Evaluation of Gene Therapy’s Long-Term Efficacy in Chronic Conditions
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy Evolution and Objectives
Gene therapy has evolved significantly since its conceptual inception in the 1970s, transitioning from theoretical possibility to clinical reality. The field experienced its first major milestone in 1990 with the treatment of adenosine deaminase deficiency, marking the beginning of human gene therapy trials. Despite early setbacks, including the tragic death of Jesse Gelsinger in 1999 that temporarily halted progress, the field has demonstrated remarkable resilience and advancement over the past two decades.
The evolution of gene therapy technologies has been characterized by three distinct generations. First-generation approaches primarily utilized viral vectors with limited targeting capabilities. Second-generation systems introduced improved vector designs with enhanced safety profiles and reduced immunogenicity. Current third-generation platforms leverage precision editing tools like CRISPR-Cas9, AAV vectors, and non-viral delivery systems, dramatically expanding therapeutic possibilities.
For chronic conditions specifically, gene therapy development has progressed from symptom management to potentially curative interventions. Early applications focused on monogenic disorders like hemophilia and cystic fibrosis, while recent advances have expanded to complex polygenic conditions including cardiovascular diseases, neurodegenerative disorders, and certain forms of cancer. This expansion represents a fundamental shift in treatment paradigms from chronic management to single-intervention approaches with long-term efficacy.
The primary objective in evaluating gene therapy's long-term efficacy for chronic conditions centers on establishing durable therapeutic effects that persist throughout a patient's lifetime without requiring repeated interventions. This goal necessitates robust assessment frameworks that can monitor efficacy beyond traditional clinical trial timeframes, potentially spanning decades rather than years.
Secondary objectives include determining optimal delivery mechanisms for specific tissue types, establishing predictive biomarkers for long-term response, quantifying the economic value proposition compared to lifetime conventional treatments, and developing standardized safety monitoring protocols for detecting delayed adverse events that may emerge years after treatment.
The technological trajectory suggests continued refinement of delivery systems, improved targeting specificity, and enhanced expression durability. Emerging research indicates potential for regulatable gene expression systems that allow post-treatment modulation of therapeutic effects, addressing concerns about irreversibility and enabling personalized dosing adjustments as chronic disease states evolve over time.
The evolution of gene therapy technologies has been characterized by three distinct generations. First-generation approaches primarily utilized viral vectors with limited targeting capabilities. Second-generation systems introduced improved vector designs with enhanced safety profiles and reduced immunogenicity. Current third-generation platforms leverage precision editing tools like CRISPR-Cas9, AAV vectors, and non-viral delivery systems, dramatically expanding therapeutic possibilities.
For chronic conditions specifically, gene therapy development has progressed from symptom management to potentially curative interventions. Early applications focused on monogenic disorders like hemophilia and cystic fibrosis, while recent advances have expanded to complex polygenic conditions including cardiovascular diseases, neurodegenerative disorders, and certain forms of cancer. This expansion represents a fundamental shift in treatment paradigms from chronic management to single-intervention approaches with long-term efficacy.
The primary objective in evaluating gene therapy's long-term efficacy for chronic conditions centers on establishing durable therapeutic effects that persist throughout a patient's lifetime without requiring repeated interventions. This goal necessitates robust assessment frameworks that can monitor efficacy beyond traditional clinical trial timeframes, potentially spanning decades rather than years.
Secondary objectives include determining optimal delivery mechanisms for specific tissue types, establishing predictive biomarkers for long-term response, quantifying the economic value proposition compared to lifetime conventional treatments, and developing standardized safety monitoring protocols for detecting delayed adverse events that may emerge years after treatment.
The technological trajectory suggests continued refinement of delivery systems, improved targeting specificity, and enhanced expression durability. Emerging research indicates potential for regulatable gene expression systems that allow post-treatment modulation of therapeutic effects, addressing concerns about irreversibility and enabling personalized dosing adjustments as chronic disease states evolve over time.
Market Analysis for Chronic Disease Gene Therapies
The global market for gene therapies targeting chronic conditions is experiencing unprecedented growth, driven by significant advancements in genomic technologies and increasing prevalence of chronic diseases worldwide. Current market valuations indicate that the gene therapy sector focused on chronic conditions reached approximately $5.6 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 16.8% through 2030. This remarkable expansion reflects both scientific progress and growing clinical acceptance of gene-based interventions for long-term disease management.
Chronic diseases represent an enormous healthcare burden globally, affecting nearly 60% of the adult population in developed nations. Conditions such as diabetes, cardiovascular diseases, neurodegenerative disorders, and certain cancers constitute primary targets for gene therapy applications. The economic impact of these conditions extends beyond direct healthcare costs to include productivity losses, estimated at $3.7 trillion annually worldwide, creating a substantial market opportunity for effective gene therapy solutions.
Patient demographics significantly influence market dynamics, with aging populations in North America, Europe, and East Asia driving increased demand for innovative therapeutic approaches. These regions currently account for over 75% of global gene therapy research and clinical trials targeting chronic conditions, though emerging markets are showing accelerated growth as healthcare infrastructure improves and regulatory frameworks evolve.
Reimbursement landscapes vary considerably across markets, presenting both opportunities and challenges. In the United States, recent policy shifts have created pathways for value-based payment models specifically designed for high-cost, potentially curative gene therapies. The European market has implemented innovative risk-sharing agreements between manufacturers and payers, while Asian markets are developing novel financing mechanisms to address affordability concerns.
Market segmentation analysis reveals that hemophilia, beta-thalassemia, and certain inherited retinal diseases currently represent the most commercially viable applications for gene therapy in chronic conditions. However, significant R&D investment is being directed toward more prevalent conditions including type 1 diabetes, Parkinson's disease, and heart failure, which could dramatically expand the addressable market within the next decade.
Consumer acceptance and awareness present interesting market dynamics. Recent surveys indicate growing patient willingness to consider gene therapy options, with 68% of chronic disease patients expressing interest in gene-based treatments if conventional therapies prove inadequate. This represents a significant shift in patient perspectives compared to survey data from just five years ago, when acceptance rates were below 40%.
Competitive analysis indicates a market currently dominated by specialized biotech firms and academic spinoffs, though major pharmaceutical companies are rapidly entering through strategic acquisitions and partnerships. This consolidation trend is expected to accelerate as more therapies demonstrate long-term efficacy in late-stage clinical trials.
Chronic diseases represent an enormous healthcare burden globally, affecting nearly 60% of the adult population in developed nations. Conditions such as diabetes, cardiovascular diseases, neurodegenerative disorders, and certain cancers constitute primary targets for gene therapy applications. The economic impact of these conditions extends beyond direct healthcare costs to include productivity losses, estimated at $3.7 trillion annually worldwide, creating a substantial market opportunity for effective gene therapy solutions.
Patient demographics significantly influence market dynamics, with aging populations in North America, Europe, and East Asia driving increased demand for innovative therapeutic approaches. These regions currently account for over 75% of global gene therapy research and clinical trials targeting chronic conditions, though emerging markets are showing accelerated growth as healthcare infrastructure improves and regulatory frameworks evolve.
Reimbursement landscapes vary considerably across markets, presenting both opportunities and challenges. In the United States, recent policy shifts have created pathways for value-based payment models specifically designed for high-cost, potentially curative gene therapies. The European market has implemented innovative risk-sharing agreements between manufacturers and payers, while Asian markets are developing novel financing mechanisms to address affordability concerns.
Market segmentation analysis reveals that hemophilia, beta-thalassemia, and certain inherited retinal diseases currently represent the most commercially viable applications for gene therapy in chronic conditions. However, significant R&D investment is being directed toward more prevalent conditions including type 1 diabetes, Parkinson's disease, and heart failure, which could dramatically expand the addressable market within the next decade.
Consumer acceptance and awareness present interesting market dynamics. Recent surveys indicate growing patient willingness to consider gene therapy options, with 68% of chronic disease patients expressing interest in gene-based treatments if conventional therapies prove inadequate. This represents a significant shift in patient perspectives compared to survey data from just five years ago, when acceptance rates were below 40%.
Competitive analysis indicates a market currently dominated by specialized biotech firms and academic spinoffs, though major pharmaceutical companies are rapidly entering through strategic acquisitions and partnerships. This consolidation trend is expected to accelerate as more therapies demonstrate long-term efficacy in late-stage clinical trials.
Current Limitations and Technical Barriers
Despite significant advancements in gene therapy for chronic conditions, several critical limitations and technical barriers continue to impede its long-term efficacy evaluation. The durability of therapeutic effects remains a primary concern, with many gene therapy interventions showing diminishing expression over time. This phenomenon, known as transgene silencing, occurs through various epigenetic mechanisms including DNA methylation and histone modifications, particularly in non-integrating vector systems.
Vector immunogenicity presents another substantial challenge, as patients often develop neutralizing antibodies against viral vectors like AAV (Adeno-Associated Virus), preventing effective re-administration and limiting treatment options for conditions requiring repeated intervention. This immune response varies significantly between individuals, complicating standardized efficacy assessments across patient populations.
The integration site specificity of viral vectors raises significant safety concerns regarding insertional mutagenesis and oncogenesis. While lentiviral vectors have improved safety profiles compared to earlier retroviral systems, the risk of disrupting essential genes or activating oncogenes remains a critical barrier to widespread clinical adoption for chronic condition management.
Delivery efficiency to target tissues represents a persistent technical challenge. Many chronic conditions affect tissues with complex barriers (like the blood-brain barrier) or involve multiple organ systems, making comprehensive therapeutic delivery exceptionally difficult. Current vector technologies demonstrate variable tropism and often insufficient penetration to achieve therapeutic thresholds in all affected tissues.
Manufacturing scalability and consistency pose significant barriers to standardized efficacy evaluation. The production of clinical-grade viral vectors remains costly and technically challenging, with batch-to-batch variations potentially affecting treatment outcomes. This inconsistency complicates long-term comparative studies and regulatory approval processes.
Dosing optimization remains poorly understood, with the relationship between vector dose, transgene expression, and therapeutic effect showing non-linear patterns across different tissues and conditions. This complexity is further compounded by patient-specific factors including age, genetic background, and disease progression stage.
Regulatory frameworks for monitoring long-term efficacy are still evolving, with insufficient standardization of endpoints and follow-up protocols. The absence of validated biomarkers for many chronic conditions further complicates objective assessment of therapeutic durability, particularly for conditions with variable natural progression.
Cost-effectiveness represents a final but critical barrier, as the extreme expense of current gene therapy approaches limits both clinical trial scope and potential market access, restricting the collection of comprehensive long-term efficacy data across diverse patient populations.
Vector immunogenicity presents another substantial challenge, as patients often develop neutralizing antibodies against viral vectors like AAV (Adeno-Associated Virus), preventing effective re-administration and limiting treatment options for conditions requiring repeated intervention. This immune response varies significantly between individuals, complicating standardized efficacy assessments across patient populations.
The integration site specificity of viral vectors raises significant safety concerns regarding insertional mutagenesis and oncogenesis. While lentiviral vectors have improved safety profiles compared to earlier retroviral systems, the risk of disrupting essential genes or activating oncogenes remains a critical barrier to widespread clinical adoption for chronic condition management.
Delivery efficiency to target tissues represents a persistent technical challenge. Many chronic conditions affect tissues with complex barriers (like the blood-brain barrier) or involve multiple organ systems, making comprehensive therapeutic delivery exceptionally difficult. Current vector technologies demonstrate variable tropism and often insufficient penetration to achieve therapeutic thresholds in all affected tissues.
Manufacturing scalability and consistency pose significant barriers to standardized efficacy evaluation. The production of clinical-grade viral vectors remains costly and technically challenging, with batch-to-batch variations potentially affecting treatment outcomes. This inconsistency complicates long-term comparative studies and regulatory approval processes.
Dosing optimization remains poorly understood, with the relationship between vector dose, transgene expression, and therapeutic effect showing non-linear patterns across different tissues and conditions. This complexity is further compounded by patient-specific factors including age, genetic background, and disease progression stage.
Regulatory frameworks for monitoring long-term efficacy are still evolving, with insufficient standardization of endpoints and follow-up protocols. The absence of validated biomarkers for many chronic conditions further complicates objective assessment of therapeutic durability, particularly for conditions with variable natural progression.
Cost-effectiveness represents a final but critical barrier, as the extreme expense of current gene therapy approaches limits both clinical trial scope and potential market access, restricting the collection of comprehensive long-term efficacy data across diverse patient populations.
Current Gene Delivery Vector Technologies
01 Long-term gene expression monitoring and maintenance strategies
Effective gene therapy requires sustained expression of therapeutic genes over extended periods. Various strategies have been developed to monitor and maintain long-term gene expression, including advanced imaging techniques, biomarkers for tracking gene expression, and controlled release systems. These approaches help ensure therapeutic efficacy by providing real-time feedback on gene expression levels and allowing for adjustments to treatment protocols when necessary.- Viral vector optimization for long-term gene expression: Optimization of viral vectors is crucial for achieving sustained gene expression in gene therapy. Modified viral vectors, such as adeno-associated viruses (AAVs) and lentiviruses, can be engineered to improve transduction efficiency, reduce immunogenicity, and enhance the durability of therapeutic gene expression. These optimized vectors allow for better targeting of specific tissues and cells, resulting in more stable and long-lasting therapeutic effects.
- Immune modulation strategies to prevent therapeutic gene silencing: Immune responses against gene therapy vectors or transgene products can significantly limit long-term efficacy. Various immune modulation strategies have been developed to overcome this challenge, including co-administration of immunosuppressive agents, use of tolerance-inducing protocols, and engineering of vectors with reduced immunogenicity. These approaches help prevent the clearance of transduced cells and maintain therapeutic gene expression over extended periods.
- Integration site selection and genomic stability for durable expression: The genomic location where therapeutic genes integrate can significantly impact long-term expression. Technologies that direct integration to safe harbor sites or use site-specific recombination systems can enhance genomic stability and reduce the risk of insertional mutagenesis. Additionally, methods to monitor integration sites and assess genomic stability over time are essential for evaluating the long-term safety and efficacy of gene therapy approaches.
- Regulated gene expression systems for sustained therapeutic effect: Regulated gene expression systems allow for controlled delivery of therapeutic proteins over extended periods. These systems include inducible promoters, small molecule-responsive elements, and feedback-regulated circuits that can adjust transgene expression based on physiological needs. Such precise control mechanisms help maintain therapeutic levels of gene products while avoiding toxicity, contributing to improved long-term efficacy of gene therapy treatments.
- Long-term efficacy assessment and monitoring methodologies: Comprehensive methodologies for assessing and monitoring the long-term efficacy of gene therapy are essential for clinical translation. These include advanced imaging techniques, biomarker analysis, functional assessments, and longitudinal studies that track therapeutic outcomes over extended periods. Such monitoring approaches help evaluate the persistence of gene expression, detect potential adverse effects, and determine the durability of clinical benefits in treated patients.
02 Vector design optimization for durable therapeutic effect
The design of gene delivery vectors significantly impacts long-term efficacy of gene therapy. Advanced vector systems incorporate elements that prevent silencing of transgene expression, reduce immunogenicity, and enable tissue-specific targeting. Innovations include self-regulating vectors, integration-deficient vectors that minimize genotoxicity risks, and hybrid vector systems that combine advantages of viral and non-viral approaches to achieve sustained therapeutic effects without compromising safety.Expand Specific Solutions03 Immune modulation approaches for sustained gene therapy
Immune responses against gene therapy vectors or transgene products can significantly limit long-term efficacy. Strategies to overcome this challenge include co-delivery of immunomodulatory molecules, transient immunosuppression during initial treatment, development of less immunogenic vectors, and induction of immune tolerance to therapeutic proteins. These approaches help prevent neutralization of vectors upon readministration and allow for persistent expression of therapeutic genes.Expand Specific Solutions04 Integration of gene therapy with tissue engineering for durability
Combining gene therapy with tissue engineering approaches enhances long-term efficacy by providing a supportive microenvironment for modified cells. Engineered scaffolds can serve as depots for sustained release of therapeutic genes or modified cells, protecting them from immune clearance and providing structural support. This integrated approach is particularly valuable for treating conditions requiring regeneration of complex tissues while maintaining therapeutic gene expression over extended periods.Expand Specific Solutions05 Clinical assessment methods for long-term gene therapy outcomes
Evaluating the long-term efficacy of gene therapy requires specialized clinical assessment methods. These include longitudinal monitoring protocols, biomarker development for early detection of efficacy decline, standardized outcome measures specific to gene therapy applications, and advanced statistical models that account for the unique kinetics of gene therapy responses. These assessment frameworks help determine the durability of therapeutic effects and guide decisions about potential retreatment.Expand Specific Solutions
Leading Companies and Research Institutions
Gene therapy for chronic conditions is currently in a transitional phase between early clinical adoption and broader market implementation. The global gene therapy market, valued at approximately $7.6 billion in 2022, is projected to grow significantly as long-term efficacy data emerges. Technical maturity varies considerably across therapeutic areas, with companies demonstrating different levels of advancement. Leading organizations like Biogen, Juno Therapeutics, and Incyte are pioneering commercial applications, while research institutions such as Fred Hutchinson Cancer Research Center, University of Zurich, and Mount Sinai are driving fundamental breakthroughs in delivery mechanisms and genetic editing technologies. Academic-industry partnerships, exemplified by collaborations between Tempus AI and research foundations, are accelerating progress toward addressing durability challenges in chronic disease applications.
Biogen MA, Inc.
Technical Solution: Biogen has developed advanced AAV (adeno-associated virus) vector platforms for long-term gene expression in chronic neurological conditions. Their approach focuses on optimized capsid design with reduced immunogenicity and enhanced blood-brain barrier penetration. For chronic conditions like spinal muscular atrophy (SMA), they've implemented one-time gene replacement therapy (Zolgensma) that has demonstrated sustained efficacy over 5+ years in clinical studies. Biogen's platform incorporates tissue-specific promoters to ensure targeted expression and minimize off-target effects. Their long-term efficacy evaluation includes comprehensive biomarker monitoring and functional outcome assessments at regular intervals (6, 12, 24, 36 months) post-treatment to track disease progression markers and therapeutic protein expression levels.
Strengths: Strong expertise in neurological disorders; established manufacturing infrastructure; robust clinical trial design for long-term follow-up. Weaknesses: High treatment costs limiting accessibility; potential for neutralizing antibodies against viral vectors reducing efficacy in some patients; challenges in demonstrating cost-effectiveness for payers despite long-term benefits.
Fondazione Telethon Ets
Technical Solution: Fondazione Telethon has established a specialized platform for evaluating gene therapy efficacy in rare genetic chronic conditions, with particular expertise in primary immunodeficiencies and metabolic disorders. Their approach centers on lentiviral vector-based ex vivo gene therapy, with proprietary vector designs optimized for long-term expression in hematopoietic stem cells. Telethon's evaluation framework includes comprehensive immune reconstitution assessment through flow cytometry panels tracking multiple cell lineages over 5+ year periods. For metabolic disorders, they employ specialized biochemical assays to monitor enzyme activity and metabolite clearance as markers of sustained therapeutic effect. Their platform incorporates regular quality-of-life assessments and disease-specific functional metrics to correlate molecular findings with clinical benefits. Telethon has pioneered integration site analysis techniques to evaluate clonal dynamics of genetically modified cells over time, providing critical safety data regarding insertional mutagenesis risks in long-term applications. Their approach has demonstrated sustained efficacy in conditions like ADA-SCID and metachromatic leukodystrophy, with follow-up data extending beyond 10 years in some patients.
Strengths: Extensive experience with rare disease patient populations; established long-term follow-up protocols; strong academic partnerships enhancing research capabilities. Weaknesses: Limited commercial infrastructure; challenges in scaling specialized approaches to larger patient populations; heavy reliance on external funding sources affecting program sustainability.
Breakthrough Research in Long-Term Expression
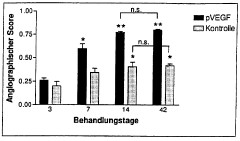
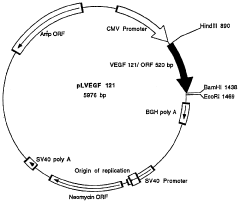
Use of a human vascular endothelial cell growth factor gene for direct genetic therapy
PatentWO1998005774A1
Innovation
- Direct gene therapy using a human vascular endothelial cell growth factor (VEGF) gene, incorporated into a functional DNA construct with regulatory signals, is introduced into cells via injection or liposome-mediated delivery to stimulate vascular growth and tissue repair.
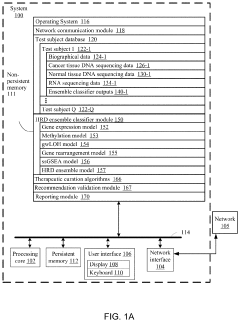
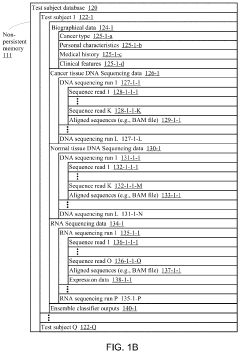
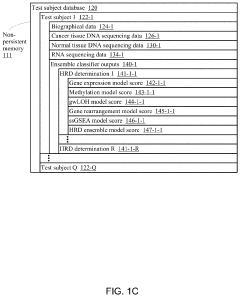
Systems and methods for predicting homologous recombination deficiency status of a specimen
PatentActiveUS20210172024A1
Innovation
- A system and method using machine-learning classifiers trained on RNA and DNA sequencing data to predict HRD status, incorporating genome-wide loss of heterozygosity and gene expression levels, enabling the identification of HRD-positive cancers without biallelic loss of BRCA1 or BRCA2.
Regulatory Framework for Gene Therapy Approval
The regulatory landscape for gene therapy approval represents a complex and evolving framework that significantly impacts the evaluation of long-term efficacy in chronic conditions. The U.S. Food and Drug Administration (FDA) has established a comprehensive pathway through the Center for Biologics Evaluation and Research (CBER), requiring rigorous preclinical testing, phased clinical trials, and post-marketing surveillance specifically designed to capture long-term outcomes.
European regulatory bodies, primarily the European Medicines Agency (EMA), have implemented the Advanced Therapy Medicinal Products (ATMP) classification, which includes specific provisions for gene therapies. This framework demands extensive documentation of manufacturing processes, quality control measures, and long-term follow-up protocols extending up to 15 years for certain genetic modifications.
Regulatory requirements for demonstrating long-term efficacy present unique challenges in chronic condition applications. Unlike conventional pharmaceuticals, gene therapies must demonstrate durability of effect across extended timeframes, often necessitating novel clinical trial designs and endpoints. Regulatory agencies increasingly require sponsors to implement Risk Evaluation and Mitigation Strategies (REMS) that include long-term patient registries and systematic follow-up protocols.
Accelerated approval pathways have emerged as critical mechanisms for promising gene therapies. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme provide expedited review processes while maintaining rigorous safety standards. These pathways typically require robust surrogate endpoints with established correlation to clinical outcomes and comprehensive plans for post-approval data collection.
International harmonization efforts, including the International Council for Harmonisation (ICH) guidelines, are working to standardize regulatory approaches across major markets. These initiatives aim to address disparities in requirements for long-term efficacy demonstration while maintaining appropriate regional considerations for different healthcare systems and patient populations.
Regulatory frameworks increasingly incorporate adaptive licensing approaches, allowing for conditional approvals based on early evidence of efficacy with requirements for ongoing data collection. This model is particularly relevant for gene therapies targeting chronic conditions, where treatment effects may evolve over extended periods and initial approvals may be based on intermediate endpoints.
The regulatory landscape continues to evolve in response to emerging scientific understanding and therapeutic applications. Recent developments include increased focus on patient-reported outcomes as complementary efficacy measures, standardization of vector characterization requirements, and refined approaches to assessing the durability of treatment effects in slowly progressing chronic conditions.
European regulatory bodies, primarily the European Medicines Agency (EMA), have implemented the Advanced Therapy Medicinal Products (ATMP) classification, which includes specific provisions for gene therapies. This framework demands extensive documentation of manufacturing processes, quality control measures, and long-term follow-up protocols extending up to 15 years for certain genetic modifications.
Regulatory requirements for demonstrating long-term efficacy present unique challenges in chronic condition applications. Unlike conventional pharmaceuticals, gene therapies must demonstrate durability of effect across extended timeframes, often necessitating novel clinical trial designs and endpoints. Regulatory agencies increasingly require sponsors to implement Risk Evaluation and Mitigation Strategies (REMS) that include long-term patient registries and systematic follow-up protocols.
Accelerated approval pathways have emerged as critical mechanisms for promising gene therapies. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme provide expedited review processes while maintaining rigorous safety standards. These pathways typically require robust surrogate endpoints with established correlation to clinical outcomes and comprehensive plans for post-approval data collection.
International harmonization efforts, including the International Council for Harmonisation (ICH) guidelines, are working to standardize regulatory approaches across major markets. These initiatives aim to address disparities in requirements for long-term efficacy demonstration while maintaining appropriate regional considerations for different healthcare systems and patient populations.
Regulatory frameworks increasingly incorporate adaptive licensing approaches, allowing for conditional approvals based on early evidence of efficacy with requirements for ongoing data collection. This model is particularly relevant for gene therapies targeting chronic conditions, where treatment effects may evolve over extended periods and initial approvals may be based on intermediate endpoints.
The regulatory landscape continues to evolve in response to emerging scientific understanding and therapeutic applications. Recent developments include increased focus on patient-reported outcomes as complementary efficacy measures, standardization of vector characterization requirements, and refined approaches to assessing the durability of treatment effects in slowly progressing chronic conditions.
Safety Monitoring and Post-Market Surveillance
Safety monitoring and post-market surveillance represent critical components in the evaluation of gene therapy's long-term efficacy for chronic conditions. The unique nature of gene therapies, which often involve permanent genetic modifications, necessitates robust surveillance systems that extend far beyond traditional pharmaceutical monitoring frameworks. Current regulatory bodies, including the FDA and EMA, have established specialized risk evaluation and mitigation strategies (REMS) specifically designed for gene therapy products, requiring manufacturers to conduct follow-up studies spanning 15 years or more.
These long-term monitoring protocols typically incorporate multiple layers of safety assessment, including patient registries that track treated individuals throughout their lifetimes. Such registries enable researchers to identify delayed adverse events that may not manifest during clinical trials, particularly those related to insertional mutagenesis or immune responses that could develop years after treatment. The FDA's guidance on long-term follow-up after administration of human gene therapy products emphasizes the importance of monitoring for delayed adverse events related to vector persistence and genomic integration.
Advanced biomarker monitoring represents another crucial element in post-market surveillance of gene therapies. This involves regular assessment of specific molecular indicators that might signal potential safety concerns before clinical symptoms appear. For chronic conditions such as hemophilia or certain lysosomal storage disorders, these biomarkers provide early warning signs of diminishing therapeutic effect or emerging safety issues, allowing for timely intervention.
Real-world evidence collection has emerged as a valuable complement to traditional clinical data. By leveraging electronic health records, wearable devices, and patient-reported outcomes, manufacturers and regulatory agencies can gather comprehensive data on gene therapy performance outside the controlled environment of clinical trials. This approach has proven particularly valuable for rare diseases where limited patient populations make traditional post-approval studies challenging.
International harmonization of safety monitoring standards represents an ongoing challenge in the field. Different regulatory jurisdictions maintain varying requirements for post-market surveillance, creating complexities for global development programs. Initiatives like the International Pharmaceutical Regulators Programme (IPRP) are working to establish consistent approaches to gene therapy monitoring, though significant differences remain in reporting requirements and follow-up protocols across major markets.
The financial sustainability of long-term monitoring programs presents another significant challenge. The cost of maintaining decades-long surveillance systems must be balanced against the need for comprehensive safety data, particularly for therapies targeting rare conditions with small patient populations. Innovative funding models, including public-private partnerships and conditional reimbursement schemes tied to ongoing data collection, are being explored to address this challenge.
These long-term monitoring protocols typically incorporate multiple layers of safety assessment, including patient registries that track treated individuals throughout their lifetimes. Such registries enable researchers to identify delayed adverse events that may not manifest during clinical trials, particularly those related to insertional mutagenesis or immune responses that could develop years after treatment. The FDA's guidance on long-term follow-up after administration of human gene therapy products emphasizes the importance of monitoring for delayed adverse events related to vector persistence and genomic integration.
Advanced biomarker monitoring represents another crucial element in post-market surveillance of gene therapies. This involves regular assessment of specific molecular indicators that might signal potential safety concerns before clinical symptoms appear. For chronic conditions such as hemophilia or certain lysosomal storage disorders, these biomarkers provide early warning signs of diminishing therapeutic effect or emerging safety issues, allowing for timely intervention.
Real-world evidence collection has emerged as a valuable complement to traditional clinical data. By leveraging electronic health records, wearable devices, and patient-reported outcomes, manufacturers and regulatory agencies can gather comprehensive data on gene therapy performance outside the controlled environment of clinical trials. This approach has proven particularly valuable for rare diseases where limited patient populations make traditional post-approval studies challenging.
International harmonization of safety monitoring standards represents an ongoing challenge in the field. Different regulatory jurisdictions maintain varying requirements for post-market surveillance, creating complexities for global development programs. Initiatives like the International Pharmaceutical Regulators Programme (IPRP) are working to establish consistent approaches to gene therapy monitoring, though significant differences remain in reporting requirements and follow-up protocols across major markets.
The financial sustainability of long-term monitoring programs presents another significant challenge. The cost of maintaining decades-long surveillance systems must be balanced against the need for comprehensive safety data, particularly for therapies targeting rare conditions with small patient populations. Innovative funding models, including public-private partnerships and conditional reimbursement schemes tied to ongoing data collection, are being explored to address this challenge.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!