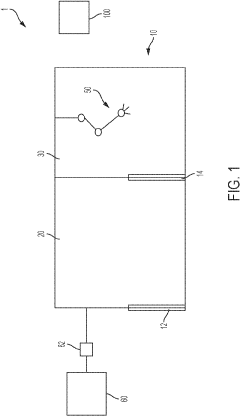
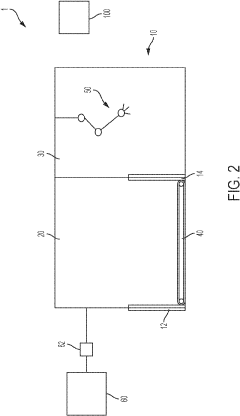
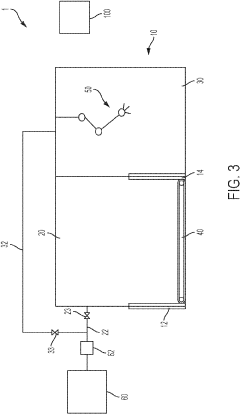
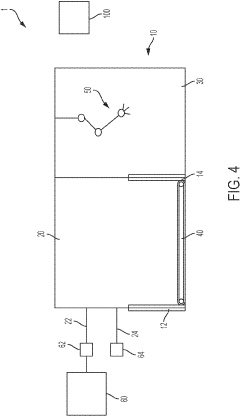
Aseptic fill/finish solutions for autologous cell therapies: modular cleanroom vs isolator-based approaches
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autologous Cell Therapy Aseptic Processing Background & Objectives
Autologous cell therapies represent a revolutionary approach in personalized medicine, where a patient's own cells are collected, modified, and reinfused to treat various diseases, particularly in oncology and rare genetic disorders. The evolution of this therapeutic modality has accelerated significantly over the past decade, with the first FDA approval of CAR-T cell therapy in 2017 marking a pivotal milestone in the field. Since then, the industry has witnessed exponential growth, with numerous clinical trials and increasing commercial applications.
The aseptic processing requirements for autologous cell therapies present unique challenges compared to traditional pharmaceutical manufacturing. Unlike allogeneic therapies or conventional biologics, autologous products involve small-batch, patient-specific processing with zero margin for error, as each batch represents a potentially life-saving treatment for a specific individual. This creates unprecedented demands on manufacturing infrastructure, particularly in the critical fill/finish operations.
Historically, cell therapy manufacturing has evolved from academic laboratory settings to more standardized GMP environments. Early approaches relied heavily on manual processes within traditional cleanrooms, but increasing regulatory scrutiny and commercialization pressures have driven the need for more robust, scalable, and contamination-controlled solutions. The industry now stands at a technological crossroads between modular cleanroom systems and isolator-based approaches.
The primary technical objective of this investigation is to comprehensively evaluate the relative merits of modular cleanroom versus isolator-based technologies for aseptic fill/finish operations in autologous cell therapy manufacturing. This assessment aims to determine which approach better addresses the unique challenges of patient-specific production while maintaining the highest standards of sterility assurance, operational efficiency, and regulatory compliance.
Secondary objectives include identifying technological trends that may influence future developments in this space, evaluating the adaptability of each approach to evolving regulatory requirements, and assessing the economic implications of implementation across different scales of operation. Additionally, we seek to understand how each technology platform might accommodate the increasing automation needs in cell therapy manufacturing while maintaining the flexibility required for personalized medicine.
The findings from this technical assessment will inform strategic decision-making regarding infrastructure investments, process development pathways, and long-term manufacturing strategies in the rapidly evolving landscape of advanced therapy medicinal products (ATMPs). As the field continues to mature, establishing optimal aseptic processing solutions will be critical to ensuring both patient safety and commercial viability of these transformative therapies.
The aseptic processing requirements for autologous cell therapies present unique challenges compared to traditional pharmaceutical manufacturing. Unlike allogeneic therapies or conventional biologics, autologous products involve small-batch, patient-specific processing with zero margin for error, as each batch represents a potentially life-saving treatment for a specific individual. This creates unprecedented demands on manufacturing infrastructure, particularly in the critical fill/finish operations.
Historically, cell therapy manufacturing has evolved from academic laboratory settings to more standardized GMP environments. Early approaches relied heavily on manual processes within traditional cleanrooms, but increasing regulatory scrutiny and commercialization pressures have driven the need for more robust, scalable, and contamination-controlled solutions. The industry now stands at a technological crossroads between modular cleanroom systems and isolator-based approaches.
The primary technical objective of this investigation is to comprehensively evaluate the relative merits of modular cleanroom versus isolator-based technologies for aseptic fill/finish operations in autologous cell therapy manufacturing. This assessment aims to determine which approach better addresses the unique challenges of patient-specific production while maintaining the highest standards of sterility assurance, operational efficiency, and regulatory compliance.
Secondary objectives include identifying technological trends that may influence future developments in this space, evaluating the adaptability of each approach to evolving regulatory requirements, and assessing the economic implications of implementation across different scales of operation. Additionally, we seek to understand how each technology platform might accommodate the increasing automation needs in cell therapy manufacturing while maintaining the flexibility required for personalized medicine.
The findings from this technical assessment will inform strategic decision-making regarding infrastructure investments, process development pathways, and long-term manufacturing strategies in the rapidly evolving landscape of advanced therapy medicinal products (ATMPs). As the field continues to mature, establishing optimal aseptic processing solutions will be critical to ensuring both patient safety and commercial viability of these transformative therapies.
Market Analysis for Cell Therapy Manufacturing Solutions
The cell therapy manufacturing market is experiencing unprecedented growth, driven by the increasing prevalence of cancer and genetic disorders, alongside substantial investments in research and development. The global cell therapy market was valued at approximately $14.5 billion in 2020 and is projected to reach $48.11 billion by 2027, growing at a CAGR of 25.6% during the forecast period. Autologous cell therapies, which utilize a patient's own cells, represent a significant portion of this market.
Manufacturing solutions for cell therapies, particularly aseptic fill/finish solutions, are critical components of the production process. The market for these solutions is expected to grow in parallel with the overall cell therapy market, with an estimated value of $1.8 billion in 2021 and projected to reach $5.2 billion by 2028. This growth is primarily driven by the increasing number of approved cell therapies and the expanding pipeline of candidates in clinical trials.
The demand for aseptic fill/finish solutions specifically designed for autologous cell therapies is intensifying due to the unique challenges associated with these therapies. Unlike traditional pharmaceuticals, autologous cell therapies require individualized processing, smaller batch sizes, and stringent contamination control measures. This has created a specialized market segment within the broader fill/finish solutions market.
Geographically, North America dominates the market for cell therapy manufacturing solutions, accounting for approximately 42% of the global market share. This dominance is attributed to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory frameworks. Europe follows with approximately 28% market share, while the Asia-Pacific region is experiencing the fastest growth rate due to increasing investments in healthcare infrastructure and research facilities.
The market is segmented based on technology type, with modular cleanroom solutions currently holding a larger market share (approximately 60%) compared to isolator-based approaches (approximately 40%). However, isolator-based technologies are gaining traction due to their enhanced contamination control capabilities and potential for cost savings in long-term operations.
End-users of these manufacturing solutions include pharmaceutical and biotechnology companies (65% market share), contract manufacturing organizations (25%), and academic and research institutions (10%). The pharmaceutical and biotechnology segment is expected to maintain its dominant position due to increasing investments in in-house manufacturing capabilities for cell therapies.
Key market drivers include the rising prevalence of chronic diseases, increasing regulatory support for cell therapy development, technological advancements in manufacturing processes, and growing investments in healthcare infrastructure. Conversely, market restraints include high manufacturing costs, complex regulatory requirements, and technical challenges associated with maintaining aseptic conditions during the manufacturing process.
Manufacturing solutions for cell therapies, particularly aseptic fill/finish solutions, are critical components of the production process. The market for these solutions is expected to grow in parallel with the overall cell therapy market, with an estimated value of $1.8 billion in 2021 and projected to reach $5.2 billion by 2028. This growth is primarily driven by the increasing number of approved cell therapies and the expanding pipeline of candidates in clinical trials.
The demand for aseptic fill/finish solutions specifically designed for autologous cell therapies is intensifying due to the unique challenges associated with these therapies. Unlike traditional pharmaceuticals, autologous cell therapies require individualized processing, smaller batch sizes, and stringent contamination control measures. This has created a specialized market segment within the broader fill/finish solutions market.
Geographically, North America dominates the market for cell therapy manufacturing solutions, accounting for approximately 42% of the global market share. This dominance is attributed to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory frameworks. Europe follows with approximately 28% market share, while the Asia-Pacific region is experiencing the fastest growth rate due to increasing investments in healthcare infrastructure and research facilities.
The market is segmented based on technology type, with modular cleanroom solutions currently holding a larger market share (approximately 60%) compared to isolator-based approaches (approximately 40%). However, isolator-based technologies are gaining traction due to their enhanced contamination control capabilities and potential for cost savings in long-term operations.
End-users of these manufacturing solutions include pharmaceutical and biotechnology companies (65% market share), contract manufacturing organizations (25%), and academic and research institutions (10%). The pharmaceutical and biotechnology segment is expected to maintain its dominant position due to increasing investments in in-house manufacturing capabilities for cell therapies.
Key market drivers include the rising prevalence of chronic diseases, increasing regulatory support for cell therapy development, technological advancements in manufacturing processes, and growing investments in healthcare infrastructure. Conversely, market restraints include high manufacturing costs, complex regulatory requirements, and technical challenges associated with maintaining aseptic conditions during the manufacturing process.
Current Aseptic Fill/Finish Technologies & Barriers
The aseptic fill/finish process represents a critical bottleneck in the manufacturing of autologous cell therapies, requiring specialized technologies to maintain product sterility while accommodating small batch sizes and personalized production. Current technologies primarily fall into two categories: traditional cleanroom-based approaches and isolator-based systems, each with distinct operational characteristics and limitations.
Traditional cleanroom environments rely on HEPA filtration, controlled airflow, and strict personnel gowning procedures to maintain aseptic conditions. While established in pharmaceutical manufacturing, these systems face significant challenges when applied to cell therapies, including high operational costs, extensive personnel training requirements, and potential for human-introduced contamination. The large footprint and fixed infrastructure of conventional cleanrooms also limit manufacturing flexibility crucial for patient-specific therapies.
Isolator technology offers enhanced contamination control through physical barriers between operators and products, typically utilizing glove ports for manipulation. These systems provide superior sterility assurance levels (SAL) compared to conventional cleanrooms and reduce dependence on operator technique. However, isolators present challenges including restricted working space, complex decontamination cycles, and limited flexibility for process changes.
Emerging hybrid solutions attempt to address these limitations through modular cleanroom designs that incorporate isolator principles. These systems feature reconfigurable layouts, integrated automation, and reduced footprints while maintaining Grade A/ISO 5 environments necessary for aseptic processing. Despite these innovations, significant barriers remain in achieving cost-effective, scalable fill/finish operations for cell therapies.
Technical barriers include the development of closed-system processing technologies compatible with living cellular products, which are highly sensitive to environmental stresses. Current filling equipment designed for traditional pharmaceuticals often proves unsuitable for fragile cell therapy products due to shear forces, temperature fluctuations, and material incompatibilities that can compromise cell viability and function.
Regulatory challenges further complicate technology adoption, as guidelines developed for traditional pharmaceuticals may not adequately address the unique aspects of personalized cell therapies. The absence of harmonized standards specifically for autologous products creates uncertainty in validation requirements and compliance pathways.
Economic barriers also persist, with high capital investment requirements for specialized equipment and facilities that may be difficult to justify for small-batch, personalized therapies. The cost-per-dose remains prohibitively high compared to traditional pharmaceutical products, limiting broader market access and commercial viability.
Traditional cleanroom environments rely on HEPA filtration, controlled airflow, and strict personnel gowning procedures to maintain aseptic conditions. While established in pharmaceutical manufacturing, these systems face significant challenges when applied to cell therapies, including high operational costs, extensive personnel training requirements, and potential for human-introduced contamination. The large footprint and fixed infrastructure of conventional cleanrooms also limit manufacturing flexibility crucial for patient-specific therapies.
Isolator technology offers enhanced contamination control through physical barriers between operators and products, typically utilizing glove ports for manipulation. These systems provide superior sterility assurance levels (SAL) compared to conventional cleanrooms and reduce dependence on operator technique. However, isolators present challenges including restricted working space, complex decontamination cycles, and limited flexibility for process changes.
Emerging hybrid solutions attempt to address these limitations through modular cleanroom designs that incorporate isolator principles. These systems feature reconfigurable layouts, integrated automation, and reduced footprints while maintaining Grade A/ISO 5 environments necessary for aseptic processing. Despite these innovations, significant barriers remain in achieving cost-effective, scalable fill/finish operations for cell therapies.
Technical barriers include the development of closed-system processing technologies compatible with living cellular products, which are highly sensitive to environmental stresses. Current filling equipment designed for traditional pharmaceuticals often proves unsuitable for fragile cell therapy products due to shear forces, temperature fluctuations, and material incompatibilities that can compromise cell viability and function.
Regulatory challenges further complicate technology adoption, as guidelines developed for traditional pharmaceuticals may not adequately address the unique aspects of personalized cell therapies. The absence of harmonized standards specifically for autologous products creates uncertainty in validation requirements and compliance pathways.
Economic barriers also persist, with high capital investment requirements for specialized equipment and facilities that may be difficult to justify for small-batch, personalized therapies. The cost-per-dose remains prohibitively high compared to traditional pharmaceutical products, limiting broader market access and commercial viability.
Modular Cleanroom vs Isolator Technical Comparison
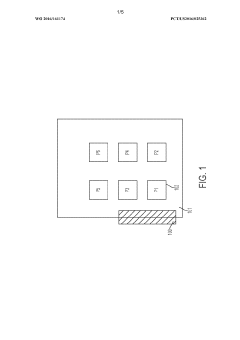
01 Aseptic filling equipment and systems
Specialized equipment and systems designed for aseptic filling operations in pharmaceutical and food industries. These systems include isolators, filling machines, and integrated production lines that maintain sterile conditions throughout the filling process. The equipment is designed to prevent contamination during the transfer of sterile products into sterile containers, ensuring product integrity and safety.- Aseptic filling equipment and systems: Advanced equipment and systems designed specifically for aseptic filling operations in pharmaceutical and food industries. These systems include specialized filling machines, isolators, and integrated production lines that maintain sterile conditions throughout the filling process. The equipment is designed to prevent contamination while efficiently handling various container types and product formulations.
- Sterilization methods for aseptic processing: Various sterilization techniques employed in aseptic processing to ensure the elimination of microorganisms from containers, equipment, and environments. These methods include heat sterilization, chemical sterilization, radiation, and filtration processes. The sterilization approaches are critical for maintaining product integrity and safety in aseptic fill/finish operations.
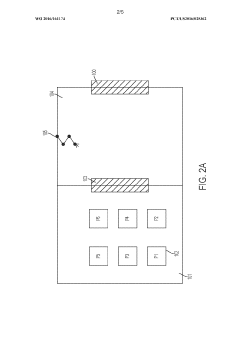
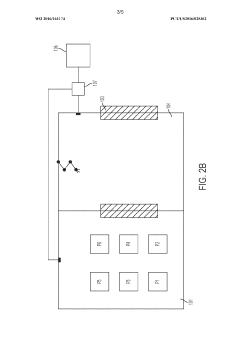
- Cleanroom and controlled environment technologies: Specialized environments and infrastructure designed to maintain sterile conditions for aseptic processing. These include HEPA filtration systems, airlocks, pressure differentials, and monitoring systems that ensure particulate and microbial control. The cleanroom technologies are essential for creating and maintaining the appropriate environment for aseptic fill/finish operations.
- Packaging innovations for aseptic products: Novel packaging solutions specifically designed for aseptically processed products. These innovations include specialized container designs, sealing technologies, and materials that maintain product sterility throughout shelf life. The packaging solutions address challenges related to product compatibility, barrier properties, and ease of aseptic filling.
- Quality control and monitoring systems: Advanced technologies and methodologies for monitoring and ensuring the integrity of aseptic processing operations. These include real-time monitoring systems, validation protocols, and testing methods that verify sterility and detect potential contamination. The quality control systems are critical for regulatory compliance and product safety in aseptic fill/finish operations.
02 Sterilization methods for aseptic processing
Various sterilization techniques employed in aseptic processing to eliminate microorganisms from packaging materials, equipment, and environments. These methods include heat treatment, chemical sterilization, radiation, and filtration. Effective sterilization is crucial for maintaining the sterility of products throughout the fill/finish process and ensuring product safety and shelf stability.Expand Specific Solutions03 Contamination control in clean rooms
Strategies and technologies for maintaining sterile environments in clean rooms where aseptic processing takes place. This includes air filtration systems, pressure differentials, personnel gowning procedures, and monitoring systems. Controlling particulate matter, microorganisms, and other contaminants is essential for successful aseptic processing operations.Expand Specific Solutions04 Packaging materials and container systems
Specialized packaging materials and container systems designed for aseptic fill/finish operations. These include pre-sterilized containers, barrier systems, and innovative packaging designs that maintain product sterility. The selection of appropriate packaging materials is critical for preserving product quality and extending shelf life in aseptic processing.Expand Specific Solutions05 Monitoring and validation of aseptic processes
Methods and technologies for monitoring, validating, and documenting aseptic processing operations. This includes environmental monitoring systems, process analytical technology, and validation protocols that ensure compliance with regulatory requirements. Continuous monitoring and validation are essential for maintaining the integrity of aseptic fill/finish operations and ensuring product safety.Expand Specific Solutions
Leading Manufacturers in Cell Therapy Fill/Finish Equipment
The autologous cell therapy aseptic fill/finish solutions market is currently in a growth phase, with increasing adoption driven by the expanding cell therapy pipeline. The market is projected to reach significant value as commercialization of cell therapies accelerates. Technology maturity varies between approaches, with isolator-based systems offering higher containment but less flexibility compared to modular cleanrooms. Key players shaping this landscape include Cytiva (Global Life Sciences Solutions) with comprehensive cell therapy workflow solutions, Vanrx Pharmasystems specializing in robotic isolator technology, Cellares developing automated end-to-end manufacturing platforms, and OPTIMA automation offering customized fill/finish systems. Emerging innovators like Cellular Origins and established medical equipment providers such as GE Healthcare are also advancing technologies to address the unique challenges of small-batch, personalized cell therapy manufacturing.
OPTIMA automation GmbH
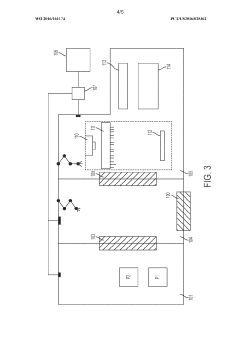
Technical Solution: OPTIMA has developed the OPTIMA MultiUse Filler, a specialized isolator-based aseptic filling system designed specifically for cell and gene therapy applications. Their solution features a compact isolator design with integrated robotic handling systems that maintain sterility while processing the small batch sizes typical of autologous therapies. The system employs a unique peristaltic pump-based filling mechanism calibrated for high-precision dispensing of small volumes with minimal product loss, critical for high-value cell therapies. OPTIMA's technology incorporates rapid VHP (vaporized hydrogen peroxide) decontamination cycles that reduce turnaround time between batches, enabling higher throughput in multi-patient settings. The system is designed with flexibility to accommodate various container formats including cryovials, syringes, and infusion bags commonly used in cell therapy applications. OPTIMA's fill/finish solution integrates with their CSPE (Comprehensive Scientific Process Engineering) methodology, which provides a streamlined validation approach specifically adapted for cell therapy manufacturing requirements [4][6]. Their isolator design includes advanced environmental monitoring systems that continuously verify aseptic conditions during the filling process.
Strengths: Purpose-built for cell therapy applications with appropriate scale and flexibility; rapid decontamination cycles improve facility utilization for multiple patient batches; high-precision filling minimizes product loss of valuable cellular material. Weaknesses: Limited throughput compared to traditional pharmaceutical filling lines; requires specialized technical expertise for operation and maintenance; integration with upstream processing equipment may present challenges in some facility configurations.
GE Healthcare UK Ltd.
Technical Solution: GE Healthcare has developed the Sefia™ Cell Processing System, which incorporates aseptic fill/finish capabilities specifically designed for autologous cell therapies. Their approach combines elements of both modular cleanroom and isolator technologies through a "hybrid" solution. The system features a closed, automated processing platform that maintains aseptic conditions throughout the manufacturing workflow, including the critical fill/finish operations. GE's technology utilizes single-use fluid paths and connection technologies that minimize contamination risks while enabling flexible manufacturing of patient-specific products. The Sefia system incorporates in-line monitoring capabilities that verify critical quality attributes during the filling process, ensuring consistent product quality across individual patient batches. GE Healthcare's solution is designed to integrate with their broader cell therapy manufacturing ecosystem, including the Xuri™ cell expansion systems and cryopreservation technologies, providing a coordinated approach to the entire manufacturing process. Their fill/finish technology emphasizes process closure and automation to reduce cleanroom classification requirements while maintaining product sterility [7][9]. The system supports various container formats and includes automated labeling capabilities essential for patient-specific therapies.
Strengths: Integrated approach that addresses the entire manufacturing workflow reduces technology transfer challenges; established global service network provides reliable support; scalable design accommodates growth from clinical to commercial manufacturing. Weaknesses: System complexity may require significant operator training; reliance on proprietary consumables could increase operational costs; less flexibility for customization compared to some modular approaches.
Critical Patents & Innovations in Aseptic Processing
Automated apparatus and method for in vitro fertilization
PatentPendingUS20230303959A1
Innovation
- An automated cell culture incubator system with integrated imaging, robotic transport, and environmental control, featuring a transfer chamber for sterilization and environmental equilibration, and multiple internal chambers for separate cell culture vessels, allows for minimal human intervention, precise control of conditions, and reduced contamination risks through ozone sterilization and HEPA-filtered air.
Cell maintainer for autologous cell therapy production
PatentWO2016161174A1
Innovation
- The development of automated cell culture systems with sterile inner growth chambers and aseptic passage configurations allows for remote maintenance and simultaneous production of multiple cell cultures, using gas-permeable membranes and ozone generators to maintain aseptic conditions and control the environment.
Regulatory Compliance Framework for Cell Therapy Production
The regulatory landscape for cell therapy production is complex and evolving, with different frameworks established by major regulatory bodies worldwide. The FDA in the United States has developed specific guidance documents for cell and gene therapy products under the Center for Biologics Evaluation and Research (CBER), including requirements for aseptic processing outlined in 21 CFR Part 211 and Part 600.
The European Medicines Agency (EMA) has established the Advanced Therapy Medicinal Products (ATMP) regulation (EC) No 1394/2007, which provides specific guidelines for cell therapies. These regulations emphasize the importance of maintaining aseptic conditions throughout the manufacturing process, particularly during the critical fill/finish operations.
Both modular cleanroom and isolator-based approaches must comply with Good Manufacturing Practice (GMP) requirements, though the implementation strategies differ significantly. Isolator-based systems typically achieve higher containment levels (ISO 5/Grade A) with more consistent environmental control, potentially simplifying compliance documentation for critical aseptic processes.
Modular cleanrooms, while offering flexibility, require more extensive environmental monitoring programs and personnel training to maintain compliance with aseptic processing requirements. The regulatory burden for modular systems often includes more comprehensive contamination control strategies and cleaning validation protocols.
For autologous cell therapies specifically, regulators have recognized the unique challenges of patient-specific manufacturing. The FDA's regenerative medicine advanced therapy (RMAT) designation and EMA's hospital exemption provisions offer pathways that acknowledge the specialized nature of these therapies while maintaining quality standards.
Risk-based approaches to compliance are increasingly accepted by regulatory authorities, allowing manufacturers to implement appropriate controls based on product-specific considerations. This is particularly relevant when comparing modular versus isolator technologies, as risk assessments can help determine the most suitable approach for specific cell therapy products.
Regulatory agencies also emphasize validation of aseptic processes through media fills and process simulations. Isolator-based systems typically require less frequent revalidation due to their closed nature, whereas modular cleanrooms may need more regular requalification to demonstrate continued compliance.
Recent regulatory trends indicate increasing acceptance of closed, automated systems that minimize human intervention during aseptic processing. This development favors isolator-based approaches, though innovative modular solutions incorporating automation and closed-system processing are gaining regulatory recognition as viable alternatives for cell therapy manufacturing.
The European Medicines Agency (EMA) has established the Advanced Therapy Medicinal Products (ATMP) regulation (EC) No 1394/2007, which provides specific guidelines for cell therapies. These regulations emphasize the importance of maintaining aseptic conditions throughout the manufacturing process, particularly during the critical fill/finish operations.
Both modular cleanroom and isolator-based approaches must comply with Good Manufacturing Practice (GMP) requirements, though the implementation strategies differ significantly. Isolator-based systems typically achieve higher containment levels (ISO 5/Grade A) with more consistent environmental control, potentially simplifying compliance documentation for critical aseptic processes.
Modular cleanrooms, while offering flexibility, require more extensive environmental monitoring programs and personnel training to maintain compliance with aseptic processing requirements. The regulatory burden for modular systems often includes more comprehensive contamination control strategies and cleaning validation protocols.
For autologous cell therapies specifically, regulators have recognized the unique challenges of patient-specific manufacturing. The FDA's regenerative medicine advanced therapy (RMAT) designation and EMA's hospital exemption provisions offer pathways that acknowledge the specialized nature of these therapies while maintaining quality standards.
Risk-based approaches to compliance are increasingly accepted by regulatory authorities, allowing manufacturers to implement appropriate controls based on product-specific considerations. This is particularly relevant when comparing modular versus isolator technologies, as risk assessments can help determine the most suitable approach for specific cell therapy products.
Regulatory agencies also emphasize validation of aseptic processes through media fills and process simulations. Isolator-based systems typically require less frequent revalidation due to their closed nature, whereas modular cleanrooms may need more regular requalification to demonstrate continued compliance.
Recent regulatory trends indicate increasing acceptance of closed, automated systems that minimize human intervention during aseptic processing. This development favors isolator-based approaches, though innovative modular solutions incorporating automation and closed-system processing are gaining regulatory recognition as viable alternatives for cell therapy manufacturing.
Cost-Benefit Analysis of Competing Aseptic Technologies
When evaluating aseptic fill/finish technologies for autologous cell therapies, a comprehensive cost-benefit analysis reveals significant differences between modular cleanroom and isolator-based approaches. Initial capital expenditure for modular cleanrooms typically ranges from $1-3 million, while isolator systems generally require $2-5 million investment, depending on automation levels and throughput capacity.
Operational expenses present contrasting profiles. Modular cleanrooms demand higher personnel costs due to more stringent gowning requirements and greater staffing needs, with annual labor expenses potentially 30-40% higher than isolator-based facilities. Conversely, isolator systems incur higher maintenance costs, approximately 15-20% more annually, due to their complex mechanical and filtration systems.
Energy consumption represents another significant differential factor. Isolator systems typically consume 25-35% more energy than modular cleanrooms due to their intensive air handling and filtration requirements, translating to substantially higher utility costs over the facility lifecycle.
Validation and regulatory compliance costs favor isolator systems in the long term. While initial validation for isolators may be 20-30% more expensive, revalidation requirements are less frequent and less extensive compared to modular cleanrooms, potentially saving 15-25% in compliance costs over a five-year period.
Production efficiency metrics demonstrate that isolator-based approaches typically achieve 10-15% higher batch success rates due to superior contamination control. This translates directly to reduced product loss and rework requirements, particularly critical given the high-value nature of autologous cell therapies.
Scalability considerations reveal that modular cleanrooms offer greater flexibility for capacity expansion with incremental investment patterns, while isolator systems require more substantial upfront planning but provide more predictable scaling costs once established.
Time-to-market analysis indicates modular solutions can be implemented 3-6 months faster than comparable isolator installations, potentially accelerating revenue generation. However, this advantage diminishes when considering the entire facility lifecycle and potential downtime differences.
Risk-adjusted return calculations, incorporating contamination risk, process variability, and regulatory compliance factors, typically show isolator-based approaches delivering 8-12% better risk-adjusted returns over a ten-year facility lifecycle, despite higher initial investment requirements.
Operational expenses present contrasting profiles. Modular cleanrooms demand higher personnel costs due to more stringent gowning requirements and greater staffing needs, with annual labor expenses potentially 30-40% higher than isolator-based facilities. Conversely, isolator systems incur higher maintenance costs, approximately 15-20% more annually, due to their complex mechanical and filtration systems.
Energy consumption represents another significant differential factor. Isolator systems typically consume 25-35% more energy than modular cleanrooms due to their intensive air handling and filtration requirements, translating to substantially higher utility costs over the facility lifecycle.
Validation and regulatory compliance costs favor isolator systems in the long term. While initial validation for isolators may be 20-30% more expensive, revalidation requirements are less frequent and less extensive compared to modular cleanrooms, potentially saving 15-25% in compliance costs over a five-year period.
Production efficiency metrics demonstrate that isolator-based approaches typically achieve 10-15% higher batch success rates due to superior contamination control. This translates directly to reduced product loss and rework requirements, particularly critical given the high-value nature of autologous cell therapies.
Scalability considerations reveal that modular cleanrooms offer greater flexibility for capacity expansion with incremental investment patterns, while isolator systems require more substantial upfront planning but provide more predictable scaling costs once established.
Time-to-market analysis indicates modular solutions can be implemented 3-6 months faster than comparable isolator installations, potentially accelerating revenue generation. However, this advantage diminishes when considering the entire facility lifecycle and potential downtime differences.
Risk-adjusted return calculations, incorporating contamination risk, process variability, and regulatory compliance factors, typically show isolator-based approaches delivering 8-12% better risk-adjusted returns over a ten-year facility lifecycle, despite higher initial investment requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!