Cold chain risk assessment for autologous cell therapy logistics: minimizing dose loss during transport
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autologous Cell Therapy Cold Chain Background and Objectives
Autologous cell therapies represent a revolutionary approach in personalized medicine, where a patient's own cells are collected, modified, and reinfused to treat various conditions, particularly in oncology and regenerative medicine. The evolution of this therapeutic modality has accelerated significantly over the past decade, with the first FDA approval of CAR-T cell therapy in 2017 marking a pivotal milestone in the field. Since then, the sector has witnessed exponential growth, with multiple approved products and hundreds more in clinical development globally.
The cold chain logistics for autologous cell therapies presents unique challenges compared to traditional pharmaceutical products. Unlike conventional medications, these living cellular products cannot be mass-produced and stockpiled; each dose is patient-specific, irreplaceable, and often represents the last therapeutic option for critically ill patients. The time-sensitive nature of these therapies creates a zero-margin-for-error scenario in their transportation and handling.
Temperature excursions during transport can significantly impact cell viability, potency, and ultimately therapeutic efficacy. Current data indicates that approximately 5-8% of autologous cell therapy doses experience some form of compromise during the logistics process, with temperature deviations being the primary culprit. This translates to potentially life-threatening consequences for patients awaiting treatment and substantial financial losses for healthcare systems and manufacturers, with each dose valued between $375,000 and $475,000.
The technical evolution in this domain has progressed from simple dry ice shipping to sophisticated cryogenic preservation systems. However, the complexity of maintaining ultra-low temperatures (-150°C or below) during multi-leg international journeys, often involving multiple handling points and transportation modes, continues to present significant challenges to the industry.
The primary objective of this technical research is to comprehensively assess the risks associated with cold chain logistics for autologous cell therapies and develop robust strategies to minimize dose loss during transport. Specifically, we aim to identify critical control points in the supply chain where temperature excursions are most likely to occur, evaluate emerging technologies for real-time monitoring and intervention, and establish evidence-based protocols for risk mitigation.
Additionally, this research seeks to explore predictive modeling approaches that can anticipate potential failures before they occur, thereby enabling proactive rather than reactive management of the cold chain. The ultimate goal is to establish a resilient logistics framework that ensures near-perfect delivery of these life-saving therapies, regardless of geographic challenges, transportation disruptions, or other external variables that might compromise product integrity.
The cold chain logistics for autologous cell therapies presents unique challenges compared to traditional pharmaceutical products. Unlike conventional medications, these living cellular products cannot be mass-produced and stockpiled; each dose is patient-specific, irreplaceable, and often represents the last therapeutic option for critically ill patients. The time-sensitive nature of these therapies creates a zero-margin-for-error scenario in their transportation and handling.
Temperature excursions during transport can significantly impact cell viability, potency, and ultimately therapeutic efficacy. Current data indicates that approximately 5-8% of autologous cell therapy doses experience some form of compromise during the logistics process, with temperature deviations being the primary culprit. This translates to potentially life-threatening consequences for patients awaiting treatment and substantial financial losses for healthcare systems and manufacturers, with each dose valued between $375,000 and $475,000.
The technical evolution in this domain has progressed from simple dry ice shipping to sophisticated cryogenic preservation systems. However, the complexity of maintaining ultra-low temperatures (-150°C or below) during multi-leg international journeys, often involving multiple handling points and transportation modes, continues to present significant challenges to the industry.
The primary objective of this technical research is to comprehensively assess the risks associated with cold chain logistics for autologous cell therapies and develop robust strategies to minimize dose loss during transport. Specifically, we aim to identify critical control points in the supply chain where temperature excursions are most likely to occur, evaluate emerging technologies for real-time monitoring and intervention, and establish evidence-based protocols for risk mitigation.
Additionally, this research seeks to explore predictive modeling approaches that can anticipate potential failures before they occur, thereby enabling proactive rather than reactive management of the cold chain. The ultimate goal is to establish a resilient logistics framework that ensures near-perfect delivery of these life-saving therapies, regardless of geographic challenges, transportation disruptions, or other external variables that might compromise product integrity.
Market Analysis for Cell Therapy Logistics Solutions
The cell therapy logistics market is experiencing unprecedented growth, driven by the increasing prevalence of cancer and genetic disorders, alongside advancements in regenerative medicine. The global cell therapy market was valued at approximately $14.5 billion in 2020 and is projected to reach $48.11 billion by 2027, with a compound annual growth rate (CAGR) of 25.6%. Within this broader market, logistics solutions specifically tailored for cell therapies represent a critical and rapidly expanding segment.
Autologous cell therapies, which utilize a patient's own cells, present unique logistical challenges compared to traditional pharmaceutical products. These therapies require an unbroken chain of identity and chain of custody throughout the entire process, from collection to manufacturing and back to patient administration. The specialized nature of these requirements has created a distinct market niche for cold chain logistics providers who can ensure temperature-controlled environments ranging from refrigerated (2-8°C) to cryogenic (-196°C) conditions.
Market demand for cell therapy logistics solutions is being driven by several factors. First, the increasing number of approved cell therapies, such as Kymriah and Yescarta, has created immediate commercial logistics needs. Second, the robust clinical pipeline, with over 1,200 ongoing cell therapy clinical trials globally, signals sustained future demand. Third, regulatory bodies like the FDA and EMA have established stringent requirements for cell therapy transport, necessitating specialized logistics solutions.
Regional analysis reveals North America currently dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing research activities, and improving regulatory frameworks in countries like China, Japan, and South Korea.
The customer base for cell therapy logistics solutions is diverse, including pharmaceutical and biotechnology companies, academic research institutions, contract research organizations (CROs), and hospitals. Each segment has distinct requirements regarding temperature control, tracking capabilities, and regulatory compliance. Pharmaceutical companies typically demand end-to-end solutions with comprehensive risk management protocols, while research institutions may prioritize cost-effectiveness and flexibility.
Price sensitivity varies significantly across market segments. While large pharmaceutical companies can absorb premium pricing for guaranteed delivery, smaller biotech firms and research institutions are more price-sensitive. This has created opportunities for tiered service offerings in the market, ranging from basic temperature-controlled shipping to comprehensive logistics management platforms with real-time monitoring and intervention capabilities.
Autologous cell therapies, which utilize a patient's own cells, present unique logistical challenges compared to traditional pharmaceutical products. These therapies require an unbroken chain of identity and chain of custody throughout the entire process, from collection to manufacturing and back to patient administration. The specialized nature of these requirements has created a distinct market niche for cold chain logistics providers who can ensure temperature-controlled environments ranging from refrigerated (2-8°C) to cryogenic (-196°C) conditions.
Market demand for cell therapy logistics solutions is being driven by several factors. First, the increasing number of approved cell therapies, such as Kymriah and Yescarta, has created immediate commercial logistics needs. Second, the robust clinical pipeline, with over 1,200 ongoing cell therapy clinical trials globally, signals sustained future demand. Third, regulatory bodies like the FDA and EMA have established stringent requirements for cell therapy transport, necessitating specialized logistics solutions.
Regional analysis reveals North America currently dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing research activities, and improving regulatory frameworks in countries like China, Japan, and South Korea.
The customer base for cell therapy logistics solutions is diverse, including pharmaceutical and biotechnology companies, academic research institutions, contract research organizations (CROs), and hospitals. Each segment has distinct requirements regarding temperature control, tracking capabilities, and regulatory compliance. Pharmaceutical companies typically demand end-to-end solutions with comprehensive risk management protocols, while research institutions may prioritize cost-effectiveness and flexibility.
Price sensitivity varies significantly across market segments. While large pharmaceutical companies can absorb premium pricing for guaranteed delivery, smaller biotech firms and research institutions are more price-sensitive. This has created opportunities for tiered service offerings in the market, ranging from basic temperature-controlled shipping to comprehensive logistics management platforms with real-time monitoring and intervention capabilities.
Current Cold Chain Challenges in Cell Therapy Transport
The transportation of autologous cell therapies presents unique cold chain challenges that significantly exceed those of traditional pharmaceutical products. Unlike conventional medications, these living cellular products are irreplaceable, patient-specific, and extremely temperature-sensitive, with each dose representing a critical, one-time therapeutic opportunity for patients often facing life-threatening conditions.
Current cold chain infrastructure, primarily designed for traditional pharmaceuticals and vaccines, proves inadequate for the stringent requirements of cell therapies. Standard temperature excursions that might be acceptable for conventional products can result in catastrophic viability loss for cellular materials. Industry data indicates that temperature deviations as brief as 30 minutes can reduce cell viability by 15-30%, potentially rendering treatments ineffective.
The absence of standardized protocols specifically designed for cell therapy transport creates significant variability in handling procedures across different facilities and geographic regions. This inconsistency introduces additional risk factors during the multiple handoffs typical in complex supply chains spanning collection centers, manufacturing facilities, and treatment centers.
Real-time monitoring capabilities remain limited, with many current systems providing only retrospective temperature data rather than actionable real-time alerts. This monitoring gap prevents immediate intervention during critical temperature excursions, often resulting in the discovery of compromised products only upon arrival at their destination.
Regulatory frameworks governing cell therapy transport continue to evolve, creating compliance uncertainties for manufacturers and logistics providers. The FDA, EMA, and other regulatory bodies are still developing specific guidance for cell therapy cold chains, resulting in varying interpretations of requirements across different markets and jurisdictions.
The financial implications of cold chain failures are particularly severe in the cell therapy context. Beyond the direct cost of product loss (often exceeding $500,000 per dose), failed deliveries result in treatment delays for critically ill patients, potentially compromising clinical outcomes and necessitating additional invasive collection procedures.
Geographical challenges further complicate cell therapy logistics, with transport routes often spanning international boundaries and diverse climate zones. Remote or underserved regions frequently lack the specialized infrastructure required for maintaining ultra-low temperatures, creating significant disparities in treatment accessibility.
The industry currently faces a critical shortage of specialized logistics personnel trained specifically in cell therapy handling requirements. This expertise gap becomes particularly problematic during transport disruptions when rapid, informed decision-making is essential to preserve product integrity.
Current cold chain infrastructure, primarily designed for traditional pharmaceuticals and vaccines, proves inadequate for the stringent requirements of cell therapies. Standard temperature excursions that might be acceptable for conventional products can result in catastrophic viability loss for cellular materials. Industry data indicates that temperature deviations as brief as 30 minutes can reduce cell viability by 15-30%, potentially rendering treatments ineffective.
The absence of standardized protocols specifically designed for cell therapy transport creates significant variability in handling procedures across different facilities and geographic regions. This inconsistency introduces additional risk factors during the multiple handoffs typical in complex supply chains spanning collection centers, manufacturing facilities, and treatment centers.
Real-time monitoring capabilities remain limited, with many current systems providing only retrospective temperature data rather than actionable real-time alerts. This monitoring gap prevents immediate intervention during critical temperature excursions, often resulting in the discovery of compromised products only upon arrival at their destination.
Regulatory frameworks governing cell therapy transport continue to evolve, creating compliance uncertainties for manufacturers and logistics providers. The FDA, EMA, and other regulatory bodies are still developing specific guidance for cell therapy cold chains, resulting in varying interpretations of requirements across different markets and jurisdictions.
The financial implications of cold chain failures are particularly severe in the cell therapy context. Beyond the direct cost of product loss (often exceeding $500,000 per dose), failed deliveries result in treatment delays for critically ill patients, potentially compromising clinical outcomes and necessitating additional invasive collection procedures.
Geographical challenges further complicate cell therapy logistics, with transport routes often spanning international boundaries and diverse climate zones. Remote or underserved regions frequently lack the specialized infrastructure required for maintaining ultra-low temperatures, creating significant disparities in treatment accessibility.
The industry currently faces a critical shortage of specialized logistics personnel trained specifically in cell therapy handling requirements. This expertise gap becomes particularly problematic during transport disruptions when rapid, informed decision-making is essential to preserve product integrity.
Current Risk Mitigation Strategies for Cell Product Transport
01 Temperature monitoring and control systems
Cold chain logistics systems require precise temperature monitoring and control to prevent dose loss. These systems utilize sensors and monitoring devices to track temperature conditions in real-time throughout the transportation and storage process. Advanced temperature control mechanisms can automatically adjust conditions when deviations occur, ensuring that temperature-sensitive products like vaccines and medications maintain their efficacy. These systems often include alert mechanisms that notify operators when temperature excursions occur, allowing for immediate corrective actions.- Temperature monitoring and control systems: Advanced temperature monitoring and control systems are essential in cold chain logistics to prevent dose loss. These systems utilize sensors and real-time tracking to maintain optimal temperature conditions throughout transportation and storage. Automated alerts notify operators when temperatures deviate from acceptable ranges, allowing for immediate corrective actions. These technologies help ensure product integrity and reduce waste due to temperature excursions.
- Smart packaging solutions: Innovative packaging solutions play a crucial role in minimizing dose loss in cold chain logistics. These include insulated containers with phase-change materials, vacuum insulation panels, and active cooling systems integrated into packaging. Smart packaging may incorporate temperature indicators and data loggers that provide visual or electronic records of temperature history. These solutions extend product shelf life and maintain efficacy during transportation across varying environmental conditions.
- IoT and blockchain integration: Integration of Internet of Things (IoT) and blockchain technologies enhances cold chain logistics management and reduces dose loss. IoT devices provide continuous monitoring of environmental conditions, while blockchain creates immutable records of the entire supply chain journey. This combination ensures data integrity, improves traceability, and enables stakeholders to verify that products have been maintained under proper conditions. These technologies support regulatory compliance and build trust among supply chain partners.
- Predictive analytics and AI-driven logistics: Artificial intelligence and predictive analytics are transforming cold chain logistics by anticipating potential issues before they cause dose loss. These systems analyze historical data, weather patterns, and transportation routes to optimize delivery schedules and storage conditions. Machine learning algorithms can predict equipment failures, identify optimal routing, and recommend preventive maintenance. This proactive approach significantly reduces the risk of product degradation and improves overall supply chain efficiency.
- Inventory management and expiration tracking systems: Sophisticated inventory management systems specifically designed for temperature-sensitive products help minimize dose loss through better stock rotation and expiration date tracking. These systems implement first-expiry-first-out protocols, automate reordering processes, and provide visibility across distribution networks. Real-time inventory tracking allows for quick identification of products approaching expiration dates, enabling timely redistribution to prevent waste. These solutions optimize resource allocation and reduce financial losses associated with expired products.
02 Smart packaging solutions
Specialized packaging plays a crucial role in preventing dose loss in cold chain logistics. Smart packaging solutions incorporate insulation materials, phase change materials, and temperature-stabilizing components that maintain required temperature ranges for extended periods. These packaging innovations can include active cooling elements or passive thermal protection systems designed specifically for pharmaceutical and biological products. Some advanced packaging solutions also integrate monitoring devices that provide visual indicators of temperature breaches, ensuring product integrity throughout the supply chain.Expand Specific Solutions03 Inventory management and tracking systems
Effective inventory management systems are essential for minimizing dose loss in cold chain logistics. These systems employ RFID technology, barcoding, and digital tracking solutions to monitor product movement and storage conditions. Advanced inventory management platforms can predict expiration dates, optimize stock rotation, and ensure that temperature-sensitive products are properly handled. These systems also facilitate batch tracking and recall management, which is critical for pharmaceutical products that may have been exposed to temperature excursions.Expand Specific Solutions04 Transportation optimization and route planning
Specialized transportation solutions and route planning are critical components in preventing dose loss during cold chain logistics. These systems optimize delivery routes to minimize transit time and reduce exposure to temperature variations. Advanced logistics platforms incorporate real-time weather data, traffic conditions, and vehicle performance metrics to ensure optimal transportation conditions. Some systems include redundant cooling mechanisms in transport vehicles and transfer protocols designed specifically for temperature-sensitive products, ensuring continuous cold chain integrity from origin to destination.Expand Specific Solutions05 Blockchain and IoT integration for cold chain verification
The integration of blockchain technology and Internet of Things (IoT) devices provides enhanced verification and transparency in cold chain logistics systems. These technologies create immutable records of temperature conditions, handling procedures, and custody transfers throughout the supply chain. IoT sensors continuously monitor environmental conditions and automatically record data to blockchain ledgers, creating tamper-proof documentation of cold chain compliance. This integration enables all stakeholders to verify that proper conditions were maintained, reducing dose loss through improved accountability and allowing for precise identification of where in the supply chain any temperature excursions may have occurred.Expand Specific Solutions
Key Industry Players in Cell Therapy Cold Chain Management
The autologous cell therapy cold chain logistics market is currently in a growth phase, with increasing demand driven by advancements in personalized medicine. The global market size is estimated to exceed $3 billion, expanding at a CAGR of 15-20% as cell therapies gain regulatory approvals. Technologically, the field is maturing rapidly with companies developing specialized solutions. Softbox Systems and MaxQ Research lead in temperature-controlled packaging innovations, while Klatu Networks and IBM offer advanced monitoring systems. Pharmaceutical logistics specialists like Medipal Holdings and Yisu Logistics provide specialized transport services. Research institutions including Case Western Reserve University and University of Porto collaborate with companies like FibroBiologics and Abeona Therapeutics to develop improved preservation methods, addressing the critical challenge of maintaining cell viability during transport.
Softbox Systems Ltd.
Technical Solution: Softbox Systems has pioneered the TempCell™ platform specifically designed for autologous cell therapy cold chain logistics. This solution combines passive temperature-controlled packaging with advanced phase change materials that maintain precise temperatures (±0.5°C accuracy) for up to 96 hours. Their technology incorporates multiple redundant temperature barriers to protect against external environmental fluctuations during transit. The packaging includes integrated electronic temperature monitoring devices that provide continuous data logging with GPS location tracking. Softbox has also developed specialized qualification protocols that simulate various transport scenarios and environmental conditions to ensure performance reliability. Their systems are designed with modular components that can be configured for different temperature ranges (from cryogenic to controlled ambient) depending on the specific cell therapy requirements. The company has implemented a risk assessment matrix tool that evaluates potential failure modes across different transport routes and conditions, allowing for route-specific packaging configurations[2][3].
Strengths: The passive system design eliminates dependence on external power sources, reducing failure points during transport. The extended temperature stability window provides greater flexibility in logistics planning and handling delays. Weaknesses: The passive nature means limited ability to actively respond to unexpected temperature excursions. The packaging solutions, while effective, add significant weight and volume compared to non-temperature-controlled options, potentially increasing shipping costs.
Shanghai Origincell Biological Cryo Equipment Co. Ltd.
Technical Solution: Shanghai Origincell has developed the CryoSafe transport system specifically engineered for autologous cell therapy logistics in challenging environments. Their technology combines advanced vacuum insulation with phase-change materials that maintain ultra-low temperatures (-196°C to -80°C) for extended periods without external power. The system incorporates a multi-sensor array that monitors temperature at different locations within the container, detecting potential cold spots or warming zones that could compromise sample integrity. Origincell's proprietary risk assessment software analyzes transport routes for potential hazards including customs delays, extreme weather patterns, and handling procedures at transfer points. Their containers feature specialized shock absorption systems that protect cellular material from vibration damage during transport, with accelerometers documenting any significant impact events. The company has implemented a dual-redundancy cooling system that automatically activates backup cooling mechanisms if primary systems show signs of failure. Their technology includes real-time location tracking integrated with temperature monitoring to correlate any temperature excursions with specific handling events or environmental conditions[8][9].
Strengths: The system's ability to maintain ultra-low temperatures without external power provides exceptional reliability in regions with unstable infrastructure. The comprehensive monitoring and documentation capabilities support regulatory compliance and quality assurance. Weaknesses: The sophisticated technology results in higher acquisition costs compared to standard cryogenic shipping containers. The specialized nature of the equipment requires dedicated training for handling personnel at all points in the logistics chain.
Critical Temperature Control Technologies and Innovations
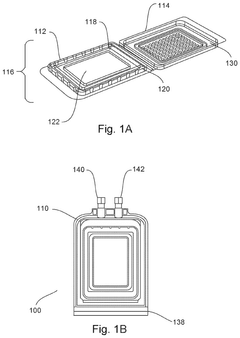
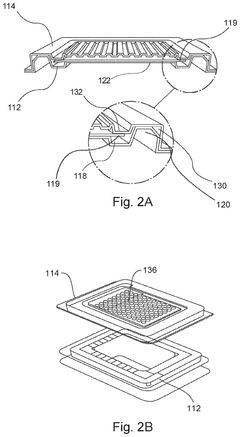
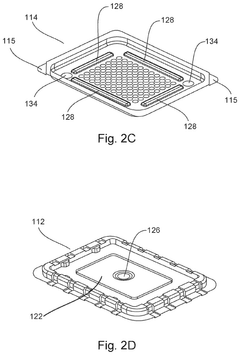
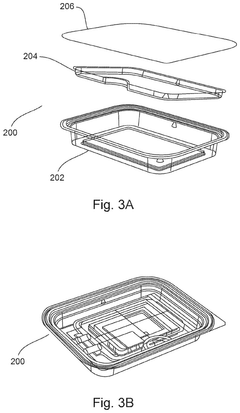
Devices, systems, and methods for packaging and transport of biological tissue
PatentPendingUS20250024833A1
Innovation
- A biological tissue packaging and transport system comprising a series of sterilized aseptic containers, including a clamshell container, a pouch, a secondary package, and a tertiary package, designed to minimize movement, maintain fluid circulation, prevent bubble formation, and ensure non-toxic materials, while allowing for sterilization and ease of use.
Regulatory Framework for Cell Therapy Transport
The regulatory landscape governing cell therapy transport is complex and multifaceted, with stringent requirements designed to ensure product integrity and patient safety. At the international level, organizations such as the International Air Transport Association (IATA) and the World Health Organization (WHO) provide overarching guidelines for the transportation of biological materials, including autologous cell therapies. These frameworks establish baseline standards for packaging, labeling, and handling procedures during transit.
In the United States, the Food and Drug Administration (FDA) regulates cell therapy products under 21 CFR Part 1271, with additional guidance documents specifically addressing chain of custody and cold chain management. The FDA's approach emphasizes risk-based strategies, requiring manufacturers to validate their shipping containers and temperature monitoring systems under various environmental conditions that simulate real-world transport scenarios.
The European Medicines Agency (EMA) implements similar but distinct requirements through its Advanced Therapy Medicinal Products (ATMP) regulation (EC No 1394/2007), which includes specific provisions for autologous products. European regulations place particular emphasis on traceability throughout the supply chain, mandating comprehensive documentation from collection site to administration.
Regulatory bodies increasingly recognize the unique challenges of autologous therapies, where each dose represents an irreplaceable, patient-specific treatment. This has led to the development of specialized guidance documents such as the PDA Technical Report No. 88, which addresses the specific cold chain requirements for cell-based products.
Compliance with Good Distribution Practice (GDP) and Good Manufacturing Practice (GMP) principles forms the foundation of regulatory expectations. These standards require continuous temperature monitoring, validated shipping containers, and detailed contingency plans for potential disruptions during transport. Recent regulatory trends show increasing focus on real-time monitoring technologies and data integrity throughout the cold chain.
Notably, regulatory frameworks are evolving to accommodate technological advancements in cryopreservation and shipping solutions. Agencies are beginning to provide pathways for implementing novel preservation methods that may extend allowable transport times or improve stability, provided manufacturers can demonstrate equivalent or superior protection against dose loss compared to traditional methods.
Understanding and navigating these regulatory requirements is essential for developing robust risk assessment protocols that not only ensure compliance but also optimize the likelihood of successful delivery of viable autologous cell therapy products to patients.
In the United States, the Food and Drug Administration (FDA) regulates cell therapy products under 21 CFR Part 1271, with additional guidance documents specifically addressing chain of custody and cold chain management. The FDA's approach emphasizes risk-based strategies, requiring manufacturers to validate their shipping containers and temperature monitoring systems under various environmental conditions that simulate real-world transport scenarios.
The European Medicines Agency (EMA) implements similar but distinct requirements through its Advanced Therapy Medicinal Products (ATMP) regulation (EC No 1394/2007), which includes specific provisions for autologous products. European regulations place particular emphasis on traceability throughout the supply chain, mandating comprehensive documentation from collection site to administration.
Regulatory bodies increasingly recognize the unique challenges of autologous therapies, where each dose represents an irreplaceable, patient-specific treatment. This has led to the development of specialized guidance documents such as the PDA Technical Report No. 88, which addresses the specific cold chain requirements for cell-based products.
Compliance with Good Distribution Practice (GDP) and Good Manufacturing Practice (GMP) principles forms the foundation of regulatory expectations. These standards require continuous temperature monitoring, validated shipping containers, and detailed contingency plans for potential disruptions during transport. Recent regulatory trends show increasing focus on real-time monitoring technologies and data integrity throughout the cold chain.
Notably, regulatory frameworks are evolving to accommodate technological advancements in cryopreservation and shipping solutions. Agencies are beginning to provide pathways for implementing novel preservation methods that may extend allowable transport times or improve stability, provided manufacturers can demonstrate equivalent or superior protection against dose loss compared to traditional methods.
Understanding and navigating these regulatory requirements is essential for developing robust risk assessment protocols that not only ensure compliance but also optimize the likelihood of successful delivery of viable autologous cell therapy products to patients.
Economic Impact of Dose Loss Prevention
The economic implications of preventing dose loss in autologous cell therapies extend far beyond the immediate cost of product replacement. Each autologous cell therapy dose represents a significant financial investment, with manufacturing costs typically ranging from $50,000 to $500,000 per dose. When considering that these therapies often target life-threatening conditions with limited treatment alternatives, the economic value transcends mere production expenses.
Risk mitigation strategies in cold chain logistics directly impact the financial sustainability of cell therapy programs. Implementation of advanced temperature monitoring systems, validated shipping containers, and redundant cooling mechanisms may require initial capital expenditure of $10,000-30,000 per shipping program, but this investment yields substantial returns through dose preservation. Industry data suggests that effective cold chain management can reduce dose loss rates from approximately 3-5% to under 1%, representing millions in saved product value annually for mid-sized therapy providers.
The economic calculation must also account for indirect costs associated with dose failures. Patient rescheduling, additional hospital stays, alternative treatment provisions, and potential litigation expenses can multiply the financial impact by 2-3 times the direct product cost. Furthermore, insurance reimbursement complications arising from therapy delays create additional administrative burdens estimated at $2,000-5,000 per incident.
From a healthcare system perspective, preventing dose loss contributes to overall cost efficiency. Each successful cell therapy administration potentially reduces long-term healthcare utilization by addressing underlying conditions more effectively than conventional treatments. Studies indicate that successful CAR-T therapies can reduce five-year healthcare costs by 15-25% compared to traditional treatment regimens for certain hematological malignancies.
The economic benefits extend to pharmaceutical companies and therapy developers as well. Improved cold chain reliability enhances scalability of autologous therapy programs, potentially reducing per-dose costs by 10-15% through operational efficiencies. Additionally, demonstrated supply chain excellence strengthens market positioning and can influence favorable reimbursement decisions from payers, directly impacting commercial viability.
Ultimately, investments in cold chain risk assessment and mitigation represent high-value allocations of resources that generate returns across multiple stakeholders in the healthcare ecosystem. As the autologous cell therapy market expands at a projected CAGR of 23.5% through 2028, the economic imperative of dose loss prevention will only increase in significance.
Risk mitigation strategies in cold chain logistics directly impact the financial sustainability of cell therapy programs. Implementation of advanced temperature monitoring systems, validated shipping containers, and redundant cooling mechanisms may require initial capital expenditure of $10,000-30,000 per shipping program, but this investment yields substantial returns through dose preservation. Industry data suggests that effective cold chain management can reduce dose loss rates from approximately 3-5% to under 1%, representing millions in saved product value annually for mid-sized therapy providers.
The economic calculation must also account for indirect costs associated with dose failures. Patient rescheduling, additional hospital stays, alternative treatment provisions, and potential litigation expenses can multiply the financial impact by 2-3 times the direct product cost. Furthermore, insurance reimbursement complications arising from therapy delays create additional administrative burdens estimated at $2,000-5,000 per incident.
From a healthcare system perspective, preventing dose loss contributes to overall cost efficiency. Each successful cell therapy administration potentially reduces long-term healthcare utilization by addressing underlying conditions more effectively than conventional treatments. Studies indicate that successful CAR-T therapies can reduce five-year healthcare costs by 15-25% compared to traditional treatment regimens for certain hematological malignancies.
The economic benefits extend to pharmaceutical companies and therapy developers as well. Improved cold chain reliability enhances scalability of autologous therapy programs, potentially reducing per-dose costs by 10-15% through operational efficiencies. Additionally, demonstrated supply chain excellence strengthens market positioning and can influence favorable reimbursement decisions from payers, directly impacting commercial viability.
Ultimately, investments in cold chain risk assessment and mitigation represent high-value allocations of resources that generate returns across multiple stakeholders in the healthcare ecosystem. As the autologous cell therapy market expands at a projected CAGR of 23.5% through 2028, the economic imperative of dose loss prevention will only increase in significance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!