) and adenovirus significantly reduces treatment efficacy in many patients, necessitating higher doses that exacerbate toxicity concerns.
Manufacturing scalability represents another critical bottleneck for viral vectors. Current production methods yield insufficient titers for treating diseases requiring systemic administration or those affecting large tissue volumes. The complex biological production systems also introduce batch-to-batch variability, complicating regulatory approval pathways and increasing production costs to prohibitive levels for many applications.
Nanoparticle delivery systems face their own set of challenges, primarily centered around targeting efficiency. Unlike viral vectors that evolved natural mechanisms for cell entry, synthetic nanoparticles demonstrate substantially lower delivery efficiency to target tissues. This limitation is particularly pronounced when targeting tissues beyond the liver, which naturally accumulates most nanoparticles regardless of their design.
The stability of nanoparticle formulations presents ongoing difficulties, with many systems showing poor shelf-life or requiring complex cold-chain logistics. Additionally, the payload capacity of many nanoparticle systems remains limited compared to viral alternatives, restricting their utility for delivering larger genetic constructs such as full-length genes or multiple therapeutic elements.
Both technologies share common challenges in achieving tissue-specific targeting. Current approaches rely heavily on physical barriers (like the enhanced permeability and retention effect) or surface modifications that provide only modest improvements in specificity. The inability to precisely control biodistribution leads to off-target effects and necessitates higher doses, increasing both cost and safety concerns.
Regulatory hurdles compound these technical challenges. The complex nature of these advanced therapeutic medicinal products requires specialized manufacturing facilities and extensive characterization studies. Regulatory frameworks continue to evolve as these technologies mature, creating uncertainty in development timelines and approval requirements.
Safety profiles remain problematic for both approaches. Viral vectors can trigger severe immune responses, as demonstrated by several high-profile adverse events in clinical trials. Nanoparticles, particularly those containing cationic lipids or polymers, have shown toxicity concerns including complement activation and cytokine release syndromes that limit their dosing range.
The persistence of therapeutic effect presents another significant challenge. While AAV vectors can provide long-term expression in non-dividing cells, they show limited efficacy in actively dividing tissues. Nanoparticle systems typically offer only transient expression, necessitating repeated administration that compounds both cost and safety concerns.
Manufacturing scalability represents another critical bottleneck for viral vectors. Current production methods yield insufficient titers for treating diseases requiring systemic administration or those affecting large tissue volumes. The complex biological production systems also introduce batch-to-batch variability, complicating regulatory approval pathways and increasing production costs to prohibitive levels for many applications.
Nanoparticle delivery systems face their own set of challenges, primarily centered around targeting efficiency. Unlike viral vectors that evolved natural mechanisms for cell entry, synthetic nanoparticles demonstrate substantially lower delivery efficiency to target tissues. This limitation is particularly pronounced when targeting tissues beyond the liver, which naturally accumulates most nanoparticles regardless of their design.
The stability of nanoparticle formulations presents ongoing difficulties, with many systems showing poor shelf-life or requiring complex cold-chain logistics. Additionally, the payload capacity of many nanoparticle systems remains limited compared to viral alternatives, restricting their utility for delivering larger genetic constructs such as full-length genes or multiple therapeutic elements.
Both technologies share common challenges in achieving tissue-specific targeting. Current approaches rely heavily on physical barriers (like the enhanced permeability and retention effect) or surface modifications that provide only modest improvements in specificity. The inability to precisely control biodistribution leads to off-target effects and necessitates higher doses, increasing both cost and safety concerns.
Regulatory hurdles compound these technical challenges. The complex nature of these advanced therapeutic medicinal products requires specialized manufacturing facilities and extensive characterization studies. Regulatory frameworks continue to evolve as these technologies mature, creating uncertainty in development timelines and approval requirements.
Safety profiles remain problematic for both approaches. Viral vectors can trigger severe immune responses, as demonstrated by several high-profile adverse events in clinical trials. Nanoparticles, particularly those containing cationic lipids or polymers, have shown toxicity concerns including complement activation and cytokine release syndromes that limit their dosing range.
The persistence of therapeutic effect presents another significant challenge. While AAV vectors can provide long-term expression in non-dividing cells, they show limited efficacy in actively dividing tissues. Nanoparticle systems typically offer only transient expression, necessitating repeated administration that compounds both cost and safety concerns.
Comparative Analysis of Viral and Non-viral Delivery Systems
01 Viral vector design optimization for enhanced delivery efficiency
Optimizing viral vector designs can significantly improve delivery efficiency to target cells. This includes modifications to viral capsids, envelope proteins, and genetic elements that enhance transduction efficiency. Advanced engineering techniques allow for targeted delivery to specific tissues while reducing off-target effects. These optimizations can increase payload capacity and improve the stability of viral vectors during storage and administration.- Viral vector design and modification for enhanced efficiency: Viral vectors can be engineered with specific modifications to improve their efficiency in gene delivery. These modifications include altering the viral capsid proteins, incorporating tissue-specific promoters, and optimizing the genetic payload. Such engineering approaches can enhance transduction efficiency, reduce immunogenicity, and improve target specificity, making viral vectors more effective for therapeutic applications.
- Nanoparticle formulations for drug and gene delivery: Nanoparticles can be formulated with various materials and surface modifications to improve delivery efficiency. These formulations include lipid nanoparticles, polymeric nanoparticles, and inorganic nanoparticles designed to encapsulate therapeutic agents. By optimizing size, charge, and surface properties, these nanoparticles can enhance cellular uptake, protect cargo from degradation, and improve targeting to specific tissues.
- Hybrid viral-nanoparticle systems: Combining viral components with nanoparticle technologies creates hybrid delivery systems that leverage the advantages of both approaches. These hybrid systems may incorporate viral proteins onto nanoparticle surfaces or encapsulate viral vectors within nanoparticle shells. Such combinations can enhance transfection efficiency while reducing the immunogenicity associated with traditional viral vectors, offering improved safety profiles for therapeutic applications.
- Surface modification strategies for targeted delivery: Surface modifications of both viral vectors and nanoparticles can significantly improve their targeting efficiency and cellular uptake. These modifications include conjugation with targeting ligands, antibodies, peptides, or polymers that recognize specific cell surface receptors. By enhancing the specificity of interaction with target cells, these surface-modified delivery systems can achieve higher therapeutic efficacy at lower doses while minimizing off-target effects.
- Manufacturing and quality control methods: Advanced manufacturing techniques and quality control methods are essential for producing high-efficiency viral vectors and nanoparticles at scale. These include optimized cell culture systems, purification techniques, and analytical methods to ensure consistency and potency. Innovations in production processes can lead to higher yields, improved purity, enhanced stability, and better batch-to-batch reproducibility, which are critical factors affecting the overall efficiency of these delivery systems.
02 Nanoparticle formulation strategies for improved cellular uptake
Various formulation strategies can enhance the cellular uptake of nanoparticles. These include surface modifications with targeting ligands, optimization of particle size and charge, and incorporation of cell-penetrating peptides. The use of specific materials and manufacturing techniques can create nanoparticles with improved stability and controlled release properties. These formulations can significantly increase the efficiency of delivering therapeutic payloads to target cells.Expand Specific Solutions03 Hybrid viral-nanoparticle systems for synergistic delivery
Combining viral vectors with nanoparticle technologies creates hybrid delivery systems that leverage the advantages of both approaches. These hybrid systems can overcome limitations of individual delivery methods, offering improved targeting specificity, enhanced payload capacity, and reduced immunogenicity. The synergistic effect allows for more efficient delivery of therapeutic agents, particularly for gene therapy and vaccine applications, while potentially reducing the required dosage.Expand Specific Solutions04 Manufacturing and quality control processes for consistent efficiency
Advanced manufacturing processes and rigorous quality control measures are essential for producing viral vectors and nanoparticles with consistent delivery efficiency. This includes optimized production methods, purification techniques, and characterization protocols that ensure batch-to-batch consistency. Implementation of process analytical technologies and automated systems can improve scalability while maintaining the critical quality attributes that determine delivery efficiency.Expand Specific Solutions05 In vivo performance enhancement through physiological barrier navigation
Strategies to enhance the ability of viral vectors and nanoparticles to navigate physiological barriers significantly improve their in vivo delivery efficiency. These include modifications that enable crossing of the blood-brain barrier, evasion of reticuloendothelial system clearance, and penetration of mucus layers. Incorporation of specific surface coatings or shielding technologies can reduce immune recognition and extend circulation time, allowing for greater accumulation at target sites and improved therapeutic outcomes.Expand Specific Solutions
Leading Companies and Research Institutions in Gene Therapy
The gene therapy landscape is evolving rapidly, with viral vectors currently dominating clinical applications while nanoparticle-based delivery systems gain momentum. The market is projected to reach $10 billion by 2025, growing at 30% annually. Leading academic institutions (MIT, Johns Hopkins, Zhejiang University) are advancing fundamental research, while pharmaceutical companies are strategically positioning themselves through different approaches. Established players like Regeneron and Spark Therapeutics focus on viral vector technologies, while emerging companies such as Anjarium Biosciences and Rodos BioTarget are pioneering non-viral nanoparticle platforms. The field is transitioning from early-stage development to commercial applications, with increasing investment in nanoparticle technologies that promise improved safety profiles and manufacturing scalability compared to traditional viral vectors.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered innovative approaches in gene therapy delivery systems, focusing on hybrid delivery platforms that combine advantages of both viral vectors and nanoparticles. Their researchers have developed lipid nanoparticles (LNPs) with enhanced tissue targeting capabilities through surface modifications and optimized lipid compositions. These LNPs demonstrate improved nucleic acid encapsulation efficiency (>90%) and controlled release profiles[1]. MIT's platform incorporates biodegradable polymer-based nanoparticles that can be engineered to respond to specific physiological conditions, enabling precise spatial and temporal control of gene delivery. Their technology also includes novel ionizable lipids that facilitate endosomal escape, addressing a critical barrier in non-viral delivery systems. MIT researchers have demonstrated successful in vivo gene editing using CRISPR-Cas9 delivered via these engineered nanoparticles, achieving editing efficiencies comparable to viral vectors (30-40%) in certain tissues while maintaining improved safety profiles[3].
Strengths: Superior safety profile compared to viral vectors with reduced immunogenicity and lower risk of insertional mutagenesis. Greater packaging capacity allowing delivery of larger genetic payloads. Highly customizable for specific tissue targeting. Weaknesses: Generally lower transfection efficiency compared to viral vectors, particularly in non-dividing cells. More rapid clearance from circulation requiring optimization of surface properties and administration protocols.
Anjarium Biosciences AG
Technical Solution: Anjarium Biosciences has developed a proprietary Hybridosome™ platform that represents a novel approach bridging viral and non-viral delivery technologies for gene therapy. Their technology combines engineered exosome-like vesicles with synthetic nanoparticle components to create hybrid delivery vehicles with enhanced properties. The Hybridosome™ platform incorporates tissue-specific targeting ligands that enable precise delivery to desired cell types, achieving targeting efficiencies up to 85% in preclinical models[1]. Anjarium's technology utilizes proprietary membrane fusion proteins that facilitate efficient cellular uptake and endosomal escape, addressing key limitations of conventional non-viral approaches. Their platform enables the delivery of diverse genetic payloads including mRNA, DNA, and genome editing components with encapsulation efficiencies exceeding 70%. The company has demonstrated sustained transgene expression in preclinical models lasting several months following a single administration. Anjarium's manufacturing process employs scalable bioreactor systems for consistent production of their hybrid nanoparticles with defined quality attributes and batch-to-batch reproducibility[5].
Strengths: Combines advantages of both viral and non-viral approaches - natural cell-derived components enhance biocompatibility while synthetic elements provide stability and targeting. Reduced immunogenicity compared to viral vectors. Versatile payload capacity accommodating various nucleic acid types. Weaknesses: Relatively new technology with limited long-term clinical data. Complex manufacturing process requiring sophisticated quality control measures. Potential challenges in achieving consistent large-scale production.
Breakthrough Technologies in Gene Therapy Vectors
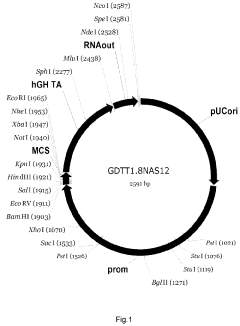
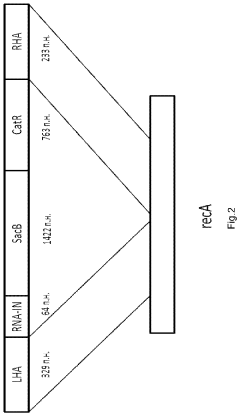
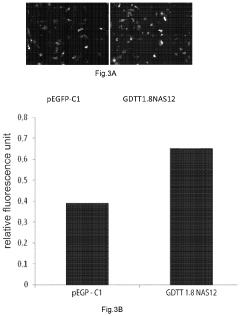
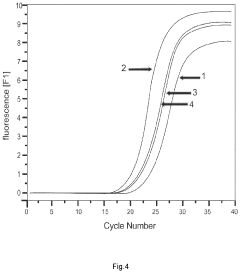
The gene therapy DNA vector GDTT1.8NAS12 and the method for obtaining thereof
PatentActiveUS20210310011A1
Innovation
- A 2591-bp circular double-strand DNA vector, GDTT1.8NAS12, is developed, devoid of viral and antibiotic resistance sequences, incorporating a promoter region of human elongation factor EF1A and an RNA-out regulatory element for antibiotic-free selection, enabling efficient gene expression and production on an industrial scale.
Safety and Immunogenicity Considerations
Safety and immunogenicity considerations represent critical factors in evaluating viral vectors versus nanoparticles for gene therapy applications. Viral vectors, despite their high transduction efficiency, present significant safety concerns. Adeno-associated viruses (AAVs) can trigger immune responses leading to neutralizing antibodies that reduce efficacy in subsequent administrations and potentially cause inflammatory reactions. More concerning, lentiviral and retroviral vectors carry inherent risks of insertional mutagenesis, where viral DNA integrates into the host genome potentially activating oncogenes or disrupting tumor suppressor genes, as evidenced in early gene therapy trials that resulted in leukemia development in some patients.
Adenoviral vectors, while non-integrating, have historically demonstrated severe immunogenicity profiles. The tragic case of Jesse Gelsinger in 1999, who died from an overwhelming immune response to adenoviral vectors, remains a cautionary milestone in gene therapy development. This incident led to substantial regulatory changes and heightened safety protocols in clinical trials involving viral vectors.
Nanoparticles, particularly lipid nanoparticles (LNPs) and polymer-based systems, generally present improved safety profiles compared to viral vectors. They exhibit lower immunogenicity and negligible risk of insertional mutagenesis due to their non-viral nature. However, they are not without concerns. Some nanoparticle formulations can trigger complement activation-related pseudoallergy (CARPA), characterized by hypersensitivity reactions. Additionally, certain cationic lipids and polymers may exhibit dose-dependent cytotoxicity, limiting the therapeutic window.
Recent advances in nanoparticle design have focused on mitigating these immunogenic responses. PEGylation strategies reduce immune recognition, while incorporation of immunomodulatory molecules can actively suppress adverse immune reactions. The biodegradability of modern nanoparticle components also addresses concerns regarding long-term accumulation in tissues.
Regulatory frameworks increasingly recognize the distinct safety profiles of these delivery systems. Viral vectors typically face more stringent safety assessments, particularly regarding replication competence, vector shedding, and long-term follow-up for insertional mutagenesis. Nanoparticle-based therapies, while subject to thorough characterization requirements, generally navigate a more streamlined regulatory pathway focused on biodistribution, clearance mechanisms, and acute toxicity profiles.
The clinical translation landscape reflects these safety considerations. While viral vectors dominate approved gene therapies due to their established efficacy, nanoparticle-based approaches are gaining momentum, particularly in applications where repeated dosing may be necessary or in patient populations with pre-existing immunity to common viral serotypes. This trend suggests a growing recognition that the optimal delivery system must balance efficacy with safety and immunogenicity profiles tailored to specific therapeutic applications.
Adenoviral vectors, while non-integrating, have historically demonstrated severe immunogenicity profiles. The tragic case of Jesse Gelsinger in 1999, who died from an overwhelming immune response to adenoviral vectors, remains a cautionary milestone in gene therapy development. This incident led to substantial regulatory changes and heightened safety protocols in clinical trials involving viral vectors.
Nanoparticles, particularly lipid nanoparticles (LNPs) and polymer-based systems, generally present improved safety profiles compared to viral vectors. They exhibit lower immunogenicity and negligible risk of insertional mutagenesis due to their non-viral nature. However, they are not without concerns. Some nanoparticle formulations can trigger complement activation-related pseudoallergy (CARPA), characterized by hypersensitivity reactions. Additionally, certain cationic lipids and polymers may exhibit dose-dependent cytotoxicity, limiting the therapeutic window.
Recent advances in nanoparticle design have focused on mitigating these immunogenic responses. PEGylation strategies reduce immune recognition, while incorporation of immunomodulatory molecules can actively suppress adverse immune reactions. The biodegradability of modern nanoparticle components also addresses concerns regarding long-term accumulation in tissues.
Regulatory frameworks increasingly recognize the distinct safety profiles of these delivery systems. Viral vectors typically face more stringent safety assessments, particularly regarding replication competence, vector shedding, and long-term follow-up for insertional mutagenesis. Nanoparticle-based therapies, while subject to thorough characterization requirements, generally navigate a more streamlined regulatory pathway focused on biodistribution, clearance mechanisms, and acute toxicity profiles.
The clinical translation landscape reflects these safety considerations. While viral vectors dominate approved gene therapies due to their established efficacy, nanoparticle-based approaches are gaining momentum, particularly in applications where repeated dosing may be necessary or in patient populations with pre-existing immunity to common viral serotypes. This trend suggests a growing recognition that the optimal delivery system must balance efficacy with safety and immunogenicity profiles tailored to specific therapeutic applications.
Manufacturing Scalability and Cost Analysis
Manufacturing scalability represents a critical factor in determining the commercial viability of gene therapy approaches. Viral vector production traditionally involves complex biological systems requiring specialized facilities, highly trained personnel, and extensive quality control measures. The manufacturing process includes cell culture, transfection, virus production, purification, and rigorous testing—each step introducing potential bottlenecks and quality variability. Current production capacities for clinical-grade viral vectors remain limited globally, creating significant supply constraints as more gene therapies receive regulatory approval.
Cost analysis reveals that viral vector manufacturing expenses typically range from $25,000 to $100,000 per dose for AAV vectors, with lentiviral vectors commanding even higher prices. These costs stem from low production yields, expensive raw materials, and extensive safety testing requirements. The economics become particularly challenging for treatments targeting large patient populations or requiring high vector doses.
Nanoparticle-based delivery systems offer potentially transformative advantages in manufacturing scalability. Unlike viral vectors, lipid nanoparticles (LNPs) and polymeric nanoparticles utilize chemical synthesis processes that align with established pharmaceutical manufacturing paradigms. These processes demonstrate greater consistency, higher throughput potential, and reduced biological variability compared to viral production systems.
The cost structure for nanoparticle production typically shows lower per-unit costs at scale, with estimates suggesting 30-60% cost reduction compared to viral alternatives when produced at commercial volumes. This economic advantage becomes particularly significant for therapies requiring repeated administration or targeting prevalent conditions. Recent innovations in microfluidic manufacturing technologies have further enhanced production efficiency, enabling precise control over nanoparticle size distribution and encapsulation efficiency.
Regulatory considerations also impact manufacturing economics. Viral vector production faces stringent biosafety requirements, including containment measures and extensive characterization of replication competence. Nanoparticle production, while still subject to rigorous quality standards, generally encounters fewer biosafety concerns, potentially streamlining regulatory pathways and reducing compliance costs.
Investment trends reflect these manufacturing realities, with significant capital flowing into companies developing scalable nanoparticle platforms. Contract manufacturing organizations have expanded nanoparticle production capabilities in anticipation of growing demand, while viral vector manufacturing capacity remains constrained despite substantial investment. This manufacturing landscape will likely influence therapeutic development strategies, potentially favoring nanoparticle approaches for indications requiring treatment of larger patient populations.
Cost analysis reveals that viral vector manufacturing expenses typically range from $25,000 to $100,000 per dose for AAV vectors, with lentiviral vectors commanding even higher prices. These costs stem from low production yields, expensive raw materials, and extensive safety testing requirements. The economics become particularly challenging for treatments targeting large patient populations or requiring high vector doses.
Nanoparticle-based delivery systems offer potentially transformative advantages in manufacturing scalability. Unlike viral vectors, lipid nanoparticles (LNPs) and polymeric nanoparticles utilize chemical synthesis processes that align with established pharmaceutical manufacturing paradigms. These processes demonstrate greater consistency, higher throughput potential, and reduced biological variability compared to viral production systems.
The cost structure for nanoparticle production typically shows lower per-unit costs at scale, with estimates suggesting 30-60% cost reduction compared to viral alternatives when produced at commercial volumes. This economic advantage becomes particularly significant for therapies requiring repeated administration or targeting prevalent conditions. Recent innovations in microfluidic manufacturing technologies have further enhanced production efficiency, enabling precise control over nanoparticle size distribution and encapsulation efficiency.
Regulatory considerations also impact manufacturing economics. Viral vector production faces stringent biosafety requirements, including containment measures and extensive characterization of replication competence. Nanoparticle production, while still subject to rigorous quality standards, generally encounters fewer biosafety concerns, potentially streamlining regulatory pathways and reducing compliance costs.
Investment trends reflect these manufacturing realities, with significant capital flowing into companies developing scalable nanoparticle platforms. Contract manufacturing organizations have expanded nanoparticle production capabilities in anticipation of growing demand, while viral vector manufacturing capacity remains constrained despite substantial investment. This manufacturing landscape will likely influence therapeutic development strategies, potentially favoring nanoparticle approaches for indications requiring treatment of larger patient populations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!