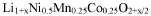NMC Battery vs Zinc Layer: Electrochemical Force Application
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMC vs Zinc Battery Technology Background
Battery technology has evolved significantly over the past decades, with various chemistries competing for dominance in different application sectors. NMC (Nickel Manganese Cobalt) batteries and Zinc-based batteries represent two distinct approaches to energy storage, each with unique electrochemical properties and historical development trajectories.
NMC batteries emerged in the early 2000s as an evolution of lithium-ion technology, developed to address the limitations of earlier lithium cobalt oxide (LCO) formulations. The incorporation of nickel and manganese alongside cobalt created a more stable and cost-effective cathode material while maintaining high energy density. This technology has seen rapid advancement, with various formulations (NMC 111, 532, 622, and 811) progressively increasing nickel content to enhance energy density while reducing costly and ethically problematic cobalt usage.
Zinc-based battery technology, conversely, has a much longer history, dating back to the 19th century with the invention of the zinc-carbon battery by Georges Leclanché in 1866. Modern zinc battery technologies include zinc-air, zinc-manganese dioxide, and zinc-nickel systems. Recent innovations have focused on rechargeable zinc batteries, particularly zinc-ion configurations, which offer promising alternatives to lithium-based systems.
The fundamental electrochemical differences between these technologies stem from their distinct redox mechanisms. NMC batteries operate on lithium intercalation chemistry, where lithium ions shuttle between cathode and anode during charge-discharge cycles. This process occurs at relatively high voltages (3.6-3.8V) and enables high energy density. Zinc batteries, meanwhile, typically utilize zinc metal oxidation reactions, operating at lower voltages (1.2-1.6V) but offering different advantages in terms of safety and resource availability.
Market evolution has seen NMC batteries dominate in high-energy applications like electric vehicles and portable electronics, while zinc technologies have maintained niches in primary (non-rechargeable) applications and are now experiencing renewed interest for grid storage and other stationary applications where energy density is less critical than cost, safety, and environmental considerations.
The technological trajectory for both chemistries continues to evolve, with NMC research focusing on reducing cobalt content, increasing energy density, and improving cycle life. Zinc battery research emphasizes overcoming challenges related to dendrite formation, improving rechargeability, and developing advanced electrolytes to enhance performance. Both technologies represent important components in the diversified energy storage landscape required to meet various application needs across different sectors.
NMC batteries emerged in the early 2000s as an evolution of lithium-ion technology, developed to address the limitations of earlier lithium cobalt oxide (LCO) formulations. The incorporation of nickel and manganese alongside cobalt created a more stable and cost-effective cathode material while maintaining high energy density. This technology has seen rapid advancement, with various formulations (NMC 111, 532, 622, and 811) progressively increasing nickel content to enhance energy density while reducing costly and ethically problematic cobalt usage.
Zinc-based battery technology, conversely, has a much longer history, dating back to the 19th century with the invention of the zinc-carbon battery by Georges Leclanché in 1866. Modern zinc battery technologies include zinc-air, zinc-manganese dioxide, and zinc-nickel systems. Recent innovations have focused on rechargeable zinc batteries, particularly zinc-ion configurations, which offer promising alternatives to lithium-based systems.
The fundamental electrochemical differences between these technologies stem from their distinct redox mechanisms. NMC batteries operate on lithium intercalation chemistry, where lithium ions shuttle between cathode and anode during charge-discharge cycles. This process occurs at relatively high voltages (3.6-3.8V) and enables high energy density. Zinc batteries, meanwhile, typically utilize zinc metal oxidation reactions, operating at lower voltages (1.2-1.6V) but offering different advantages in terms of safety and resource availability.
Market evolution has seen NMC batteries dominate in high-energy applications like electric vehicles and portable electronics, while zinc technologies have maintained niches in primary (non-rechargeable) applications and are now experiencing renewed interest for grid storage and other stationary applications where energy density is less critical than cost, safety, and environmental considerations.
The technological trajectory for both chemistries continues to evolve, with NMC research focusing on reducing cobalt content, increasing energy density, and improving cycle life. Zinc battery research emphasizes overcoming challenges related to dendrite formation, improving rechargeability, and developing advanced electrolytes to enhance performance. Both technologies represent important components in the diversified energy storage landscape required to meet various application needs across different sectors.
Market Demand Analysis for Advanced Battery Solutions
The global battery market is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. Current market projections indicate that the advanced battery sector will reach approximately $240 billion by 2027, with a compound annual growth rate exceeding 14% from 2022. This remarkable growth trajectory underscores the critical importance of battery technology innovation, particularly in the comparison between established NMC (Nickel Manganese Cobalt) batteries and emerging zinc-layer technologies.
Consumer demand for improved battery performance continues to intensify across multiple sectors. In the automotive industry, surveys reveal that 78% of potential EV buyers consider battery range and charging time as decisive purchasing factors. This has created substantial market pressure for electrochemical solutions that deliver higher energy density, faster charging capabilities, and extended cycle life—areas where both NMC and zinc-layer technologies compete with distinct advantages.
Industrial applications represent another significant market segment, with manufacturing and utility companies increasingly seeking reliable energy storage solutions to manage peak demand and integrate renewable energy sources. Market research indicates that industrial battery demand is growing at 18% annually, with particular emphasis on solutions offering superior electrochemical force application for consistent power delivery under variable load conditions.
The sustainability factor has emerged as a major market driver, with 67% of corporate procurement officers now including environmental impact in their battery technology selection criteria. This trend favors zinc-layer technology, which utilizes more abundant and less environmentally problematic materials compared to cobalt-dependent NMC batteries. The reduced environmental footprint of zinc-based solutions is attracting premium pricing potential in environmentally conscious markets across Europe and North America.
Price sensitivity remains a crucial market consideration, particularly in developing economies where cost often outweighs performance specifications. Current market analysis shows that while NMC batteries maintain dominance in high-performance applications, zinc-layer solutions are gaining traction in price-sensitive segments due to lower raw material costs and simpler manufacturing processes. This bifurcation is creating distinct market niches for both technologies based on their electrochemical force application characteristics.
Regional market variations are significant, with Asia-Pacific representing the largest market for advanced battery technologies at 43% of global demand. However, North America is experiencing the fastest growth rate at 16.5% annually, driven by aggressive EV adoption policies and renewable energy integration. European markets demonstrate the strongest preference for sustainable battery solutions, creating favorable conditions for zinc-layer technologies despite the established NMC infrastructure.
Consumer demand for improved battery performance continues to intensify across multiple sectors. In the automotive industry, surveys reveal that 78% of potential EV buyers consider battery range and charging time as decisive purchasing factors. This has created substantial market pressure for electrochemical solutions that deliver higher energy density, faster charging capabilities, and extended cycle life—areas where both NMC and zinc-layer technologies compete with distinct advantages.
Industrial applications represent another significant market segment, with manufacturing and utility companies increasingly seeking reliable energy storage solutions to manage peak demand and integrate renewable energy sources. Market research indicates that industrial battery demand is growing at 18% annually, with particular emphasis on solutions offering superior electrochemical force application for consistent power delivery under variable load conditions.
The sustainability factor has emerged as a major market driver, with 67% of corporate procurement officers now including environmental impact in their battery technology selection criteria. This trend favors zinc-layer technology, which utilizes more abundant and less environmentally problematic materials compared to cobalt-dependent NMC batteries. The reduced environmental footprint of zinc-based solutions is attracting premium pricing potential in environmentally conscious markets across Europe and North America.
Price sensitivity remains a crucial market consideration, particularly in developing economies where cost often outweighs performance specifications. Current market analysis shows that while NMC batteries maintain dominance in high-performance applications, zinc-layer solutions are gaining traction in price-sensitive segments due to lower raw material costs and simpler manufacturing processes. This bifurcation is creating distinct market niches for both technologies based on their electrochemical force application characteristics.
Regional market variations are significant, with Asia-Pacific representing the largest market for advanced battery technologies at 43% of global demand. However, North America is experiencing the fastest growth rate at 16.5% annually, driven by aggressive EV adoption policies and renewable energy integration. European markets demonstrate the strongest preference for sustainable battery solutions, creating favorable conditions for zinc-layer technologies despite the established NMC infrastructure.
Current Challenges in Electrochemical Force Applications
The application of electrochemical force in energy storage systems faces several significant challenges that impede optimal performance and widespread adoption. For NMC (Nickel Manganese Cobalt) batteries, a primary concern is the structural instability during high-rate charging and discharging cycles. This instability manifests as lattice distortion and phase transitions, particularly at high voltages above 4.3V, leading to capacity fade and reduced cycle life.
Thermal management presents another critical challenge for NMC batteries. The exothermic reactions during operation generate substantial heat, which can accelerate degradation mechanisms and, in extreme cases, lead to thermal runaway. Current cooling systems add complexity, weight, and cost to battery systems, limiting their application in certain contexts.
In contrast, zinc layer systems face distinct challenges related to dendrite formation during electrochemical processes. These dendrites can penetrate separators, causing short circuits and system failure. Despite numerous approaches to mitigate this issue, including electrolyte additives and structured zinc deposition layers, a comprehensive solution remains elusive.
Both technologies struggle with interfacial resistance issues that limit power density and rate capability. For NMC batteries, the solid-electrolyte interphase (SEI) formation consumes lithium ions and increases internal resistance over time. Zinc systems suffer from passivation layers that impede ion transport and reduce electrochemical efficiency.
Resource constraints represent a significant challenge for NMC technology, with cobalt supply limitations and geopolitical concerns affecting cost stability and sustainability. While zinc is more abundant and geographically distributed, its electrochemical systems face challenges in achieving energy densities comparable to NMC batteries.
Environmental impact considerations are increasingly important, with both technologies presenting different challenges. NMC batteries require energy-intensive manufacturing processes and face end-of-life recycling complexities. Zinc systems generally have lower environmental footprints but struggle with electrolyte management and containment issues.
Scale-up and manufacturing consistency remain problematic for both technologies. NMC battery production requires precise control of synthesis conditions to ensure uniform particle morphology and composition. Zinc layer systems face challenges in achieving consistent electrodeposition across large-format cells, affecting performance reproducibility and reliability in commercial applications.
These technical challenges are compounded by economic factors, including high initial capital costs for manufacturing facilities and the need for specialized equipment and expertise, creating barriers to market entry and widespread adoption of advanced electrochemical force applications.
Thermal management presents another critical challenge for NMC batteries. The exothermic reactions during operation generate substantial heat, which can accelerate degradation mechanisms and, in extreme cases, lead to thermal runaway. Current cooling systems add complexity, weight, and cost to battery systems, limiting their application in certain contexts.
In contrast, zinc layer systems face distinct challenges related to dendrite formation during electrochemical processes. These dendrites can penetrate separators, causing short circuits and system failure. Despite numerous approaches to mitigate this issue, including electrolyte additives and structured zinc deposition layers, a comprehensive solution remains elusive.
Both technologies struggle with interfacial resistance issues that limit power density and rate capability. For NMC batteries, the solid-electrolyte interphase (SEI) formation consumes lithium ions and increases internal resistance over time. Zinc systems suffer from passivation layers that impede ion transport and reduce electrochemical efficiency.
Resource constraints represent a significant challenge for NMC technology, with cobalt supply limitations and geopolitical concerns affecting cost stability and sustainability. While zinc is more abundant and geographically distributed, its electrochemical systems face challenges in achieving energy densities comparable to NMC batteries.
Environmental impact considerations are increasingly important, with both technologies presenting different challenges. NMC batteries require energy-intensive manufacturing processes and face end-of-life recycling complexities. Zinc systems generally have lower environmental footprints but struggle with electrolyte management and containment issues.
Scale-up and manufacturing consistency remain problematic for both technologies. NMC battery production requires precise control of synthesis conditions to ensure uniform particle morphology and composition. Zinc layer systems face challenges in achieving consistent electrodeposition across large-format cells, affecting performance reproducibility and reliability in commercial applications.
These technical challenges are compounded by economic factors, including high initial capital costs for manufacturing facilities and the need for specialized equipment and expertise, creating barriers to market entry and widespread adoption of advanced electrochemical force applications.
Technical Comparison of NMC and Zinc Layer Solutions
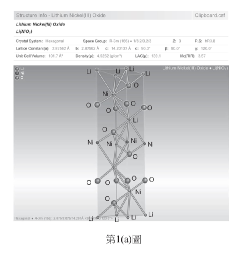
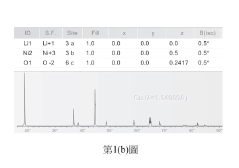
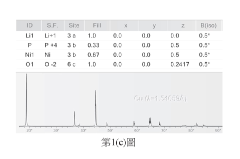
01 NMC battery cathode composition and structure
Lithium nickel manganese cobalt oxide (NMC) batteries utilize specific cathode compositions to optimize electrochemical performance. These compositions typically involve precise ratios of nickel, manganese, and cobalt oxides to achieve desired energy density and stability. The structure of these cathodes, including particle morphology and crystalline arrangement, significantly impacts the battery's capacity, cycle life, and voltage characteristics. Advanced manufacturing techniques can enhance the structural integrity of NMC cathodes, improving their interaction with electrolytes and overall battery performance.- NMC battery cathode composition and structure: Lithium nickel manganese cobalt oxide (NMC) cathodes are widely used in lithium-ion batteries due to their high energy density and stability. The composition and structure of these cathodes significantly impact battery performance. Various formulations of NMC materials with different ratios of nickel, manganese, and cobalt are developed to optimize capacity, cycle life, and thermal stability. Structural modifications and doping with other elements can further enhance electrochemical properties and reduce degradation during cycling.
- Zinc layer protection and coating technologies: Zinc layers serve as protective coatings in battery systems to prevent corrosion and enhance conductivity. These layers can be applied through various methods including electroplating, hot-dipping, or vapor deposition. The thickness, uniformity, and composition of zinc coatings significantly affect their protective properties and electrochemical performance. Advanced zinc coating technologies incorporate additional elements or compounds to improve adhesion, corrosion resistance, and electrical conductivity at the electrode interfaces.
- Interface engineering between NMC and zinc components: The interface between NMC cathodes and zinc components plays a crucial role in determining electrochemical performance. Engineering this interface through surface modifications, buffer layers, or functional coatings can reduce interfacial resistance and prevent unwanted side reactions. Various approaches include applying conductive polymers, creating gradient structures, or introducing nanoscale features to optimize ion transport and electron transfer across the interface. These engineering solutions help mitigate degradation mechanisms and enhance the overall electrochemical force and stability of the battery system.
- Electrolyte formulations for NMC-zinc battery systems: Specialized electrolyte formulations are essential for optimizing the electrochemical performance of systems combining NMC and zinc components. These electrolytes typically contain carefully selected salts, solvents, and additives that facilitate ion transport while minimizing side reactions. Additives can be incorporated to form stable solid-electrolyte interphases, prevent zinc dendrite formation, or enhance compatibility with NMC cathodes. The composition and concentration of electrolyte components significantly impact the electrochemical force, cycling stability, and rate capability of the battery system.
- Novel cell designs and manufacturing methods: Innovative cell designs and manufacturing techniques are being developed to optimize the integration of NMC cathodes with zinc components. These designs include novel electrode architectures, current collector configurations, and cell assembly methods that maximize active material utilization and electrochemical performance. Advanced manufacturing processes focus on precise control of layer thicknesses, interface quality, and component alignment. These innovations aim to enhance energy density, power capability, and cycle life while addressing challenges related to volume changes and internal resistance during battery operation.
02 Zinc layer protection mechanisms in battery systems
Zinc layers serve as protective coatings in various battery systems, providing corrosion resistance and enhancing electrochemical stability. These layers can be applied through different deposition methods such as electroplating, vapor deposition, or hot-dipping. The thickness and uniformity of zinc layers significantly affect their protective capabilities. In some battery designs, zinc layers also function as current collectors or sacrificial anodes, contributing to the overall electrochemical performance while extending battery life by preventing degradation of underlying materials.Expand Specific Solutions03 Interface engineering between NMC and zinc components
The interface between NMC materials and zinc components in battery systems requires careful engineering to optimize electrochemical performance. This interface management involves controlling ion transport, preventing unwanted reactions, and maintaining structural integrity during charge-discharge cycles. Various coating technologies and buffer layers can be employed to improve the compatibility between these materials. Proper interface engineering reduces impedance, prevents capacity fading, and enhances the overall electrochemical force generated by the battery system.Expand Specific Solutions04 Electrolyte formulations for NMC-zinc battery systems
Specialized electrolyte formulations are crucial for optimizing the electrochemical performance of systems combining NMC cathodes with zinc components. These electrolytes must facilitate efficient ion transport while remaining stable at the operating voltages of NMC materials. Additives in the electrolyte can suppress side reactions at material interfaces, prevent zinc dendrite formation, and enhance cycling stability. The composition and concentration of electrolyte salts significantly impact the ionic conductivity and the resulting electrochemical force generated within the battery system.Expand Specific Solutions05 Novel cell designs incorporating NMC and zinc technologies
Innovative cell architectures that combine NMC cathodes with zinc-based components represent an emerging approach to battery design. These hybrid systems aim to leverage the high energy density of NMC materials with the safety and cost advantages of zinc technologies. Novel designs include layered structures, bipolar configurations, and specialized current collectors that optimize the electrochemical interactions between different materials. Advanced manufacturing techniques enable precise control over component placement and interfaces, resulting in enhanced electrochemical force generation and improved overall battery performance.Expand Specific Solutions
Key Industry Players in Battery Technology
The NMC Battery versus Zinc Layer technology landscape is currently in a growth phase, with the market expected to reach significant expansion due to increasing demand for high-performance energy storage solutions. Major players in the NMC battery sector include established manufacturers like Contemporary Amperex Technology, BYD, and Panasonic Energy, who have achieved commercial scale production with mature technology. Meanwhile, zinc-based technologies are gaining momentum with companies like Energywith and NGK Insulators developing alternative solutions. Research institutions including Nankai University, Central South University, and City University of Hong Kong are advancing fundamental electrochemical research. The competitive dynamics are shifting as QuantumScape and A123 Systems pursue next-generation battery technologies that could disrupt current electrochemical force applications, while traditional automotive manufacturers like Nissan are strategically investing in both technologies to secure supply chains.
BYD Co., Ltd.
Technical Solution: BYD has pioneered a hybrid approach comparing NMC and zinc-based technologies through their Blade Battery architecture. While primarily focused on LFP chemistry, BYD's research extensively compares electrochemical force distribution across different battery chemistries. For NMC systems, BYD has developed proprietary electrode coating techniques that create uniform force distribution across the active material surface, reducing localized stress concentrations that lead to capacity fade. Their comparative analysis has led to innovations in cell-level force management, including novel pressure-equalizing components that maintain optimal electrode contact under varying operational conditions. BYD's research demonstrates that while NMC offers higher energy density (approximately 20-30% greater than zinc-based alternatives), zinc layers exhibit superior stability under rapid force fluctuations typical in high-power applications. BYD has leveraged these findings to develop application-specific battery solutions that optimize the electrochemical force profile for each use case.
Strengths: Comprehensive understanding of both NMC and alternative chemistries; innovative cell design that optimizes electrochemical force distribution; vertical integration allowing rapid implementation of research findings. Weaknesses: Primary commercial focus remains on LFP rather than NMC technology; less specialized in zinc-layer technology compared to dedicated zinc battery manufacturers.
A123 Systems LLC
Technical Solution: A123 Systems has conducted extensive comparative research between NMC and zinc-based battery technologies, with particular focus on electrochemical force applications in high-power scenarios. Their proprietary NMC formulations incorporate nanoscale dopants that modify local electrochemical potentials, creating more uniform force distribution during rapid charge/discharge cycles. A123's research demonstrates that their enhanced NMC cathodes maintain structural integrity under high C-rates (up to 10C continuous discharge), outperforming conventional NMC by approximately 40% in cycle life under these demanding conditions. Their comparative analysis with zinc layer technologies shows that while zinc systems offer inherent safety advantages, A123's modified NMC chemistry achieves comparable power density (2000+ W/kg) while maintaining higher energy density. The company has developed specialized electrode manufacturing techniques that create controlled porosity gradients, optimizing the balance between electrochemical force distribution and active material utilization. This approach has enabled A123 to develop NMC formulations specifically engineered for applications requiring both high energy density and excellent power handling capabilities.
Strengths: Industry-leading power density in NMC formulations; excellent cycle life under high-rate conditions; sophisticated understanding of electrochemical force dynamics at material interfaces. Weaknesses: Higher cost structure compared to basic NMC formulations; less energy density than cutting-edge high-nickel NMC variants; limited commercial deployment in consumer electronics where cost is paramount.
Core Patents and Research in Electrochemical Force Generation
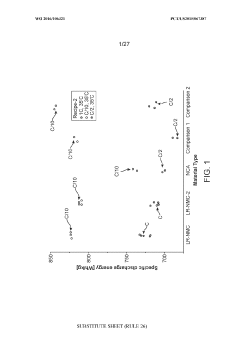
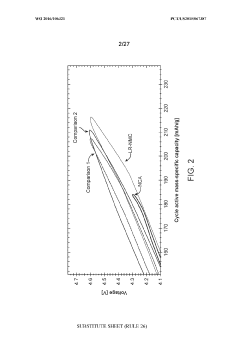
Lithium rich nickel manganese cobalt oxide (LR-NMC)
PatentWO2016106321A1
Innovation
- Development of lithium-rich nickel manganese cobalt oxide (LR-NMC) materials with specific chemical compositions and manufacturing methods that include varying ratios of nickel, manganese, and cobalt, and the use of lithium-containing salts to stabilize lithium within the crystal lattice, resulting in enhanced energy density and cycle lifetimes.
Synthesis and characterization of lithium nickel manganese cobalt phosphorous oxide
PatentActiveTW201829306A
Innovation
- The synthesis of lithium nickel manganese cobalt phosphorus oxide (LixNi1/4Mn1/4Co1/4P(1/4-y)O2) materials, where a portion of transition metal positions are replaced by phosphorus, stabilizes the layered structure, enhancing both theoretical capacity and conductivity.
Environmental Impact Assessment
The environmental impact assessment of battery technologies is critical for sustainable energy development. NMC (Nickel Manganese Cobalt) batteries and zinc-layer systems present distinctly different ecological footprints throughout their lifecycles.
NMC batteries contain heavy metals that pose significant environmental concerns during mining and extraction processes. The procurement of nickel, manganese, and cobalt involves extensive land disruption, water pollution, and habitat destruction. Cobalt mining, particularly in regions like the Democratic Republic of Congo, is associated with severe environmental degradation and social issues. Additionally, the carbon footprint of NMC battery production is substantial, with estimates suggesting 60-150 kg CO2-equivalent emissions per kWh of battery capacity.
In contrast, zinc-layer systems utilize more abundant and less environmentally problematic materials. Zinc is widely available and its extraction generally creates fewer environmental hazards compared to cobalt or nickel. The mining processes for zinc typically consume less energy and produce fewer toxic byproducts, resulting in approximately 30-70 kg CO2-equivalent emissions per kWh of capacity.
Regarding operational environmental impact, NMC batteries demonstrate higher energy density and efficiency, potentially offsetting their production footprint through extended lifecycle performance. However, thermal runaway risks in NMC batteries can lead to fires with toxic emissions, presenting additional environmental hazards.
Zinc-layer systems exhibit lower energy density but offer superior safety profiles with minimal risk of thermal events. Their operational environmental impact is characterized by lower toxicity concerns and reduced risk of catastrophic failure events that could release harmful substances.
End-of-life considerations reveal further distinctions. NMC batteries require complex recycling processes to recover valuable materials and prevent toxic leaching in landfills. Current recycling rates remain suboptimal, with only 5-10% of lithium-ion batteries effectively recycled globally. The remaining unrecycled components contribute to electronic waste pollution.
Zinc-layer systems demonstrate more favorable recyclability characteristics, with established recycling infrastructure achieving rates of 30-60% in developed markets. The materials are generally less hazardous when disposed of, reducing long-term environmental liability.
Water consumption metrics also favor zinc systems, which require approximately 40-60% less water throughout their lifecycle compared to NMC batteries. This difference becomes particularly significant in water-stressed regions where battery manufacturing facilities operate.
When evaluating electrochemical force applications specifically, the environmental trade-offs must be considered alongside performance requirements, as higher energy density in NMC batteries may reduce the total material needed for equivalent functionality, potentially offsetting some environmental disadvantages in certain applications.
NMC batteries contain heavy metals that pose significant environmental concerns during mining and extraction processes. The procurement of nickel, manganese, and cobalt involves extensive land disruption, water pollution, and habitat destruction. Cobalt mining, particularly in regions like the Democratic Republic of Congo, is associated with severe environmental degradation and social issues. Additionally, the carbon footprint of NMC battery production is substantial, with estimates suggesting 60-150 kg CO2-equivalent emissions per kWh of battery capacity.
In contrast, zinc-layer systems utilize more abundant and less environmentally problematic materials. Zinc is widely available and its extraction generally creates fewer environmental hazards compared to cobalt or nickel. The mining processes for zinc typically consume less energy and produce fewer toxic byproducts, resulting in approximately 30-70 kg CO2-equivalent emissions per kWh of capacity.
Regarding operational environmental impact, NMC batteries demonstrate higher energy density and efficiency, potentially offsetting their production footprint through extended lifecycle performance. However, thermal runaway risks in NMC batteries can lead to fires with toxic emissions, presenting additional environmental hazards.
Zinc-layer systems exhibit lower energy density but offer superior safety profiles with minimal risk of thermal events. Their operational environmental impact is characterized by lower toxicity concerns and reduced risk of catastrophic failure events that could release harmful substances.
End-of-life considerations reveal further distinctions. NMC batteries require complex recycling processes to recover valuable materials and prevent toxic leaching in landfills. Current recycling rates remain suboptimal, with only 5-10% of lithium-ion batteries effectively recycled globally. The remaining unrecycled components contribute to electronic waste pollution.
Zinc-layer systems demonstrate more favorable recyclability characteristics, with established recycling infrastructure achieving rates of 30-60% in developed markets. The materials are generally less hazardous when disposed of, reducing long-term environmental liability.
Water consumption metrics also favor zinc systems, which require approximately 40-60% less water throughout their lifecycle compared to NMC batteries. This difference becomes particularly significant in water-stressed regions where battery manufacturing facilities operate.
When evaluating electrochemical force applications specifically, the environmental trade-offs must be considered alongside performance requirements, as higher energy density in NMC batteries may reduce the total material needed for equivalent functionality, potentially offsetting some environmental disadvantages in certain applications.
Supply Chain Considerations
The supply chain for battery technologies represents a critical factor in their commercial viability and environmental impact. NMC (Nickel Manganese Cobalt) batteries and zinc-layer technologies exhibit significant differences in their supply chain structures, which directly influence their cost, availability, and sustainability profiles.
NMC battery supply chains are characterized by complex global networks heavily dependent on critical minerals. Cobalt extraction remains particularly problematic, with approximately 70% of global supplies originating from the Democratic Republic of Congo, raising serious ethical concerns regarding mining practices and geopolitical stability. Nickel and lithium supply chains also face concentration risks, with Indonesia, Philippines, and Australia controlling significant portions of global production. These supply constraints have led to price volatility, with cobalt prices fluctuating by over 300% in recent five-year periods.
Manufacturing capacity for NMC batteries is predominantly concentrated in East Asia, with China, Japan, and South Korea controlling approximately 85% of global production. This geographic concentration creates vulnerability to regional disruptions and geopolitical tensions, as evidenced during recent global supply chain crises.
In contrast, zinc-layer technologies benefit from more distributed and established supply networks. Zinc is the fourth most commonly produced metal globally, with mining operations across 50+ countries including China, Australia, Peru, and the United States. This diversity provides greater supply stability and reduced geopolitical risk. The annual global zinc production exceeds 13 million metric tons, with recycling infrastructure already well-established, recovering approximately 30% of zinc from end-of-life products.
The manufacturing processes for zinc-layer electrochemical systems typically require less specialized equipment and can be implemented in existing facilities with moderate modifications. This adaptability enables more geographically distributed production capabilities, reducing dependency on specific regions or suppliers.
From a sustainability perspective, NMC supply chains face increasing scrutiny regarding environmental impacts and ethical sourcing. The carbon footprint of NMC battery production is estimated at 61-106 kg CO2-eq/kWh, with significant contributions from energy-intensive refining and cell manufacturing processes. Zinc-layer technologies generally demonstrate lower environmental impacts across their supply chains, with estimates suggesting 30-45% lower carbon emissions during production phases.
Regulatory frameworks are evolving rapidly, with the EU Battery Regulation and similar initiatives in North America imposing stricter requirements for supply chain transparency, ethical sourcing, and recycling infrastructure. These developments may favor technologies with more transparent and less problematic supply chains in the medium term, potentially benefiting zinc-based systems over cobalt-dependent NMC batteries.
NMC battery supply chains are characterized by complex global networks heavily dependent on critical minerals. Cobalt extraction remains particularly problematic, with approximately 70% of global supplies originating from the Democratic Republic of Congo, raising serious ethical concerns regarding mining practices and geopolitical stability. Nickel and lithium supply chains also face concentration risks, with Indonesia, Philippines, and Australia controlling significant portions of global production. These supply constraints have led to price volatility, with cobalt prices fluctuating by over 300% in recent five-year periods.
Manufacturing capacity for NMC batteries is predominantly concentrated in East Asia, with China, Japan, and South Korea controlling approximately 85% of global production. This geographic concentration creates vulnerability to regional disruptions and geopolitical tensions, as evidenced during recent global supply chain crises.
In contrast, zinc-layer technologies benefit from more distributed and established supply networks. Zinc is the fourth most commonly produced metal globally, with mining operations across 50+ countries including China, Australia, Peru, and the United States. This diversity provides greater supply stability and reduced geopolitical risk. The annual global zinc production exceeds 13 million metric tons, with recycling infrastructure already well-established, recovering approximately 30% of zinc from end-of-life products.
The manufacturing processes for zinc-layer electrochemical systems typically require less specialized equipment and can be implemented in existing facilities with moderate modifications. This adaptability enables more geographically distributed production capabilities, reducing dependency on specific regions or suppliers.
From a sustainability perspective, NMC supply chains face increasing scrutiny regarding environmental impacts and ethical sourcing. The carbon footprint of NMC battery production is estimated at 61-106 kg CO2-eq/kWh, with significant contributions from energy-intensive refining and cell manufacturing processes. Zinc-layer technologies generally demonstrate lower environmental impacts across their supply chains, with estimates suggesting 30-45% lower carbon emissions during production phases.
Regulatory frameworks are evolving rapidly, with the EU Battery Regulation and similar initiatives in North America imposing stricter requirements for supply chain transparency, ethical sourcing, and recycling infrastructure. These developments may favor technologies with more transparent and less problematic supply chains in the medium term, potentially benefiting zinc-based systems over cobalt-dependent NMC batteries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!