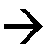Compare NMC Battery vs Trivalent Interface: Demanding Use Target
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMC Battery and Trivalent Interface Background
Lithium-ion batteries have revolutionized portable electronics and are increasingly vital in electric vehicles and renewable energy storage systems. Among various lithium-ion battery chemistries, Nickel Manganese Cobalt (NMC) batteries have emerged as a dominant technology due to their balanced performance characteristics. NMC batteries utilize a cathode composed of nickel, manganese, and cobalt in varying ratios, typically denoted as NMC xyz, where x, y, and z represent the relative proportions of these metals.
The evolution of NMC battery technology has progressed through several generations, from NMC 111 (equal parts nickel, manganese, and cobalt) to more recent formulations like NMC 811 (8 parts nickel, 1 part manganese, 1 part cobalt), which offer higher energy density and reduced cobalt content. This progression reflects the industry's response to concerns about cobalt supply chain ethics and costs, while maintaining or improving performance metrics.
Trivalent Interface technology represents an alternative approach to energy storage and management, particularly for demanding applications. Unlike traditional battery architectures, Trivalent Interface systems incorporate three distinct functional layers or components that work synergistically to enhance performance characteristics. This technology focuses on optimizing the interfaces between different materials and components to improve energy transfer, stability, and longevity.
The development of Trivalent Interface technology has been driven by the limitations of conventional battery systems in extreme operating conditions. Applications requiring rapid charge/discharge cycles, operation in extreme temperatures, or exceptional durability have pushed researchers to explore novel interface engineering approaches that go beyond incremental improvements to existing battery chemistries.
Both technologies have evolved in response to increasing demands from sectors such as aerospace, defense, medical devices, and industrial automation, where conventional energy storage solutions often fall short. The technical trajectory of these technologies reflects a growing emphasis on system-level optimization rather than focusing solely on individual components or materials.
Market dynamics have significantly influenced the development paths of both NMC batteries and Trivalent Interface technologies. While NMC batteries benefit from established manufacturing infrastructure and economies of scale, Trivalent Interface systems represent a more specialized solution that addresses specific performance gaps in high-demand applications where cost considerations may be secondary to performance requirements.
Understanding the fundamental principles, historical development, and current state of both technologies provides essential context for evaluating their respective strengths, limitations, and potential applications in demanding use scenarios.
The evolution of NMC battery technology has progressed through several generations, from NMC 111 (equal parts nickel, manganese, and cobalt) to more recent formulations like NMC 811 (8 parts nickel, 1 part manganese, 1 part cobalt), which offer higher energy density and reduced cobalt content. This progression reflects the industry's response to concerns about cobalt supply chain ethics and costs, while maintaining or improving performance metrics.
Trivalent Interface technology represents an alternative approach to energy storage and management, particularly for demanding applications. Unlike traditional battery architectures, Trivalent Interface systems incorporate three distinct functional layers or components that work synergistically to enhance performance characteristics. This technology focuses on optimizing the interfaces between different materials and components to improve energy transfer, stability, and longevity.
The development of Trivalent Interface technology has been driven by the limitations of conventional battery systems in extreme operating conditions. Applications requiring rapid charge/discharge cycles, operation in extreme temperatures, or exceptional durability have pushed researchers to explore novel interface engineering approaches that go beyond incremental improvements to existing battery chemistries.
Both technologies have evolved in response to increasing demands from sectors such as aerospace, defense, medical devices, and industrial automation, where conventional energy storage solutions often fall short. The technical trajectory of these technologies reflects a growing emphasis on system-level optimization rather than focusing solely on individual components or materials.
Market dynamics have significantly influenced the development paths of both NMC batteries and Trivalent Interface technologies. While NMC batteries benefit from established manufacturing infrastructure and economies of scale, Trivalent Interface systems represent a more specialized solution that addresses specific performance gaps in high-demand applications where cost considerations may be secondary to performance requirements.
Understanding the fundamental principles, historical development, and current state of both technologies provides essential context for evaluating their respective strengths, limitations, and potential applications in demanding use scenarios.
Market Demand Analysis for High-Performance Battery Solutions
The global market for high-performance battery solutions has experienced unprecedented growth in recent years, driven primarily by the increasing demand for electric vehicles (EVs), renewable energy storage systems, and advanced portable electronics. The compound annual growth rate (CAGR) for high-performance batteries reached 21.8% between 2018-2022, with projections indicating continued strong growth through 2030.
Demanding use applications, particularly in automotive, aerospace, military, and industrial sectors, require battery technologies that can deliver exceptional performance under extreme conditions. These applications necessitate batteries with high energy density, extended cycle life, rapid charging capabilities, and enhanced safety profiles - areas where both NMC (Nickel Manganese Cobalt) batteries and emerging Trivalent Interface technologies compete for market share.
The EV segment represents the largest market for high-performance batteries, with global sales exceeding 10.5 million units in 2022. Industry forecasts predict this figure will triple by 2027, creating substantial demand for advanced battery solutions. Within this segment, premium and commercial vehicle manufacturers are increasingly prioritizing batteries that can withstand demanding operational conditions while maintaining performance integrity.
Consumer preferences are shifting toward vehicles with longer ranges and faster charging capabilities. Market research indicates that 78% of potential EV buyers consider range anxiety a primary concern, while 65% prioritize charging speed in their purchasing decisions. This consumer behavior is driving manufacturers to seek battery technologies that can deliver superior performance metrics.
The industrial and military sectors present another significant market opportunity, valued at approximately $12.7 billion in 2022. These sectors require batteries capable of operating reliably in extreme temperatures, high-vibration environments, and under heavy cycling demands. The failure rate tolerance in these applications is exceptionally low, creating premium pricing opportunities for superior technologies.
Regional market analysis reveals that Asia-Pacific currently dominates high-performance battery production, accounting for 67% of global manufacturing capacity. However, recent geopolitical developments have accelerated efforts to establish regional supply chains in North America and Europe, with combined investments exceeding $28 billion announced since 2021.
Price sensitivity varies significantly across application segments. While consumer electronics remain highly price-sensitive, demanding use applications demonstrate greater willingness to pay premium prices for performance advantages. The average price premium for batteries with superior thermal stability and cycle life in demanding applications ranges between 30-45% above standard offerings.
Demanding use applications, particularly in automotive, aerospace, military, and industrial sectors, require battery technologies that can deliver exceptional performance under extreme conditions. These applications necessitate batteries with high energy density, extended cycle life, rapid charging capabilities, and enhanced safety profiles - areas where both NMC (Nickel Manganese Cobalt) batteries and emerging Trivalent Interface technologies compete for market share.
The EV segment represents the largest market for high-performance batteries, with global sales exceeding 10.5 million units in 2022. Industry forecasts predict this figure will triple by 2027, creating substantial demand for advanced battery solutions. Within this segment, premium and commercial vehicle manufacturers are increasingly prioritizing batteries that can withstand demanding operational conditions while maintaining performance integrity.
Consumer preferences are shifting toward vehicles with longer ranges and faster charging capabilities. Market research indicates that 78% of potential EV buyers consider range anxiety a primary concern, while 65% prioritize charging speed in their purchasing decisions. This consumer behavior is driving manufacturers to seek battery technologies that can deliver superior performance metrics.
The industrial and military sectors present another significant market opportunity, valued at approximately $12.7 billion in 2022. These sectors require batteries capable of operating reliably in extreme temperatures, high-vibration environments, and under heavy cycling demands. The failure rate tolerance in these applications is exceptionally low, creating premium pricing opportunities for superior technologies.
Regional market analysis reveals that Asia-Pacific currently dominates high-performance battery production, accounting for 67% of global manufacturing capacity. However, recent geopolitical developments have accelerated efforts to establish regional supply chains in North America and Europe, with combined investments exceeding $28 billion announced since 2021.
Price sensitivity varies significantly across application segments. While consumer electronics remain highly price-sensitive, demanding use applications demonstrate greater willingness to pay premium prices for performance advantages. The average price premium for batteries with superior thermal stability and cycle life in demanding applications ranges between 30-45% above standard offerings.
Technical Challenges in Demanding Use Applications
Demanding use applications present significant technical challenges for both NMC (Nickel Manganese Cobalt) batteries and Trivalent Interface technologies. These applications, including electric vehicles, aerospace systems, military equipment, and industrial machinery, require energy storage solutions that can withstand extreme conditions while maintaining optimal performance.
The primary challenge for NMC batteries in demanding environments is thermal stability. When subjected to high temperatures exceeding 60°C, NMC cathodes can undergo structural degradation, leading to capacity loss and potential thermal runaway. This issue becomes particularly critical in applications where cooling systems may be compromised or environmental temperatures are naturally elevated.
Cycle life degradation represents another significant hurdle. NMC batteries typically experience capacity fade of 20-30% after 1,000 cycles under standard conditions, but this deterioration accelerates dramatically in demanding applications with deep discharge cycles and high C-rates. The nickel-rich variants (NMC 811), while offering higher energy density, show even faster degradation patterns under stress.
Safety concerns persist despite advancements in battery management systems. The exothermic nature of thermal runaway in NMC cells can trigger cascading failures in battery packs, necessitating sophisticated thermal management solutions that add weight and complexity to the overall system.
Trivalent Interface technology faces its own set of challenges. The interface stability between electrodes and electrolyte deteriorates under extreme temperature fluctuations, creating micro-cracks that increase internal resistance and reduce power output. This becomes particularly problematic in applications requiring rapid power delivery under varying environmental conditions.
Manufacturing consistency presents a significant barrier for Trivalent Interface implementation. The precise control required for uniform interface formation across production batches remains difficult to achieve at scale, resulting in performance variability that is unacceptable for mission-critical applications.
Material compatibility issues arise when integrating Trivalent Interface technology with existing battery architectures. The chemical interactions between interface materials and conventional electrode components can lead to unexpected degradation mechanisms that only manifest after extended use in demanding conditions.
Both technologies struggle with low-temperature performance limitations. At temperatures below -20°C, ion mobility decreases dramatically, resulting in significant power loss and increased internal resistance. This poses particular challenges for applications in cold environments or space operations where temperature control is limited.
Cost-performance optimization remains an ongoing challenge, as the materials and manufacturing processes required for enhancing performance in demanding applications significantly increase production costs, creating barriers to widespread adoption in price-sensitive markets.
The primary challenge for NMC batteries in demanding environments is thermal stability. When subjected to high temperatures exceeding 60°C, NMC cathodes can undergo structural degradation, leading to capacity loss and potential thermal runaway. This issue becomes particularly critical in applications where cooling systems may be compromised or environmental temperatures are naturally elevated.
Cycle life degradation represents another significant hurdle. NMC batteries typically experience capacity fade of 20-30% after 1,000 cycles under standard conditions, but this deterioration accelerates dramatically in demanding applications with deep discharge cycles and high C-rates. The nickel-rich variants (NMC 811), while offering higher energy density, show even faster degradation patterns under stress.
Safety concerns persist despite advancements in battery management systems. The exothermic nature of thermal runaway in NMC cells can trigger cascading failures in battery packs, necessitating sophisticated thermal management solutions that add weight and complexity to the overall system.
Trivalent Interface technology faces its own set of challenges. The interface stability between electrodes and electrolyte deteriorates under extreme temperature fluctuations, creating micro-cracks that increase internal resistance and reduce power output. This becomes particularly problematic in applications requiring rapid power delivery under varying environmental conditions.
Manufacturing consistency presents a significant barrier for Trivalent Interface implementation. The precise control required for uniform interface formation across production batches remains difficult to achieve at scale, resulting in performance variability that is unacceptable for mission-critical applications.
Material compatibility issues arise when integrating Trivalent Interface technology with existing battery architectures. The chemical interactions between interface materials and conventional electrode components can lead to unexpected degradation mechanisms that only manifest after extended use in demanding conditions.
Both technologies struggle with low-temperature performance limitations. At temperatures below -20°C, ion mobility decreases dramatically, resulting in significant power loss and increased internal resistance. This poses particular challenges for applications in cold environments or space operations where temperature control is limited.
Cost-performance optimization remains an ongoing challenge, as the materials and manufacturing processes required for enhancing performance in demanding applications significantly increase production costs, creating barriers to widespread adoption in price-sensitive markets.
Current Technical Solutions Comparison
01 NMC cathode material composition and structure
Nickel-Manganese-Cobalt (NMC) cathode materials are widely used in lithium-ion batteries due to their high energy density and stability. The composition typically involves lithium nickel manganese cobalt oxide with various ratios of the transition metals. The crystal structure and morphology of these materials significantly impact battery performance, including capacity, cycling stability, and rate capability. Optimization of the NMC composition and structure is crucial for enhancing the electrochemical properties at the electrode-electrolyte interface.- NMC cathode material composition and structure: Lithium nickel manganese cobalt oxide (NMC) cathode materials are widely used in lithium-ion batteries due to their high energy density and stability. The composition typically involves varying ratios of nickel, manganese, and cobalt to optimize performance characteristics. The crystal structure and morphology of these materials significantly impact battery performance, with research focusing on controlling particle size, distribution, and crystallinity to enhance electrochemical properties and stability at the electrode-electrolyte interface.
- Trivalent interface engineering in NMC batteries: Interface engineering involving trivalent elements at the cathode-electrolyte interface plays a crucial role in improving NMC battery performance. These interfaces can be modified using trivalent metal ions (such as Al3+, Cr3+, or Fe3+) to create protective layers that prevent unwanted side reactions, reduce transition metal dissolution, and enhance structural stability. This approach helps maintain capacity retention during cycling and improves the overall lifespan of NMC batteries by stabilizing the critical interfaces where degradation often occurs.
- Surface coating and doping strategies for NMC materials: Surface modification techniques including coating and doping are employed to enhance the performance of NMC battery materials. These strategies involve applying protective layers or incorporating foreign elements into the crystal structure to stabilize the cathode-electrolyte interface. Common coating materials include metal oxides, phosphates, and fluorides, while doping often involves introducing small amounts of elements that can improve structural stability, ionic conductivity, and thermal properties, ultimately leading to better cycling performance and safety characteristics.
- Electrolyte formulations for stable interfaces in NMC batteries: Advanced electrolyte formulations are designed to form stable solid-electrolyte interfaces (SEI) with NMC cathode materials. These formulations may include additives that promote the formation of protective films at the electrode surface, preventing continuous electrolyte decomposition and cathode degradation. Functional additives can react preferentially at the interface to create a stable passivation layer that allows lithium-ion transport while blocking unwanted side reactions, thereby improving the cycling stability and high-voltage performance of NMC batteries.
- Manufacturing processes affecting interface properties in NMC batteries: Manufacturing techniques significantly influence the formation and properties of interfaces in NMC batteries. Parameters such as synthesis temperature, precursor selection, calcination conditions, and electrode preparation methods all affect the surface chemistry and interfacial characteristics of NMC materials. Advanced manufacturing approaches focus on controlling particle morphology, surface defects, and grain boundaries to optimize the electrode-electrolyte interface. Post-synthesis treatments may also be employed to modify surface properties and enhance the electrochemical performance and stability of NMC battery systems.
02 Trivalent interface engineering for NMC batteries
Engineering the trivalent interface in NMC batteries involves modifying the boundary between the cathode material, electrolyte, and conductive additives. This interface plays a critical role in ion transport, electron transfer, and overall battery performance. Various approaches include surface coatings, dopants, and functional additives that can stabilize the interface, reduce side reactions, and improve the electrochemical stability of NMC materials. Proper interface engineering can mitigate capacity fading and enhance the cycle life of batteries.Expand Specific Solutions03 Surface modification techniques for NMC cathodes
Surface modification of NMC cathode materials is employed to improve the stability of the electrode-electrolyte interface. Techniques include coating with metal oxides, phosphates, or fluorides to create a protective layer that prevents direct contact between the cathode and electrolyte. These modifications can effectively suppress undesirable side reactions, reduce transition metal dissolution, and enhance structural stability during cycling. The modified surface acts as a buffer zone that improves the electrochemical performance and thermal stability of NMC batteries.Expand Specific Solutions04 Electrolyte formulations for improved NMC interface stability
Specialized electrolyte formulations can significantly enhance the stability of the trivalent interface in NMC batteries. These formulations may include additives that form stable solid electrolyte interphase (SEI) layers, solvents with high oxidation resistance, and lithium salts that promote efficient ion transport. The electrolyte composition directly affects the chemical reactions at the electrode surface, influencing the formation and properties of the interface layer. Optimized electrolyte systems can reduce impedance growth, prevent cathode degradation, and extend battery lifespan.Expand Specific Solutions05 Advanced characterization methods for NMC interfaces
Understanding the complex nature of trivalent interfaces in NMC batteries requires sophisticated characterization techniques. Methods such as X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and in-situ/operando spectroscopic techniques provide valuable insights into the chemical composition, structure, and evolution of these interfaces during battery operation. Advanced characterization enables researchers to identify degradation mechanisms, evaluate the effectiveness of interface engineering strategies, and guide the development of next-generation NMC battery materials with enhanced performance and durability.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The NMC battery versus trivalent interface technology landscape is currently in a growth phase, with the market for high-performance batteries expanding rapidly due to increasing demand in electric vehicles and energy storage applications. Companies like Micron Technology, Panasonic, and Wanxiang 123 are leading innovation in NMC battery technology, while research institutions such as Interuniversitair Micro-Electronica Centrum and Fudan University are advancing trivalent interface solutions. Chinese manufacturers including Hefei Guoxuan and Jiangsu Zenergy are scaling production capabilities, particularly for demanding applications. The technology maturity varies significantly - NMC batteries represent a more established commercial technology with ongoing refinements, while trivalent interface solutions remain in earlier development stages but show promising performance characteristics for extreme operating conditions.
Jingmen Gem Co., Ltd.
Technical Solution: Jingmen Gem has focused on developing sustainable NMC battery technology with enhanced performance for demanding applications. Their "EcoNMC" technology utilizes recycled materials in cathode production while maintaining performance comparable to batteries made from virgin materials. The company has pioneered a novel trivalent interface modification approach that incorporates aluminum and titanium dopants at precise concentrations to stabilize the cathode-electrolyte interface. This technique creates a protective surface layer that prevents electrolyte oxidation at high voltages and temperatures. For applications requiring extended cycle life, Jingmen Gem employs a gradient concentration cathode structure where nickel content decreases toward the particle surface, enhancing structural stability during cycling. Their batteries feature a specialized electrolyte formulation with flame-retardant additives that improve safety without compromising performance, achieving over 1500 cycles at 80% capacity retention under accelerated aging conditions.
Strengths: Environmentally sustainable production, excellent safety characteristics, and good cycle life make these batteries suitable for stationary storage and commercial electric vehicles. Weaknesses: Slightly lower energy density compared to highest-nickel NMC formulations, and the recycled material supply chain can introduce variability in performance.
International Battery Co., Inc.
Technical Solution: International Battery has developed a distinctive approach to NMC battery technology through their "Tri-Core" architecture, which features a modified trivalent interface design. Their solution incorporates a gradient functional layer between the NMC cathode and electrolyte that significantly reduces interfacial resistance and improves ion transport kinetics. This design utilizes a proprietary blend of fluorinated compounds and lithium salts that form a protective layer on the cathode surface, preventing transition metal dissolution even under high voltage operation (up to 4.5V). For demanding applications, International Battery employs a silicon-graphite composite anode that increases energy density by approximately 25% compared to conventional graphite anodes. Their cells demonstrate remarkable rate capability, delivering up to 80% of nominal capacity at 5C discharge rates, while maintaining thermal stability through an advanced thermal management system integrated into the cell design.
Strengths: High voltage stability enables greater energy density, excellent rate capability for high-power applications, and improved thermal management for safer operation. Weaknesses: Higher manufacturing complexity and cost, plus the silicon-composite anode may experience more significant volume changes during cycling.
Core Patents and Innovations Analysis
Lithium battery and use of triphenylphosphine oxide as an electrolyte additive therein
PatentWO2018224167A1
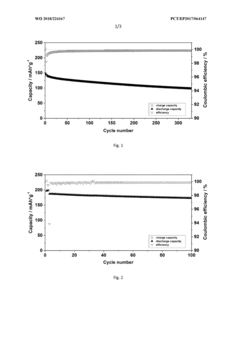
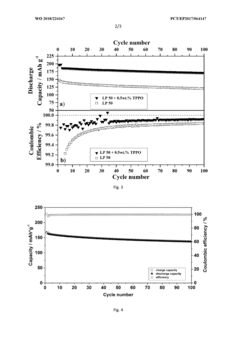
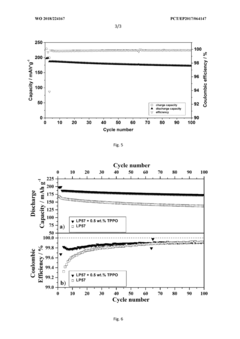
Innovation
- Incorporating triphenylphosphine oxide as an electrolyte additive in lithium-ion batteries with NMC cathodes, which forms a passivation layer that kinetically inhibits oxidative decomposition and metal release, enhancing cycle stability and service life.
Active material for cathode of lithium-ion battery, cathode comprising said active material, and method for preparing said cathode
PatentWO2023170449A1
Innovation
- A cathode active material is developed by combining lithium manganese oxide (LMO) with lithium nickel manganese cobalt oxide (NMC) in specific mole ratios, enhancing stability and cycle life, and incorporating a binder and conductive material for improved electron and ion transfer, with the mixture's mass ratio optimized for high energy density and long cycle life.
Environmental Impact and Sustainability Assessment
The environmental impact of battery technologies has become a critical consideration in their adoption for demanding applications. NMC (Nickel Manganese Cobalt) batteries present significant environmental challenges throughout their lifecycle. The mining of cobalt, a key component in NMC batteries, is particularly problematic, with documented issues of habitat destruction, water pollution, and human rights concerns in major mining regions like the Democratic Republic of Congo. Additionally, the extraction of nickel and manganese contributes to substantial carbon emissions and ecological disruption.
In contrast, Trivalent Interface technology demonstrates promising environmental advantages. This technology typically requires fewer rare earth elements and toxic materials, resulting in a reduced mining footprint. The manufacturing process for Trivalent Interface systems generally consumes less energy and produces fewer harmful byproducts compared to traditional NMC battery production, which involves energy-intensive electrode coating and cell assembly processes.
From a lifecycle perspective, NMC batteries currently have more established recycling infrastructure, with recovery rates for cobalt reaching up to 80% in advanced facilities. However, the recycling process itself is energy-intensive and can release harmful substances if not properly controlled. Trivalent Interface systems, while newer to market, are being designed with end-of-life considerations, potentially offering more straightforward material separation and recovery.
Carbon footprint assessments reveal that NMC batteries generate approximately 75-200 kg CO2-equivalent per kWh of storage capacity during production, depending on manufacturing location and energy sources. Preliminary studies suggest Trivalent Interface technology may reduce this impact by 30-40%, primarily through decreased material intensity and simplified manufacturing processes.
Water usage presents another significant environmental factor. NMC battery production typically requires 400-600 liters of water per kWh, while Trivalent Interface systems may reduce this requirement by up to 50% through more efficient processing techniques and alternative material compositions that require less intensive purification.
For demanding applications where frequent replacement might be necessary, the cumulative environmental impact becomes particularly relevant. The potentially longer service life of Trivalent Interface technology could significantly reduce waste generation and resource consumption over time, despite the current advantages in recycling infrastructure for NMC batteries.
Regulatory trends globally are increasingly favoring technologies with lower environmental impacts, with the EU Battery Directive and similar frameworks in Asia and North America imposing stricter sustainability requirements. This regulatory landscape may accelerate the transition toward more environmentally benign technologies like Trivalent Interface systems for demanding applications where performance and sustainability must be balanced.
In contrast, Trivalent Interface technology demonstrates promising environmental advantages. This technology typically requires fewer rare earth elements and toxic materials, resulting in a reduced mining footprint. The manufacturing process for Trivalent Interface systems generally consumes less energy and produces fewer harmful byproducts compared to traditional NMC battery production, which involves energy-intensive electrode coating and cell assembly processes.
From a lifecycle perspective, NMC batteries currently have more established recycling infrastructure, with recovery rates for cobalt reaching up to 80% in advanced facilities. However, the recycling process itself is energy-intensive and can release harmful substances if not properly controlled. Trivalent Interface systems, while newer to market, are being designed with end-of-life considerations, potentially offering more straightforward material separation and recovery.
Carbon footprint assessments reveal that NMC batteries generate approximately 75-200 kg CO2-equivalent per kWh of storage capacity during production, depending on manufacturing location and energy sources. Preliminary studies suggest Trivalent Interface technology may reduce this impact by 30-40%, primarily through decreased material intensity and simplified manufacturing processes.
Water usage presents another significant environmental factor. NMC battery production typically requires 400-600 liters of water per kWh, while Trivalent Interface systems may reduce this requirement by up to 50% through more efficient processing techniques and alternative material compositions that require less intensive purification.
For demanding applications where frequent replacement might be necessary, the cumulative environmental impact becomes particularly relevant. The potentially longer service life of Trivalent Interface technology could significantly reduce waste generation and resource consumption over time, despite the current advantages in recycling infrastructure for NMC batteries.
Regulatory trends globally are increasingly favoring technologies with lower environmental impacts, with the EU Battery Directive and similar frameworks in Asia and North America imposing stricter sustainability requirements. This regulatory landscape may accelerate the transition toward more environmentally benign technologies like Trivalent Interface systems for demanding applications where performance and sustainability must be balanced.
Safety Standards and Regulatory Compliance
The regulatory landscape for battery technologies in demanding applications is complex and continuously evolving, with safety standards forming the cornerstone of market acceptance and commercial viability. NMC (Nickel Manganese Cobalt) batteries and Trivalent Interface technologies are subject to different regulatory frameworks depending on their application domains and geographical markets.
For NMC batteries, the primary safety standards include IEC 62133 for portable applications and UN 38.3 for transportation safety. These batteries must undergo rigorous testing for thermal stability, with particular emphasis on preventing thermal runaway events that have historically plagued lithium-ion chemistries. The UL 1642 standard specifically addresses cell-level safety requirements, while UL 2054 covers battery pack assemblies. NMC batteries in automotive applications must additionally comply with ISO 26262 for functional safety and SAE J2464 for abuse testing.
Trivalent Interface technology, being a newer entrant in the energy storage domain, faces evolving regulatory frameworks. Its compliance pathway typically includes IEC 61508 for functional safety systems and additional standards specific to its electrochemical properties. The technology's enhanced thermal stability characteristics potentially simplify compliance with thermal runaway prevention requirements, though comprehensive long-term safety data remains under development.
Regional variations in regulatory requirements present significant challenges for global deployment. The European Union's Battery Directive 2006/66/EC and its recent updates impose strict requirements on battery recycling and hazardous material content, affecting both technologies differently based on their material composition. Similarly, China's GB/T standards and North America's NFPA requirements create a complex compliance matrix that manufacturers must navigate.
For demanding use applications such as aerospace, military, or critical infrastructure, additional standards including MIL-STD-810 for environmental performance and DO-311A for airborne equipment apply. These specialized domains often require customized safety protocols beyond standard certifications, with particular emphasis on failure mode analysis and redundancy systems.
Emerging regulatory trends indicate increasing scrutiny of battery lifecycle management, with emphasis on end-of-life handling and environmental impact. The EU's proposed Battery Regulation aims to replace the current Directive with more stringent requirements for carbon footprint declaration and responsible sourcing of materials, potentially creating competitive advantages for technologies with lower environmental impacts and higher recyclability profiles.
For NMC batteries, the primary safety standards include IEC 62133 for portable applications and UN 38.3 for transportation safety. These batteries must undergo rigorous testing for thermal stability, with particular emphasis on preventing thermal runaway events that have historically plagued lithium-ion chemistries. The UL 1642 standard specifically addresses cell-level safety requirements, while UL 2054 covers battery pack assemblies. NMC batteries in automotive applications must additionally comply with ISO 26262 for functional safety and SAE J2464 for abuse testing.
Trivalent Interface technology, being a newer entrant in the energy storage domain, faces evolving regulatory frameworks. Its compliance pathway typically includes IEC 61508 for functional safety systems and additional standards specific to its electrochemical properties. The technology's enhanced thermal stability characteristics potentially simplify compliance with thermal runaway prevention requirements, though comprehensive long-term safety data remains under development.
Regional variations in regulatory requirements present significant challenges for global deployment. The European Union's Battery Directive 2006/66/EC and its recent updates impose strict requirements on battery recycling and hazardous material content, affecting both technologies differently based on their material composition. Similarly, China's GB/T standards and North America's NFPA requirements create a complex compliance matrix that manufacturers must navigate.
For demanding use applications such as aerospace, military, or critical infrastructure, additional standards including MIL-STD-810 for environmental performance and DO-311A for airborne equipment apply. These specialized domains often require customized safety protocols beyond standard certifications, with particular emphasis on failure mode analysis and redundancy systems.
Emerging regulatory trends indicate increasing scrutiny of battery lifecycle management, with emphasis on end-of-life handling and environmental impact. The EU's proposed Battery Regulation aims to replace the current Directive with more stringent requirements for carbon footprint declaration and responsible sourcing of materials, potentially creating competitive advantages for technologies with lower environmental impacts and higher recyclability profiles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!