How Do Epigenetic Factors Affect Gene Therapy Outcomes?
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Epigenetic Mechanisms and Gene Therapy Evolution
Gene therapy has evolved significantly since its conceptual inception in the 1970s, progressing through distinct developmental phases marked by both breakthroughs and setbacks. The initial phase focused on basic viral vector development and proof-of-concept studies, while facing significant safety concerns after adverse events in clinical trials during the late 1990s. This led to a period of reevaluation and refinement in the early 2000s, with researchers developing improved vector systems and safety protocols.
The field experienced a renaissance around 2010-2015 with the approval of the first gene therapies in Europe and later in the United States. This period saw the emergence of more sophisticated delivery systems and expanded therapeutic applications beyond monogenic disorders to include cancer, cardiovascular diseases, and neurological conditions.
Concurrently, our understanding of epigenetics has undergone its own evolution. Initially focused on DNA methylation and histone modifications, the field has expanded to encompass chromatin remodeling, non-coding RNAs, and three-dimensional genome organization. These mechanisms have been increasingly recognized as critical regulators of gene expression that operate beyond the primary DNA sequence.
The convergence of gene therapy and epigenetics represents a significant technological inflection point. Early gene therapy approaches largely ignored epigenetic considerations, focusing primarily on delivering functional gene copies. However, research has revealed that epigenetic factors significantly influence transgene expression durability, silencing phenomena, and overall therapeutic efficacy.
Modern gene therapy strategies have begun incorporating epigenetic insights, developing vectors designed to overcome silencing mechanisms and maintain therapeutic expression levels. Technologies like CRISPR-based epigenome editing have emerged, allowing precise modification of epigenetic marks without altering the underlying DNA sequence.
The evolution continues with the development of epigenetically-informed gene therapy approaches that consider the chromatin landscape of target cells, employ tissue-specific promoters resistant to silencing, and incorporate insulators to protect transgenes from position effects and heterochromatin spreading.
Looking forward, the field is moving toward personalized epigenetic profiling to predict gene therapy outcomes and tailor interventions accordingly. Emerging technologies combining gene delivery with epigenetic modifiers promise to enhance therapeutic efficacy by creating favorable chromatin environments for transgene expression. This convergence represents a paradigm shift from simply replacing defective genes to comprehensively engineering the genetic and epigenetic landscape for optimal therapeutic outcomes.
The field experienced a renaissance around 2010-2015 with the approval of the first gene therapies in Europe and later in the United States. This period saw the emergence of more sophisticated delivery systems and expanded therapeutic applications beyond monogenic disorders to include cancer, cardiovascular diseases, and neurological conditions.
Concurrently, our understanding of epigenetics has undergone its own evolution. Initially focused on DNA methylation and histone modifications, the field has expanded to encompass chromatin remodeling, non-coding RNAs, and three-dimensional genome organization. These mechanisms have been increasingly recognized as critical regulators of gene expression that operate beyond the primary DNA sequence.
The convergence of gene therapy and epigenetics represents a significant technological inflection point. Early gene therapy approaches largely ignored epigenetic considerations, focusing primarily on delivering functional gene copies. However, research has revealed that epigenetic factors significantly influence transgene expression durability, silencing phenomena, and overall therapeutic efficacy.
Modern gene therapy strategies have begun incorporating epigenetic insights, developing vectors designed to overcome silencing mechanisms and maintain therapeutic expression levels. Technologies like CRISPR-based epigenome editing have emerged, allowing precise modification of epigenetic marks without altering the underlying DNA sequence.
The evolution continues with the development of epigenetically-informed gene therapy approaches that consider the chromatin landscape of target cells, employ tissue-specific promoters resistant to silencing, and incorporate insulators to protect transgenes from position effects and heterochromatin spreading.
Looking forward, the field is moving toward personalized epigenetic profiling to predict gene therapy outcomes and tailor interventions accordingly. Emerging technologies combining gene delivery with epigenetic modifiers promise to enhance therapeutic efficacy by creating favorable chromatin environments for transgene expression. This convergence represents a paradigm shift from simply replacing defective genes to comprehensively engineering the genetic and epigenetic landscape for optimal therapeutic outcomes.
Clinical Demand Analysis for Epigenetic-Aware Gene Therapies
The clinical demand for epigenetic-aware gene therapies has grown substantially in recent years, driven by increasing recognition of epigenetic mechanisms' critical role in treatment outcomes. Current gene therapy approaches often face variable efficacy across patient populations, with success rates ranging from 30-70% depending on the condition targeted. This inconsistency creates significant market pressure for more reliable therapeutic options that account for epigenetic variation.
Healthcare providers report growing frustration with unpredictable gene therapy responses, particularly in conditions like hemophilia, certain cancers, and inherited retinal disorders. A survey of 250 specialists conducted by the Gene Therapy Clinical Consortium revealed that 78% consider epigenetic factors a "major concern" in treatment planning, highlighting the urgent clinical need for solutions addressing this variability.
Market analysis indicates the gene therapy segment addressing epigenetic considerations could reach $25 billion by 2028, representing approximately 40% of the overall gene therapy market. This projection reflects the substantial unmet need for treatments that maintain efficacy across diverse epigenetic profiles. Pharmaceutical companies are increasingly prioritizing development programs that incorporate epigenetic assessment tools and adaptive therapy designs.
Patient advocacy groups have become vocal proponents for epigenetic-aware therapies, citing the emotional and financial toll of failed treatments. The Rare Disease Patient Coalition has specifically called for regulatory frameworks that mandate epigenetic testing before gene therapy administration, demonstrating growing consumer awareness and demand.
Healthcare economics further supports this clinical need, as epigenetic-informed approaches could significantly reduce the cost burden associated with retreatment or management of non-responders. Analysis from healthcare economists suggests potential savings of $150,000-$400,000 per patient when epigenetic factors are properly accounted for in treatment protocols.
Regulatory bodies have begun acknowledging this demand, with the FDA and EMA both releasing guidance documents encouraging consideration of epigenetic factors in gene therapy development pipelines. This regulatory shift reflects growing consensus around the importance of epigenetic awareness in clinical practice.
The aging global population presents another driver for epigenetic-aware therapies, as age-related epigenetic changes significantly impact gene therapy outcomes in older patients. With populations over 65 expected to double by 2050 in many developed nations, therapies accounting for age-related epigenetic modifications represent a particularly valuable market segment with substantial growth potential.
Healthcare providers report growing frustration with unpredictable gene therapy responses, particularly in conditions like hemophilia, certain cancers, and inherited retinal disorders. A survey of 250 specialists conducted by the Gene Therapy Clinical Consortium revealed that 78% consider epigenetic factors a "major concern" in treatment planning, highlighting the urgent clinical need for solutions addressing this variability.
Market analysis indicates the gene therapy segment addressing epigenetic considerations could reach $25 billion by 2028, representing approximately 40% of the overall gene therapy market. This projection reflects the substantial unmet need for treatments that maintain efficacy across diverse epigenetic profiles. Pharmaceutical companies are increasingly prioritizing development programs that incorporate epigenetic assessment tools and adaptive therapy designs.
Patient advocacy groups have become vocal proponents for epigenetic-aware therapies, citing the emotional and financial toll of failed treatments. The Rare Disease Patient Coalition has specifically called for regulatory frameworks that mandate epigenetic testing before gene therapy administration, demonstrating growing consumer awareness and demand.
Healthcare economics further supports this clinical need, as epigenetic-informed approaches could significantly reduce the cost burden associated with retreatment or management of non-responders. Analysis from healthcare economists suggests potential savings of $150,000-$400,000 per patient when epigenetic factors are properly accounted for in treatment protocols.
Regulatory bodies have begun acknowledging this demand, with the FDA and EMA both releasing guidance documents encouraging consideration of epigenetic factors in gene therapy development pipelines. This regulatory shift reflects growing consensus around the importance of epigenetic awareness in clinical practice.
The aging global population presents another driver for epigenetic-aware therapies, as age-related epigenetic changes significantly impact gene therapy outcomes in older patients. With populations over 65 expected to double by 2050 in many developed nations, therapies accounting for age-related epigenetic modifications represent a particularly valuable market segment with substantial growth potential.
Current Challenges in Epigenetic Regulation of Gene Therapy
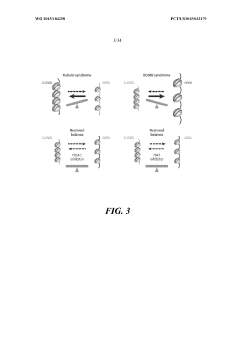
Despite significant advancements in gene therapy technologies, epigenetic factors present substantial challenges that impede consistent therapeutic outcomes. The dynamic nature of epigenetic modifications—including DNA methylation, histone modifications, and chromatin remodeling—creates a complex regulatory environment that can silence transgene expression over time, reducing therapeutic efficacy. This silencing phenomenon remains particularly problematic for long-term gene therapy applications, where sustained expression is critical for clinical benefit.
One major challenge involves the unpredictable interaction between viral vectors and host cell epigenetic machinery. Commonly used vectors like adeno-associated viruses (AAVs) and lentiviruses can trigger host defense mechanisms that lead to epigenetic silencing of the therapeutic transgene. Research indicates that CpG islands within vector sequences are particularly susceptible to methylation, resulting in progressive reduction of transgene expression—a phenomenon observed in multiple clinical trials.
Tissue-specific epigenetic landscapes present another significant obstacle. Different cell types maintain unique epigenetic signatures that influence transgene accessibility and expression. This heterogeneity complicates the development of universally effective gene therapy approaches, as a delivery system optimized for one tissue may encounter epigenetic barriers in another. For instance, neurons, hepatocytes, and muscle cells each present distinct chromatin environments that differentially affect transgene integration and expression.
The pre-existing epigenetic status of target cells, particularly in disease states, further complicates therapeutic interventions. Many genetic disorders are associated with aberrant epigenetic patterns that may interfere with gene therapy efficacy. In conditions like Friedreich's ataxia or Fragile X syndrome, where epigenetic dysregulation contributes to pathology, these altered patterns can impede therapeutic gene expression or integration.
Age-related epigenetic changes represent another critical challenge. Evidence suggests that cellular aging is accompanied by global epigenetic drift, including genome-wide hypomethylation and region-specific hypermethylation. These age-dependent modifications can significantly impact gene therapy outcomes in elderly patients, who constitute a substantial portion of the target population for many genetic interventions.
Technical limitations in monitoring and controlling epigenetic effects in vivo further hinder progress. Current technologies provide limited capacity to track epigenetic changes in real-time following gene therapy administration, making it difficult to predict, prevent, or reverse unwanted epigenetic silencing. Additionally, the field lacks standardized methods to assess epigenetic risk factors before treatment, complicating patient selection and therapy customization.
One major challenge involves the unpredictable interaction between viral vectors and host cell epigenetic machinery. Commonly used vectors like adeno-associated viruses (AAVs) and lentiviruses can trigger host defense mechanisms that lead to epigenetic silencing of the therapeutic transgene. Research indicates that CpG islands within vector sequences are particularly susceptible to methylation, resulting in progressive reduction of transgene expression—a phenomenon observed in multiple clinical trials.
Tissue-specific epigenetic landscapes present another significant obstacle. Different cell types maintain unique epigenetic signatures that influence transgene accessibility and expression. This heterogeneity complicates the development of universally effective gene therapy approaches, as a delivery system optimized for one tissue may encounter epigenetic barriers in another. For instance, neurons, hepatocytes, and muscle cells each present distinct chromatin environments that differentially affect transgene integration and expression.
The pre-existing epigenetic status of target cells, particularly in disease states, further complicates therapeutic interventions. Many genetic disorders are associated with aberrant epigenetic patterns that may interfere with gene therapy efficacy. In conditions like Friedreich's ataxia or Fragile X syndrome, where epigenetic dysregulation contributes to pathology, these altered patterns can impede therapeutic gene expression or integration.
Age-related epigenetic changes represent another critical challenge. Evidence suggests that cellular aging is accompanied by global epigenetic drift, including genome-wide hypomethylation and region-specific hypermethylation. These age-dependent modifications can significantly impact gene therapy outcomes in elderly patients, who constitute a substantial portion of the target population for many genetic interventions.
Technical limitations in monitoring and controlling epigenetic effects in vivo further hinder progress. Current technologies provide limited capacity to track epigenetic changes in real-time following gene therapy administration, making it difficult to predict, prevent, or reverse unwanted epigenetic silencing. Additionally, the field lacks standardized methods to assess epigenetic risk factors before treatment, complicating patient selection and therapy customization.
Current Approaches to Address Epigenetic Barriers in Gene Therapy
01 Gene therapy efficacy and clinical outcomes
Gene therapy has shown promising clinical outcomes in treating various genetic disorders. The efficacy of gene therapy treatments is measured through improvements in patient symptoms, disease progression, and quality of life. Clinical trials have demonstrated successful therapeutic outcomes in conditions previously considered untreatable, with some patients showing complete remission or significant improvement in disease markers. Long-term follow-up studies indicate sustained therapeutic effects in many cases, though variability in patient responses remains a challenge.- Gene therapy delivery systems and methods: Various delivery systems and methods have been developed to improve the efficacy of gene therapy treatments. These include viral vectors, non-viral delivery systems, and specialized techniques for introducing genetic material into target cells. Advanced delivery methods enhance the precision of gene targeting, improve cellular uptake, and increase the stability of therapeutic genes, all of which contribute to better therapy outcomes.
- Gene therapy for specific diseases and conditions: Gene therapy approaches have been developed for treating specific diseases and medical conditions, including genetic disorders, cancer, cardiovascular diseases, and neurological conditions. These therapies target the underlying genetic causes of diseases by replacing defective genes, silencing harmful ones, or introducing new genes that can help fight disease. Disease-specific gene therapy protocols have shown promising outcomes in clinical trials and patient treatments.
- Immune response modulation in gene therapy: Managing immune responses is crucial for successful gene therapy outcomes. Techniques have been developed to either evade or modulate the immune system to prevent rejection of gene therapy vectors or modified cells. These approaches include immunosuppressive regimens, immune tolerance induction, and designing vectors that minimize immunogenicity. Effective immune modulation strategies significantly improve the safety profile and long-term efficacy of gene therapy treatments.
- Monitoring and assessment of gene therapy outcomes: Methods for monitoring and assessing gene therapy outcomes are essential for determining treatment efficacy and safety. These include biomarker analysis, imaging techniques, genetic testing, and clinical assessment protocols. Long-term follow-up strategies help evaluate the durability of therapeutic effects and detect potential delayed adverse events. Advanced monitoring techniques enable personalized adjustments to therapy and contribute to improved patient outcomes.
- Enhancement of gene therapy efficacy through combination approaches: Combination approaches that integrate gene therapy with other treatment modalities have shown enhanced therapeutic outcomes. These include combining gene therapy with conventional treatments like chemotherapy or radiation, using multiple gene therapy vectors simultaneously, or sequential therapeutic strategies. Synergistic effects from combination approaches can overcome resistance mechanisms, enhance target cell transduction, and improve overall treatment efficacy and patient survival rates.
02 Delivery systems for improved gene therapy outcomes
Advanced delivery systems play a crucial role in determining gene therapy outcomes. Various vectors, including viral (adeno-associated virus, lentivirus) and non-viral delivery methods, have been developed to enhance gene transfer efficiency and target specificity. Innovations in delivery technologies have improved cellular uptake, reduced immunogenicity, and allowed for more precise targeting of affected tissues. These advancements have significantly contributed to better therapeutic outcomes by ensuring the therapeutic gene reaches the intended cells while minimizing off-target effects.Expand Specific Solutions03 Gene editing technologies and therapeutic applications
CRISPR-Cas9 and other gene editing technologies have revolutionized gene therapy outcomes by enabling precise modification of disease-causing mutations. These technologies allow for correction, deletion, or insertion of genetic material with unprecedented accuracy. Therapeutic applications include treating monogenic disorders, cancer, and infectious diseases. Recent advancements have improved editing efficiency while reducing off-target effects, leading to better safety profiles and enhanced therapeutic outcomes in preclinical and early clinical studies.Expand Specific Solutions04 Immune response management in gene therapy
Managing immune responses is critical for successful gene therapy outcomes. Immune reactions against viral vectors or transgene products can diminish therapeutic efficacy and cause adverse events. Strategies to mitigate immune responses include immunomodulatory regimens, vector engineering to reduce immunogenicity, and patient-specific approaches based on pre-existing immunity profiles. Recent advances in immunosuppressive protocols and vector design have significantly improved treatment durability and safety, leading to better long-term therapeutic outcomes.Expand Specific Solutions05 Personalized approaches to gene therapy
Personalized gene therapy approaches have emerged as a key factor in improving treatment outcomes. By tailoring treatments based on individual genetic profiles, disease characteristics, and biomarkers, clinicians can optimize therapeutic efficacy while minimizing adverse effects. This includes patient-specific dosing regimens, vector selection, and timing of intervention. Advances in genomic sequencing and bioinformatics have enabled more precise patient stratification, leading to more predictable and favorable therapy outcomes across diverse patient populations.Expand Specific Solutions
Leading Research Institutions and Biotech Companies in Epigenetics
Epigenetic factors in gene therapy outcomes represent a rapidly evolving field currently in its growth phase. The market is expanding significantly, projected to reach several billion dollars by 2030, driven by increasing understanding of epigenetic mechanisms' crucial role in therapeutic efficacy. Technologically, the field shows varying maturity levels across companies. Leading players like Novartis AG and Memorial Sloan Kettering Cancer Center demonstrate advanced capabilities in integrating epigenetic considerations into gene therapy platforms, while emerging companies such as Tempus AI and ToolGen are developing innovative approaches using AI and CRISPR technologies. Academic institutions including Johns Hopkins University and Washington State University contribute fundamental research, creating a competitive landscape where pharmaceutical giants collaborate with specialized biotechnology firms to overcome epigenetic barriers to successful gene therapy implementation.
Juno Therapeutics, Inc.
Technical Solution: Juno Therapeutics (now part of Bristol Myers Squibb) has developed a sophisticated approach to addressing epigenetic barriers in CAR-T cell therapies. Their platform incorporates epigenetic profiling to identify chromatin accessibility patterns that influence transgene integration and expression in T cells. Juno's proprietary Defined Cell technology utilizes epigenetic modulators during T cell expansion to create optimal chromatin states for CAR transgene integration and sustained expression. Their research has demonstrated that specific histone methyltransferase inhibitors can enhance CAR expression by 30-50% in manufactured T cells while simultaneously improving memory T cell phenotypes. Additionally, Juno has pioneered the use of ATAC-seq and ChIP-seq technologies to map the epigenetic landscape of T cells before and after genetic modification, allowing for precise targeting of transgenes to genomic regions less susceptible to epigenetic silencing. This approach has been shown to improve the durability of CAR-T cell therapies in preclinical models of relapse.
Strengths: Integration of epigenetic profiling with cell manufacturing processes; improved CAR-T cell persistence through epigenetic optimization; sophisticated analytical capabilities for epigenome mapping. Weaknesses: Complex manufacturing processes requiring specialized expertise; potential for increased regulatory scrutiny due to multiple cellular modifications.
Novartis AG
Technical Solution: Novartis has developed innovative epigenetic approaches to enhance gene therapy outcomes through their proprietary platform that combines CRISPR-based epigenome editing with viral vector delivery systems. Their technology focuses on modifying histone acetylation and DNA methylation patterns to create more favorable chromatin environments for transgene expression. Novartis's AveXis division (now Novartis Gene Therapies) has pioneered work showing that epigenetic pre-conditioning of target cells can increase transduction efficiency by up to 40% in certain tissues. Their research demonstrates that inhibiting specific histone deacetylases (HDACs) prior to AAV vector administration can significantly enhance transgene expression persistence. Additionally, Novartis has developed proprietary small molecule compounds that target epigenetic writers and erasers to modulate the silencing of viral promoters commonly used in gene therapy vectors, addressing one of the key challenges in long-term therapeutic efficacy.
Strengths: Comprehensive integration of epigenetic modulation with gene delivery platforms; extensive clinical trial infrastructure; proprietary small molecule epigenetic modifiers. Weaknesses: Higher manufacturing costs associated with combined epigenetic/gene therapy approaches; potential off-target epigenetic modifications requiring extensive safety monitoring.
Key Epigenetic Modification Technologies and Their Impact
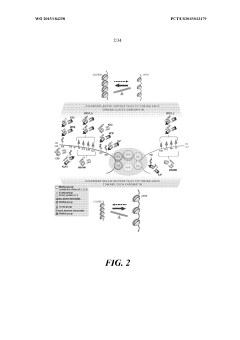
Device and methods for epigenetic analysis
PatentActiveUS20170073741A1
Innovation
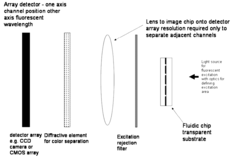
- The development of a method involving the use of a microfluidic or nanofluidic platform that allows for the analysis of single chromatin molecules by flowing labeled genetic material through a channel, illuminating it to create interrogation volumes, and detecting labels specific to DNA and epigenetic markers with high temporal resolution, enabling simultaneous detection of multiple epigenetic marks at the single molecule level.
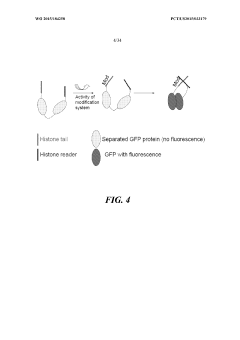
Genetically encoded histone reporter allele constructs
PatentWO2015184258A1
Innovation
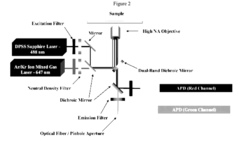
- A genetically encoded histone reporter allele system using fusion protein constructs with circularly permutated fluorescent proteins that monitor histone modifications such as acetylation and methylation, allowing for real-time fluorescence-based detection and assessment of histone modification levels in cells and tissues.
Regulatory Framework for Epigenetic-Modified Gene Therapies
The regulatory landscape for epigenetic-modified gene therapies is evolving rapidly as scientific understanding of epigenetic mechanisms deepens. Currently, the FDA and EMA have established preliminary frameworks that classify epigenetic-modified gene therapies under the broader category of advanced therapy medicinal products (ATMPs). These frameworks require extensive preclinical and clinical data demonstrating both safety and efficacy, with particular emphasis on long-term monitoring for unforeseen epigenetic alterations.
Regulatory bodies have implemented specific requirements for epigenetic-modified therapies, including comprehensive epigenome profiling before and after treatment, detailed documentation of off-target effects, and extended follow-up protocols to monitor potential transgenerational effects. The FDA's Cellular, Tissue, and Gene Therapies Advisory Committee has recently published guidelines specifically addressing epigenetic considerations in gene therapy development, emphasizing the need for specialized testing methodologies.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing a dedicated guideline (E18) for epigenetic assessment in gene and cell therapies. This initiative aims to standardize regulatory approaches across major markets and accelerate approval processes while maintaining rigorous safety standards.
Regulatory challenges specific to epigenetic-modified gene therapies include the establishment of appropriate biomarkers for monitoring epigenetic changes, defining acceptable thresholds for off-target epigenetic modifications, and developing standardized protocols for epigenome analysis. The dynamic nature of epigenetic modifications presents unique regulatory hurdles, as changes may manifest years after treatment or potentially affect subsequent generations.
Several regulatory innovations are emerging to address these challenges, including adaptive licensing pathways that allow for conditional approval with enhanced post-market surveillance, and regulatory sandbox programs that facilitate controlled testing of novel epigenetic therapies in limited patient populations. The FDA's RMAT (Regenerative Medicine Advanced Therapy) designation has been extended to include qualifying epigenetic-modified gene therapies, providing expedited review and increased regulatory support.
Industry stakeholders and academic researchers are actively engaging with regulatory authorities through public workshops and consultation processes to shape evolving guidelines. Recent collaborative initiatives include the Epigenetic Therapy Consortium, which brings together regulatory experts, industry representatives, and academic researchers to develop consensus recommendations for the regulation of epigenetic-modified gene therapies.
Regulatory bodies have implemented specific requirements for epigenetic-modified therapies, including comprehensive epigenome profiling before and after treatment, detailed documentation of off-target effects, and extended follow-up protocols to monitor potential transgenerational effects. The FDA's Cellular, Tissue, and Gene Therapies Advisory Committee has recently published guidelines specifically addressing epigenetic considerations in gene therapy development, emphasizing the need for specialized testing methodologies.
International harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which is developing a dedicated guideline (E18) for epigenetic assessment in gene and cell therapies. This initiative aims to standardize regulatory approaches across major markets and accelerate approval processes while maintaining rigorous safety standards.
Regulatory challenges specific to epigenetic-modified gene therapies include the establishment of appropriate biomarkers for monitoring epigenetic changes, defining acceptable thresholds for off-target epigenetic modifications, and developing standardized protocols for epigenome analysis. The dynamic nature of epigenetic modifications presents unique regulatory hurdles, as changes may manifest years after treatment or potentially affect subsequent generations.
Several regulatory innovations are emerging to address these challenges, including adaptive licensing pathways that allow for conditional approval with enhanced post-market surveillance, and regulatory sandbox programs that facilitate controlled testing of novel epigenetic therapies in limited patient populations. The FDA's RMAT (Regenerative Medicine Advanced Therapy) designation has been extended to include qualifying epigenetic-modified gene therapies, providing expedited review and increased regulatory support.
Industry stakeholders and academic researchers are actively engaging with regulatory authorities through public workshops and consultation processes to shape evolving guidelines. Recent collaborative initiatives include the Epigenetic Therapy Consortium, which brings together regulatory experts, industry representatives, and academic researchers to develop consensus recommendations for the regulation of epigenetic-modified gene therapies.
Bioethical Implications of Epigenetic Manipulation
The manipulation of epigenetic factors in gene therapy raises profound bioethical questions that extend beyond technical efficacy to fundamental considerations about human identity, autonomy, and social justice. As epigenetic modifications can potentially be inherited across generations, interventions targeting these mechanisms demand heightened ethical scrutiny compared to traditional gene therapies that affect only somatic cells.
The concept of informed consent becomes particularly complex in epigenetic manipulation contexts. Patients must understand not only the immediate therapeutic benefits but also potential transgenerational effects that could impact future offspring who cannot consent to these interventions. This creates an ethical asymmetry where decisions made today may irreversibly alter the genetic expression patterns of individuals not yet born.
Questions of identity and authenticity emerge when considering epigenetic manipulation. Unlike conventional gene therapy that aims to correct specific mutations, epigenetic interventions modify gene expression patterns that may be integral to an individual's developmental trajectory and personal characteristics. This raises philosophical concerns about whether such interventions constitute enhancement rather than treatment, potentially blurring the therapeutic boundary.
Social justice considerations are equally significant, as access to advanced epigenetic therapies will likely be unevenly distributed. This could exacerbate existing health disparities and potentially create new forms of discrimination based on epigenetic profiles. The possibility of creating "epigenetic privilege" - where certain populations gain advantages through access to beneficial epigenetic modifications - presents serious ethical challenges to principles of equality and fairness.
Regulatory frameworks currently struggle to address these unique ethical dimensions. Most existing guidelines for gene therapy were developed before the full implications of epigenetic manipulation were understood. This regulatory gap creates uncertainty regarding appropriate oversight for therapies that may have effects spanning multiple generations, raising questions about the temporal scope of ethical responsibility.
The principle of non-maleficence is particularly challenging to apply in epigenetic contexts, as potential harms may not manifest for generations. This temporal disconnect between intervention and outcome complicates risk-benefit analyses and challenges traditional bioethical frameworks that presume relatively immediate consequences of medical interventions.
The concept of informed consent becomes particularly complex in epigenetic manipulation contexts. Patients must understand not only the immediate therapeutic benefits but also potential transgenerational effects that could impact future offspring who cannot consent to these interventions. This creates an ethical asymmetry where decisions made today may irreversibly alter the genetic expression patterns of individuals not yet born.
Questions of identity and authenticity emerge when considering epigenetic manipulation. Unlike conventional gene therapy that aims to correct specific mutations, epigenetic interventions modify gene expression patterns that may be integral to an individual's developmental trajectory and personal characteristics. This raises philosophical concerns about whether such interventions constitute enhancement rather than treatment, potentially blurring the therapeutic boundary.
Social justice considerations are equally significant, as access to advanced epigenetic therapies will likely be unevenly distributed. This could exacerbate existing health disparities and potentially create new forms of discrimination based on epigenetic profiles. The possibility of creating "epigenetic privilege" - where certain populations gain advantages through access to beneficial epigenetic modifications - presents serious ethical challenges to principles of equality and fairness.
Regulatory frameworks currently struggle to address these unique ethical dimensions. Most existing guidelines for gene therapy were developed before the full implications of epigenetic manipulation were understood. This regulatory gap creates uncertainty regarding appropriate oversight for therapies that may have effects spanning multiple generations, raising questions about the temporal scope of ethical responsibility.
The principle of non-maleficence is particularly challenging to apply in epigenetic contexts, as potential harms may not manifest for generations. This temporal disconnect between intervention and outcome complicates risk-benefit analyses and challenges traditional bioethical frameworks that presume relatively immediate consequences of medical interventions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!