Automated dose preparation and labeling systems to support high-throughput clinical sites
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Automated Dose Preparation Evolution and Objectives
Automated dose preparation systems have evolved significantly over the past three decades, transforming from manual processes to increasingly sophisticated automated solutions. The initial systems emerged in the 1990s as basic mechanical aids for pharmacists, primarily focused on reducing human error in medication dispensing. By the early 2000s, these systems incorporated barcode verification technology, representing a crucial step toward enhancing patient safety through accurate medication identification.
The mid-2000s witnessed the integration of robotic components capable of handling vials, syringes, and other medication containers with minimal human intervention. This period marked the transition from semi-automated to fully automated systems in controlled environments. The 2010s brought significant advancements with the incorporation of computer vision, machine learning algorithms, and precision robotics, enabling systems to adapt to various medication types and container formats.
Recent technological developments have focused on creating end-to-end solutions that manage the entire medication preparation workflow, from initial order receipt to final verification and documentation. These modern systems now incorporate real-time monitoring, automated quality control checks, and seamless integration with electronic health records and inventory management systems.
The primary objective of automated dose preparation technology is to enhance patient safety by minimizing human error in medication preparation. Studies consistently demonstrate that automated systems can reduce medication errors by 60-97% compared to manual processes, particularly for high-risk medications requiring precise dosing. This improvement directly translates to better patient outcomes and reduced adverse events.
Efficiency improvement represents another critical objective, as high-throughput clinical sites face increasing patient volumes and medication complexity. Automated systems can process medications 3-5 times faster than manual methods while maintaining consistent quality, enabling healthcare facilities to meet growing demands without proportional staffing increases.
Cost reduction through waste minimization constitutes a significant goal, with automated systems precisely measuring medications to reduce overfill and waste. This precision is particularly valuable for expensive biologics and specialty medications, where even small reductions in waste translate to substantial cost savings.
Data capture and compliance represent emerging objectives, as regulatory requirements for medication traceability continue to expand. Modern systems automatically document each step of the preparation process, creating comprehensive audit trails that satisfy regulatory requirements while reducing administrative burden on clinical staff.
Looking forward, the technology aims to achieve greater flexibility and scalability to accommodate diverse clinical settings, from large hospital systems to ambulatory care centers, while maintaining consistent performance across varying workloads and medication types.
The mid-2000s witnessed the integration of robotic components capable of handling vials, syringes, and other medication containers with minimal human intervention. This period marked the transition from semi-automated to fully automated systems in controlled environments. The 2010s brought significant advancements with the incorporation of computer vision, machine learning algorithms, and precision robotics, enabling systems to adapt to various medication types and container formats.
Recent technological developments have focused on creating end-to-end solutions that manage the entire medication preparation workflow, from initial order receipt to final verification and documentation. These modern systems now incorporate real-time monitoring, automated quality control checks, and seamless integration with electronic health records and inventory management systems.
The primary objective of automated dose preparation technology is to enhance patient safety by minimizing human error in medication preparation. Studies consistently demonstrate that automated systems can reduce medication errors by 60-97% compared to manual processes, particularly for high-risk medications requiring precise dosing. This improvement directly translates to better patient outcomes and reduced adverse events.
Efficiency improvement represents another critical objective, as high-throughput clinical sites face increasing patient volumes and medication complexity. Automated systems can process medications 3-5 times faster than manual methods while maintaining consistent quality, enabling healthcare facilities to meet growing demands without proportional staffing increases.
Cost reduction through waste minimization constitutes a significant goal, with automated systems precisely measuring medications to reduce overfill and waste. This precision is particularly valuable for expensive biologics and specialty medications, where even small reductions in waste translate to substantial cost savings.
Data capture and compliance represent emerging objectives, as regulatory requirements for medication traceability continue to expand. Modern systems automatically document each step of the preparation process, creating comprehensive audit trails that satisfy regulatory requirements while reducing administrative burden on clinical staff.
Looking forward, the technology aims to achieve greater flexibility and scalability to accommodate diverse clinical settings, from large hospital systems to ambulatory care centers, while maintaining consistent performance across varying workloads and medication types.
Clinical Site Demand Analysis for Automation Solutions
The demand for automated dose preparation and labeling systems in clinical settings has experienced significant growth over the past decade. This surge is primarily driven by the increasing complexity of clinical trials, the rising number of participants, and the growing emphasis on precision medicine requiring exact dosing. Clinical sites conducting high-throughput trials face mounting pressure to maintain accuracy while increasing efficiency, creating a substantial market opportunity for automation solutions.
Research indicates that large academic medical centers and contract research organizations (CROs) are leading the adoption curve, with approximately 65% of high-volume sites expressing interest in implementing automated dose preparation systems. The primary drivers behind this demand include the need to reduce medication errors, enhance standardization across multi-site trials, and address the shortage of qualified pharmacy staff experienced in clinical trial medication management.
The market demand is particularly pronounced in oncology trials, where complex dose calculations based on patient-specific factors such as body surface area, weight, and laboratory values create significant room for human error. Immunotherapy trials also demonstrate strong demand due to the precise handling requirements and short stability windows of many biological products.
From an operational perspective, clinical sites report spending between 2-4 hours daily on manual dose preparation and labeling tasks. This represents a significant resource allocation that could be redirected to patient care and other value-added activities. Sites conducting more than 20 clinical trials simultaneously report the highest interest in automation solutions, citing workflow bottlenecks during peak enrollment periods.
Regulatory factors are also driving demand, as health authorities worldwide increasingly emphasize Good Manufacturing Practices (GMP) compliance in clinical trial medication preparation. Automated systems offer enhanced documentation, audit trails, and quality control measures that align with these regulatory expectations.
Geographic analysis reveals that North American and European markets currently demonstrate the strongest demand, with Asia-Pacific regions showing rapid growth potential as clinical trial activities expand in these territories. Urban centers with concentrated research hospitals and pharmaceutical company presence exhibit particularly strong market potential.
Financial considerations reveal that clinical sites are seeking solutions with demonstrable return on investment within 24-36 months. The primary economic drivers include reduced waste of expensive investigational products, decreased labor costs, and mitigation of potential costs associated with dosing errors.
Research indicates that large academic medical centers and contract research organizations (CROs) are leading the adoption curve, with approximately 65% of high-volume sites expressing interest in implementing automated dose preparation systems. The primary drivers behind this demand include the need to reduce medication errors, enhance standardization across multi-site trials, and address the shortage of qualified pharmacy staff experienced in clinical trial medication management.
The market demand is particularly pronounced in oncology trials, where complex dose calculations based on patient-specific factors such as body surface area, weight, and laboratory values create significant room for human error. Immunotherapy trials also demonstrate strong demand due to the precise handling requirements and short stability windows of many biological products.
From an operational perspective, clinical sites report spending between 2-4 hours daily on manual dose preparation and labeling tasks. This represents a significant resource allocation that could be redirected to patient care and other value-added activities. Sites conducting more than 20 clinical trials simultaneously report the highest interest in automation solutions, citing workflow bottlenecks during peak enrollment periods.
Regulatory factors are also driving demand, as health authorities worldwide increasingly emphasize Good Manufacturing Practices (GMP) compliance in clinical trial medication preparation. Automated systems offer enhanced documentation, audit trails, and quality control measures that align with these regulatory expectations.
Geographic analysis reveals that North American and European markets currently demonstrate the strongest demand, with Asia-Pacific regions showing rapid growth potential as clinical trial activities expand in these territories. Urban centers with concentrated research hospitals and pharmaceutical company presence exhibit particularly strong market potential.
Financial considerations reveal that clinical sites are seeking solutions with demonstrable return on investment within 24-36 months. The primary economic drivers include reduced waste of expensive investigational products, decreased labor costs, and mitigation of potential costs associated with dosing errors.
Current Challenges in High-Throughput Dose Preparation
Despite significant advancements in clinical trial operations, high-throughput dose preparation remains plagued by several critical challenges that impede efficiency, accuracy, and safety. Manual dose preparation processes continue to dominate many clinical sites, introducing considerable variability and human error risks. These manual workflows typically require multiple verification steps, creating bottlenecks that significantly limit throughput capacity and delay patient treatment schedules.
Cross-contamination risks represent another substantial challenge, particularly when preparing multiple drug formulations in shared spaces. Current manual processes often lack robust isolation mechanisms, potentially compromising both patient safety and data integrity. This issue becomes increasingly problematic as clinical trials grow in complexity and scale.
Dosing accuracy presents persistent difficulties, especially for precision medicine applications requiring patient-specific formulations. Manual calculations and preparations introduce error margins that can impact therapeutic outcomes and trial data reliability. The absence of real-time verification systems compounds this problem, as errors may not be detected until after administration.
Documentation and traceability deficiencies further complicate high-throughput operations. Paper-based recording systems remain common in many clinical settings, creating information silos that hinder comprehensive audit trails and regulatory compliance. The disconnect between preparation activities and electronic health records introduces additional verification burdens and potential transcription errors.
Resource constraints significantly impact dose preparation capabilities. Specialized pharmacy staff trained in clinical trial protocols are often in short supply, creating scheduling conflicts that limit throughput capacity. Physical space limitations in many clinical sites further restrict the implementation of dedicated preparation areas that could enhance efficiency and safety.
Regulatory compliance presents evolving challenges as standards become increasingly stringent. Current manual processes struggle to maintain consistent documentation that satisfies regulatory requirements across different jurisdictions. This regulatory complexity often necessitates redundant verification steps that further reduce throughput capacity.
Integration with existing clinical workflows represents a significant operational hurdle. Many clinical sites operate with fragmented systems that don't effectively communicate between pharmacy operations and patient administration points. This lack of integration creates information gaps that compromise efficiency and introduce additional verification requirements.
The financial implications of these challenges are substantial, with manual dose preparation driving up per-patient costs through labor-intensive processes and potential waste from preparation errors. These economic factors create significant barriers to scaling clinical trial operations, particularly for smaller research organizations and emerging markets.
Cross-contamination risks represent another substantial challenge, particularly when preparing multiple drug formulations in shared spaces. Current manual processes often lack robust isolation mechanisms, potentially compromising both patient safety and data integrity. This issue becomes increasingly problematic as clinical trials grow in complexity and scale.
Dosing accuracy presents persistent difficulties, especially for precision medicine applications requiring patient-specific formulations. Manual calculations and preparations introduce error margins that can impact therapeutic outcomes and trial data reliability. The absence of real-time verification systems compounds this problem, as errors may not be detected until after administration.
Documentation and traceability deficiencies further complicate high-throughput operations. Paper-based recording systems remain common in many clinical settings, creating information silos that hinder comprehensive audit trails and regulatory compliance. The disconnect between preparation activities and electronic health records introduces additional verification burdens and potential transcription errors.
Resource constraints significantly impact dose preparation capabilities. Specialized pharmacy staff trained in clinical trial protocols are often in short supply, creating scheduling conflicts that limit throughput capacity. Physical space limitations in many clinical sites further restrict the implementation of dedicated preparation areas that could enhance efficiency and safety.
Regulatory compliance presents evolving challenges as standards become increasingly stringent. Current manual processes struggle to maintain consistent documentation that satisfies regulatory requirements across different jurisdictions. This regulatory complexity often necessitates redundant verification steps that further reduce throughput capacity.
Integration with existing clinical workflows represents a significant operational hurdle. Many clinical sites operate with fragmented systems that don't effectively communicate between pharmacy operations and patient administration points. This lack of integration creates information gaps that compromise efficiency and introduce additional verification requirements.
The financial implications of these challenges are substantial, with manual dose preparation driving up per-patient costs through labor-intensive processes and potential waste from preparation errors. These economic factors create significant barriers to scaling clinical trial operations, particularly for smaller research organizations and emerging markets.
Existing Automated Dose Preparation Methodologies
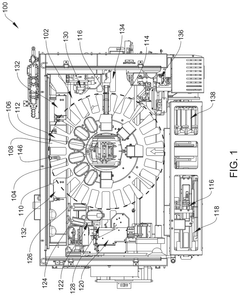
01 Automated medication dispensing and labeling systems
These systems automate the process of preparing, dispensing, and labeling medications in healthcare settings. They incorporate robotic technology to accurately measure and dispense medications according to prescribed doses, while automatically generating and applying appropriate labels. This automation reduces human error, increases efficiency, and ensures compliance with medication safety protocols.- Automated medication dispensing and labeling systems: These systems automate the process of preparing, dispensing, and labeling medications in healthcare settings. They incorporate robotic technology to accurately measure and dispense prescribed doses, while also generating appropriate labels with patient information, dosage instructions, and other relevant details. These automated systems help reduce medication errors, improve efficiency, and ensure compliance with regulatory requirements in pharmacies and hospitals.
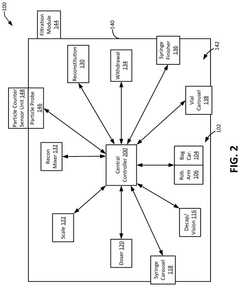
- Software control systems for dose preparation automation: Software platforms that control and manage automated dose preparation workflows. These systems provide interfaces for prescription input, verification, and tracking while coordinating the activities of robotic dispensing hardware. The software includes features for dose calculation, inventory management, quality control, and integration with electronic health records to ensure accurate and efficient medication preparation.
- Robotic systems for pharmaceutical compounding: Specialized robotic systems designed for the precise compounding of pharmaceuticals, particularly for sterile preparations such as intravenous medications, chemotherapy drugs, and parenteral nutrition. These systems operate in controlled environments to maintain sterility while automating the measuring, mixing, and preparation of complex drug formulations according to specific patient requirements.
- Automated verification and quality control in dose preparation: Systems that incorporate verification technologies such as barcode scanning, image recognition, weight verification, and spectroscopic analysis to ensure the accuracy of prepared doses. These quality control mechanisms automatically check medication identity, concentration, and volume before dispensing and labeling, creating multiple layers of verification to prevent medication errors and enhance patient safety.
- Integration of automated dose systems with healthcare information networks: Technologies that enable automated dose preparation and labeling systems to communicate with broader healthcare information networks, including electronic medical records, pharmacy management systems, and hospital information systems. These integrations allow for seamless data flow from prescription to preparation to administration, supporting closed-loop medication management and enhancing traceability throughout the medication use process.
02 Software control systems for dose preparation automation
Advanced software platforms that control and manage automated dose preparation systems. These software solutions coordinate workflow, monitor system operations, validate dosage accuracy, and integrate with healthcare information systems. They provide user interfaces for healthcare professionals to input prescriptions, track preparation status, and generate documentation, while ensuring regulatory compliance through audit trails and data security measures.Expand Specific Solutions03 Barcode and RFID technology for medication tracking and verification
Integration of barcode and RFID technologies in automated dose preparation systems to enable accurate tracking and verification of medications throughout the preparation and dispensing process. These technologies help verify that the correct medication is being prepared for the right patient, track inventory levels, and document the complete chain of custody from preparation to administration.Expand Specific Solutions04 Robotic systems for sterile compounding of medications
Specialized robotic systems designed for the sterile compounding of medications, particularly for intravenous preparations, chemotherapy drugs, and other sterile products. These systems operate in controlled environments to maintain sterility while automating precise measurement, mixing, and packaging of medications. They incorporate advanced safety features to protect both patients and healthcare workers from contamination and exposure to hazardous substances.Expand Specific Solutions05 Quality control and verification systems in automated dose preparation
Integrated quality control mechanisms that verify the accuracy and safety of automatically prepared medications. These systems employ technologies such as computer vision, weight verification, spectroscopic analysis, and barcode scanning to confirm medication identity, concentration, and dosage. They document each verification step, creating comprehensive audit trails that support regulatory compliance and patient safety initiatives.Expand Specific Solutions
Leading Manufacturers and Market Competition
The automated dose preparation and labeling systems market is in a growth phase, driven by increasing demand for high-throughput clinical solutions. The global market is expanding rapidly, with projections indicating significant growth as healthcare facilities seek to improve efficiency and reduce medication errors. Technologically, the field is advancing from semi-automated to fully integrated systems. Key players demonstrate varying levels of maturity: Omnicell and B. Braun Melsungen offer established enterprise solutions, while Synergy Medical BRG and Precision System Science provide specialized automation platforms. Emerging players like Opio Connect are developing niche solutions for specific clinical applications. Companies such as Chemspeed Technologies and National Instruments contribute complementary technologies that enhance system capabilities, indicating a market that is consolidating while still allowing for innovative entrants.
Omnicell, Inc.
Technical Solution: Omnicell has developed comprehensive automated medication management systems that integrate dose preparation and labeling for high-throughput clinical environments. Their XR2 Automated Central Pharmacy System utilizes robotic technology to automate the medication dispensing process, capable of handling 90% of a hospital's non-IV medication inventory. The system incorporates barcode verification technology to ensure accurate medication selection and labeling, with 100% barcode scanning for medication verification. Omnicell's Central Pharmacy IV Compounding Service combines robotic technology with advanced software algorithms to automate the preparation of IV medications, reducing manual handling by up to 80%. Their XT Automated Dispensing Cabinets feature integrated label printing capabilities that automatically generate patient-specific medication labels during the dispensing process. The company's Unity platform provides a centralized database that tracks medication inventory, usage patterns, and preparation workflows across multiple clinical sites, enabling enterprise-level coordination of medication management.
Strengths: Comprehensive end-to-end solution that integrates with existing hospital systems; proven track record in medication management automation with significant market penetration; strong focus on safety with multiple verification steps. Weaknesses: High initial implementation costs; requires significant physical space for installation; dependency on proprietary software ecosystems may limit integration flexibility with some third-party systems.
B. Braun Melsungen AG
Technical Solution: B. Braun has pioneered the Space Automated Infusion System, which incorporates automated dose preparation and labeling capabilities specifically designed for high-volume clinical settings. Their OncoSafety system utilizes gravimetric verification technology to ensure precise dosing during preparation of hazardous medications like chemotherapy drugs, with accuracy rates exceeding 99.5%. The company's Cyto-Set system provides closed-system transfer devices that integrate with automated compounding systems to minimize exposure risks during preparation. B. Braun's Compounding Manager software coordinates the entire preparation workflow, from physician order to final product labeling, reducing preparation time by approximately 30% compared to manual processes. Their automated systems incorporate RFID technology for tracking medications throughout the preparation process, and their labeling systems comply with international standards including GS1 and ISBT 128. B. Braun's solutions emphasize modular design, allowing facilities to scale automation based on throughput requirements while maintaining consistent labeling and preparation protocols across multiple clinical sites.
Strengths: Strong focus on safety features particularly for hazardous drug handling; modular approach allows scalable implementation based on facility needs; extensive experience in infusion technology provides deep understanding of clinical workflows. Weaknesses: Solutions primarily focused on infusion medications rather than comprehensive medication management; integration with non-B. Braun systems may require additional customization; higher ongoing consumable costs compared to some competitors.
Key Patents in Medication Automation Technology
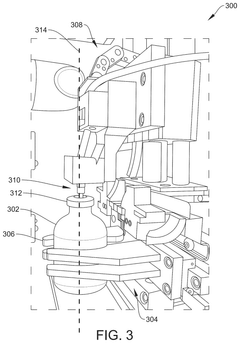
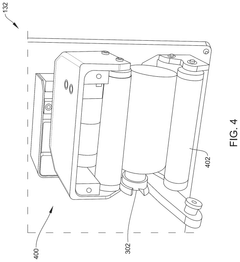
Systems and methods for parallel preparation processing
PatentActiveUS12125574B2
Innovation
- An automated dosing device with multiple stations and a central controller that uses a robotic arm and bag carousel to perform parallel processing, iteratively assigning tasks and directing the transport of medication containers between stations to create dosed medication delivery containers, while maintaining sterility and cleanliness through controlled environments and continuous quality monitoring.
Device and method for developing formulations
PatentWO2017023154A2
Innovation
- An automated system with multiple screening lines, analyzers, and a robotic arm for high-throughput screening and formulation, which includes storage tanks, dosing systems, formulation chambers, sensors, and actuators to rapidly prepare and test numerous ingredient combinations, reducing manual labor and enhancing precision.
Regulatory Compliance and Safety Standards
Automated dose preparation and labeling systems operate within a complex regulatory framework that varies across global regions. In the United States, these systems must comply with FDA regulations, particularly 21 CFR Part 211 for pharmaceutical manufacturing and 21 CFR Part 11 for electronic records. The European Medicines Agency (EMA) enforces similar but distinct requirements through EU GMP Annex 11 and Annex 15, focusing on computerized systems validation and qualification processes.
Safety standards for these automated systems are multifaceted, encompassing both operator safety and patient protection. ISO 13485 certification is typically required for medical device quality management systems, while IEC 62304 addresses software lifecycle requirements. For systems handling radiopharmaceuticals, additional regulations such as those from the Nuclear Regulatory Commission (NRC) in the US or equivalent bodies internationally must be considered.
Risk management frameworks, particularly ISO 14971, are essential for identifying and mitigating potential hazards. Manufacturers must conduct comprehensive risk assessments covering mechanical safety, electrical safety, software reliability, and cross-contamination prevention. These assessments must be documented and regularly updated throughout the system's lifecycle.
Data integrity requirements present significant challenges for automated dose preparation systems. ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be embedded in system design. This necessitates robust audit trail capabilities, electronic signature compliance, and secure data storage solutions.
Validation protocols for these systems are particularly stringent, requiring Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) documentation. For high-throughput clinical sites, the validation must specifically address volume processing capabilities while maintaining accuracy and sterility.
Emerging regulatory trends indicate increasing scrutiny of artificial intelligence and machine learning components in automated systems. Regulatory bodies are developing frameworks for evaluating AI-driven decision-making in critical healthcare applications, which will impact next-generation dose preparation systems incorporating these technologies.
Harmonization efforts between major regulatory bodies are gradually reducing compliance complexity, though significant regional variations persist. Manufacturers developing global solutions must implement configurable compliance features that can adapt to different regulatory environments while maintaining core safety and performance standards.
Safety standards for these automated systems are multifaceted, encompassing both operator safety and patient protection. ISO 13485 certification is typically required for medical device quality management systems, while IEC 62304 addresses software lifecycle requirements. For systems handling radiopharmaceuticals, additional regulations such as those from the Nuclear Regulatory Commission (NRC) in the US or equivalent bodies internationally must be considered.
Risk management frameworks, particularly ISO 14971, are essential for identifying and mitigating potential hazards. Manufacturers must conduct comprehensive risk assessments covering mechanical safety, electrical safety, software reliability, and cross-contamination prevention. These assessments must be documented and regularly updated throughout the system's lifecycle.
Data integrity requirements present significant challenges for automated dose preparation systems. ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) must be embedded in system design. This necessitates robust audit trail capabilities, electronic signature compliance, and secure data storage solutions.
Validation protocols for these systems are particularly stringent, requiring Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) documentation. For high-throughput clinical sites, the validation must specifically address volume processing capabilities while maintaining accuracy and sterility.
Emerging regulatory trends indicate increasing scrutiny of artificial intelligence and machine learning components in automated systems. Regulatory bodies are developing frameworks for evaluating AI-driven decision-making in critical healthcare applications, which will impact next-generation dose preparation systems incorporating these technologies.
Harmonization efforts between major regulatory bodies are gradually reducing compliance complexity, though significant regional variations persist. Manufacturers developing global solutions must implement configurable compliance features that can adapt to different regulatory environments while maintaining core safety and performance standards.
Cost-Benefit Analysis of Implementation
The implementation of automated dose preparation and labeling systems represents a significant capital investment for clinical sites. Initial costs include equipment acquisition (ranging from $150,000 to $500,000 depending on system complexity), facility modifications ($50,000-$100,000), software integration ($30,000-$80,000), and staff training ($10,000-$25,000). Ongoing expenses encompass maintenance contracts (typically 10-15% of equipment cost annually), consumables, software updates, and periodic recertification.
Despite substantial upfront investments, the long-term financial benefits are compelling. Labor cost reduction is significant, with automated systems reducing pharmacist and technician time by 40-60% for dose preparation activities. A high-throughput clinical site preparing 100 doses daily could save approximately $120,000-$180,000 annually in labor costs alone.
Error reduction generates substantial savings by minimizing costly medication errors. Studies indicate automated systems can reduce preparation errors by up to 97%, potentially saving $500,000-$1,000,000 annually in error-related costs for large clinical sites. Additionally, waste reduction from precise dosing saves 8-12% in medication costs, particularly valuable for expensive biologics and specialized pharmaceuticals.
Throughput improvements enable clinical sites to increase patient capacity by 30-45% without proportional staffing increases. This operational efficiency translates to approximately $200,000-$400,000 in additional annual revenue for medium to large clinical sites.
Return on investment analysis indicates most facilities achieve breakeven within 18-24 months. Five-year ROI calculations typically show returns of 150-300%, with higher returns for larger facilities handling greater volumes. The net present value of implementation generally ranges from $500,000 to $2,000,000 depending on facility size and patient volume.
Non-financial benefits include enhanced regulatory compliance, improved documentation accuracy, and increased patient satisfaction through reduced wait times. These factors, while difficult to quantify directly, contribute significantly to institutional reputation and operational excellence.
Risk mitigation considerations include implementation disruption, integration challenges with existing systems, and potential resistance to workflow changes. Successful implementations typically allocate 15-20% of the project budget to change management and contingency planning to address these concerns.
Despite substantial upfront investments, the long-term financial benefits are compelling. Labor cost reduction is significant, with automated systems reducing pharmacist and technician time by 40-60% for dose preparation activities. A high-throughput clinical site preparing 100 doses daily could save approximately $120,000-$180,000 annually in labor costs alone.
Error reduction generates substantial savings by minimizing costly medication errors. Studies indicate automated systems can reduce preparation errors by up to 97%, potentially saving $500,000-$1,000,000 annually in error-related costs for large clinical sites. Additionally, waste reduction from precise dosing saves 8-12% in medication costs, particularly valuable for expensive biologics and specialized pharmaceuticals.
Throughput improvements enable clinical sites to increase patient capacity by 30-45% without proportional staffing increases. This operational efficiency translates to approximately $200,000-$400,000 in additional annual revenue for medium to large clinical sites.
Return on investment analysis indicates most facilities achieve breakeven within 18-24 months. Five-year ROI calculations typically show returns of 150-300%, with higher returns for larger facilities handling greater volumes. The net present value of implementation generally ranges from $500,000 to $2,000,000 depending on facility size and patient volume.
Non-financial benefits include enhanced regulatory compliance, improved documentation accuracy, and increased patient satisfaction through reduced wait times. These factors, while difficult to quantify directly, contribute significantly to institutional reputation and operational excellence.
Risk mitigation considerations include implementation disruption, integration challenges with existing systems, and potential resistance to workflow changes. Successful implementations typically allocate 15-20% of the project budget to change management and contingency planning to address these concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!