Optimizing NMC Battery's Graphitic Composition in Cell Designs
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMC Battery Technology Evolution and Objectives
Lithium-ion batteries have undergone significant evolution since their commercial introduction in the early 1990s. The development of NMC (Nickel Manganese Cobalt) cathode materials represents one of the most important advancements in this field, offering improved energy density, cycle life, and thermal stability compared to earlier lithium cobalt oxide (LCO) chemistries. The first generation of NMC batteries emerged in the early 2000s with compositions typically denoted as NMC 111, indicating equal parts nickel, manganese, and cobalt.
The technological trajectory has since focused on increasing nickel content while reducing costly and ethically problematic cobalt, leading to the development of NMC 532, NMC 622, and most recently NMC 811 formulations. This evolution has been driven by the need for higher energy density, longer cycle life, and reduced production costs to meet the growing demands of electric vehicles and energy storage systems.
Graphitic materials have been the predominant anode material in commercial lithium-ion batteries, including NMC systems. The optimization of graphitic composition in NMC cell designs represents a critical frontier in battery technology development. Traditional graphite anodes typically contain a mixture of natural and synthetic graphite particles with specific surface areas, particle sizes, and morphologies that significantly impact battery performance.
Recent research has focused on enhancing the graphitic anode's capacity, rate capability, and cycle stability through various approaches, including particle engineering, surface modification, and composite formation with silicon or other high-capacity materials. The interaction between the optimized graphitic anode and NMC cathode materials is particularly important, as it affects the overall cell performance, safety, and longevity.
The primary technical objectives in optimizing NMC battery's graphitic composition include: increasing the specific capacity beyond the theoretical limit of graphite (372 mAh/g); improving fast-charging capabilities without lithium plating; enhancing low-temperature performance; extending cycle life to match vehicle lifetimes (>1000 cycles); and ensuring thermal stability under various operating conditions.
Additionally, sustainability objectives have gained prominence, with efforts directed toward reducing the environmental footprint of battery production, improving recyclability, and decreasing dependence on critical raw materials. These objectives align with global initiatives to transition toward cleaner energy systems and reduce greenhouse gas emissions.
The convergence of advanced materials science, electrochemistry, and manufacturing innovations is expected to drive the next generation of NMC batteries with optimized graphitic compositions, potentially enabling electric vehicles with ranges exceeding 500 km on a single charge and lifespans comparable to conventional vehicles.
The technological trajectory has since focused on increasing nickel content while reducing costly and ethically problematic cobalt, leading to the development of NMC 532, NMC 622, and most recently NMC 811 formulations. This evolution has been driven by the need for higher energy density, longer cycle life, and reduced production costs to meet the growing demands of electric vehicles and energy storage systems.
Graphitic materials have been the predominant anode material in commercial lithium-ion batteries, including NMC systems. The optimization of graphitic composition in NMC cell designs represents a critical frontier in battery technology development. Traditional graphite anodes typically contain a mixture of natural and synthetic graphite particles with specific surface areas, particle sizes, and morphologies that significantly impact battery performance.
Recent research has focused on enhancing the graphitic anode's capacity, rate capability, and cycle stability through various approaches, including particle engineering, surface modification, and composite formation with silicon or other high-capacity materials. The interaction between the optimized graphitic anode and NMC cathode materials is particularly important, as it affects the overall cell performance, safety, and longevity.
The primary technical objectives in optimizing NMC battery's graphitic composition include: increasing the specific capacity beyond the theoretical limit of graphite (372 mAh/g); improving fast-charging capabilities without lithium plating; enhancing low-temperature performance; extending cycle life to match vehicle lifetimes (>1000 cycles); and ensuring thermal stability under various operating conditions.
Additionally, sustainability objectives have gained prominence, with efforts directed toward reducing the environmental footprint of battery production, improving recyclability, and decreasing dependence on critical raw materials. These objectives align with global initiatives to transition toward cleaner energy systems and reduce greenhouse gas emissions.
The convergence of advanced materials science, electrochemistry, and manufacturing innovations is expected to drive the next generation of NMC batteries with optimized graphitic compositions, potentially enabling electric vehicles with ranges exceeding 500 km on a single charge and lifespans comparable to conventional vehicles.
Market Demand Analysis for Advanced NMC Batteries
The global market for advanced NMC (Nickel Manganese Cobalt) batteries is experiencing robust growth, driven primarily by the rapid expansion of electric vehicle (EV) adoption worldwide. Market research indicates that the NMC battery segment is projected to grow at a compound annual growth rate of 18.5% through 2030, with particular acceleration in regions implementing stringent carbon emission regulations.
Consumer demand for EVs with longer range capabilities has created significant pressure for battery manufacturers to optimize energy density while maintaining safety standards. This has directly influenced the focus on graphitic composition optimization in NMC cell designs, as the graphite anode material significantly impacts overall battery performance. Industry surveys reveal that consumers consistently rank driving range as the second most important factor in EV purchasing decisions, just behind price point.
Commercial fleet operators represent another substantial market segment driving demand for advanced NMC batteries. These customers prioritize total cost of ownership metrics, where battery longevity and fast-charging capabilities deliver competitive advantages. The optimization of graphitic composition directly addresses these requirements by enhancing cycle life and charge acceptance rates.
The stationary energy storage market presents an additional growth vector for optimized NMC batteries. Grid-scale applications require batteries with excellent thermal stability and long service life, attributes that can be significantly enhanced through graphitic composition refinement. This market segment is expanding at 22% annually as renewable energy integration accelerates globally.
Supply chain considerations are increasingly influencing market dynamics for NMC batteries. Recent geopolitical tensions have highlighted vulnerabilities in critical mineral supply chains, particularly for cobalt and nickel. This has intensified interest in NMC formulations that can maintain performance while reducing dependency on these materials, with graphitic composition optimization offering a promising pathway.
Regulatory frameworks are evolving to address battery sustainability, with the European Union's proposed Battery Regulation establishing requirements for carbon footprint declarations and recycled content. These regulations are expected to favor NMC battery designs with optimized graphitic compositions that enable easier end-of-life recycling and reduced environmental impact.
Price sensitivity remains a critical market factor, with automotive OEMs targeting battery pack costs below $100/kWh to achieve price parity with internal combustion vehicles. Graphitic composition optimization offers a potential pathway to cost reduction through improved manufacturing yields and enhanced performance with less expensive materials.
Consumer demand for EVs with longer range capabilities has created significant pressure for battery manufacturers to optimize energy density while maintaining safety standards. This has directly influenced the focus on graphitic composition optimization in NMC cell designs, as the graphite anode material significantly impacts overall battery performance. Industry surveys reveal that consumers consistently rank driving range as the second most important factor in EV purchasing decisions, just behind price point.
Commercial fleet operators represent another substantial market segment driving demand for advanced NMC batteries. These customers prioritize total cost of ownership metrics, where battery longevity and fast-charging capabilities deliver competitive advantages. The optimization of graphitic composition directly addresses these requirements by enhancing cycle life and charge acceptance rates.
The stationary energy storage market presents an additional growth vector for optimized NMC batteries. Grid-scale applications require batteries with excellent thermal stability and long service life, attributes that can be significantly enhanced through graphitic composition refinement. This market segment is expanding at 22% annually as renewable energy integration accelerates globally.
Supply chain considerations are increasingly influencing market dynamics for NMC batteries. Recent geopolitical tensions have highlighted vulnerabilities in critical mineral supply chains, particularly for cobalt and nickel. This has intensified interest in NMC formulations that can maintain performance while reducing dependency on these materials, with graphitic composition optimization offering a promising pathway.
Regulatory frameworks are evolving to address battery sustainability, with the European Union's proposed Battery Regulation establishing requirements for carbon footprint declarations and recycled content. These regulations are expected to favor NMC battery designs with optimized graphitic compositions that enable easier end-of-life recycling and reduced environmental impact.
Price sensitivity remains a critical market factor, with automotive OEMs targeting battery pack costs below $100/kWh to achieve price parity with internal combustion vehicles. Graphitic composition optimization offers a potential pathway to cost reduction through improved manufacturing yields and enhanced performance with less expensive materials.
Graphite Composition Challenges in Current NMC Cells
Current NMC (Nickel Manganese Cobalt) battery cells face significant challenges related to their graphitic composition, which directly impacts overall cell performance, longevity, and safety characteristics. The graphite anode material, typically accounting for 10-15% of a cell's weight, serves as the host structure for lithium ions during charging cycles, making its composition critically important to battery functionality.
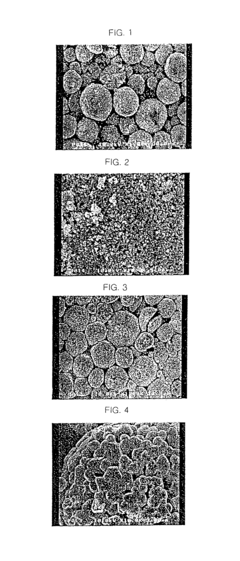
One primary challenge involves the particle size distribution of graphite materials. Conventional NMC cells utilize graphite with particle sizes ranging from 10-25 micrometers, creating a delicate balance between surface area and ion diffusion pathways. Smaller particles offer enhanced surface area for lithium-ion interaction but can lead to increased solid-electrolyte interphase (SEI) formation, while larger particles reduce specific capacity and rate capability.
The crystallinity and orientation of graphite layers present another significant hurdle. Ideal graphite anodes require highly ordered crystalline structures with minimal defects to facilitate efficient lithium intercalation. However, manufacturing processes often introduce structural imperfections that create "dead zones" where lithium becomes trapped, contributing to capacity fade over multiple charge-discharge cycles.
Surface modification challenges also plague current graphite compositions. The reactive nature of fresh graphite surfaces with electrolytes necessitates protective coatings or surface treatments. Current approaches using carbon coating or silicon-oxide layers often result in non-uniform coverage, creating vulnerability points for electrolyte decomposition and subsequent cell degradation.
The graphite-binder interaction represents another critical challenge. PVDF (polyvinylidene fluoride) remains the dominant binder in commercial cells, but its interaction with graphite particles creates inconsistent adhesion properties. This leads to electrode expansion/contraction issues during cycling, ultimately compromising structural integrity and electrical connectivity within the electrode matrix.
Electrolyte compatibility issues further complicate graphite composition optimization. Standard carbonate-based electrolytes react with graphite surfaces to form SEI layers that, while necessary for cell function, consume lithium inventory and increase internal resistance when formed non-uniformly or excessively.
Additionally, the thermal conductivity limitations of current graphite compositions create heat management challenges. With thermal conductivity values typically between 5-10 W/m·K for compressed graphite electrodes, heat dissipation during fast charging or high-power applications remains suboptimal, increasing the risk of thermal runaway events and accelerated degradation mechanisms.
One primary challenge involves the particle size distribution of graphite materials. Conventional NMC cells utilize graphite with particle sizes ranging from 10-25 micrometers, creating a delicate balance between surface area and ion diffusion pathways. Smaller particles offer enhanced surface area for lithium-ion interaction but can lead to increased solid-electrolyte interphase (SEI) formation, while larger particles reduce specific capacity and rate capability.
The crystallinity and orientation of graphite layers present another significant hurdle. Ideal graphite anodes require highly ordered crystalline structures with minimal defects to facilitate efficient lithium intercalation. However, manufacturing processes often introduce structural imperfections that create "dead zones" where lithium becomes trapped, contributing to capacity fade over multiple charge-discharge cycles.
Surface modification challenges also plague current graphite compositions. The reactive nature of fresh graphite surfaces with electrolytes necessitates protective coatings or surface treatments. Current approaches using carbon coating or silicon-oxide layers often result in non-uniform coverage, creating vulnerability points for electrolyte decomposition and subsequent cell degradation.
The graphite-binder interaction represents another critical challenge. PVDF (polyvinylidene fluoride) remains the dominant binder in commercial cells, but its interaction with graphite particles creates inconsistent adhesion properties. This leads to electrode expansion/contraction issues during cycling, ultimately compromising structural integrity and electrical connectivity within the electrode matrix.
Electrolyte compatibility issues further complicate graphite composition optimization. Standard carbonate-based electrolytes react with graphite surfaces to form SEI layers that, while necessary for cell function, consume lithium inventory and increase internal resistance when formed non-uniformly or excessively.
Additionally, the thermal conductivity limitations of current graphite compositions create heat management challenges. With thermal conductivity values typically between 5-10 W/m·K for compressed graphite electrodes, heat dissipation during fast charging or high-power applications remains suboptimal, increasing the risk of thermal runaway events and accelerated degradation mechanisms.
Current Graphitic Optimization Approaches for NMC Cells
01 Graphite composition for NMC battery electrodes
Graphite materials are commonly used in NMC battery electrodes due to their excellent electrical conductivity and structural stability. These compositions typically include specific types of graphite with controlled particle size distribution and surface modifications to enhance lithium-ion intercalation. The graphite composition significantly affects the battery's charge/discharge efficiency, cycle life, and overall performance when paired with NMC cathode materials.- Graphite composition for NMC battery electrodes: Graphite materials are commonly used in NMC battery electrodes due to their excellent electrical conductivity and structural stability. These compositions typically include natural or synthetic graphite particles with specific particle sizes, shapes, and surface treatments to enhance performance. The graphite component serves as the primary active material in the negative electrode, providing intercalation sites for lithium ions during charging and discharging cycles.
- NMC cathode material formulations: Lithium nickel manganese cobalt oxide (NMC) cathode materials are formulated with specific ratios of nickel, manganese, and cobalt to optimize battery performance. These formulations can be adjusted to enhance energy density, cycle life, thermal stability, and rate capability. Various synthesis methods and post-processing techniques are employed to control the crystal structure, particle morphology, and surface properties of NMC materials, which directly impact battery performance.
- Composite anode structures with graphite: Composite anode structures incorporate graphite with other materials to enhance battery performance. These composites may combine graphite with silicon, tin, or other high-capacity materials to increase energy density while maintaining stability. Binders, conductive additives, and surface coatings are used to improve the mechanical integrity and electrochemical performance of these composite structures. The graphite component provides structural stability while the additional materials contribute to increased capacity.
- Surface modification of graphite for NMC batteries: Surface modifications of graphite materials are employed to improve the interface between the electrode and electrolyte in NMC batteries. These modifications include coatings, functional groups, or dopants that enhance the solid electrolyte interphase formation, reduce irreversible capacity loss, and improve cycling stability. Various techniques such as chemical vapor deposition, wet chemical methods, or thermal treatments are used to modify the graphite surface properties, resulting in improved battery performance and longevity.
- Electrolyte additives for graphite-NMC systems: Specialized electrolyte additives are developed for graphite-NMC battery systems to enhance performance and safety. These additives can improve the formation of stable solid electrolyte interphase layers on graphite surfaces, prevent electrolyte decomposition, and enhance lithium-ion transport. Film-forming additives, redox shuttle compounds, and flame retardants are commonly used to address specific performance and safety challenges in NMC batteries with graphite anodes.
02 Silicon-graphite composite anodes for NMC batteries
Silicon-graphite composite materials are used to enhance the capacity of anodes in NMC batteries. Silicon provides higher theoretical capacity than graphite alone, while the graphite component maintains structural stability and conductivity. These composites typically incorporate silicon nanoparticles or silicon-based compounds dispersed within a graphitic matrix, often with binders and conductive additives to improve cycling performance and mitigate silicon's volume expansion issues.Expand Specific Solutions03 Surface coating and modification of graphite for NMC batteries
Surface treatments and coatings are applied to graphite materials used in NMC batteries to improve their electrochemical performance and stability. These modifications include carbon coating, metal oxide deposition, or polymer treatments that enhance the solid electrolyte interphase formation, reduce irreversible capacity loss, and improve cycling stability. Modified graphite surfaces also help prevent electrolyte decomposition and enhance the overall safety and longevity of NMC battery systems.Expand Specific Solutions04 Doped graphite materials for enhanced NMC battery performance
Doping graphite with various elements or compounds can significantly improve its performance in NMC battery applications. Common dopants include nitrogen, boron, phosphorus, or metal elements that modify the electronic properties of graphite. These doped materials exhibit enhanced conductivity, improved lithium-ion diffusion kinetics, and better structural stability during cycling. The strategic incorporation of dopants can lead to NMC batteries with higher energy density, faster charging capabilities, and extended cycle life.Expand Specific Solutions05 Manufacturing processes for graphitic components in NMC batteries
Specialized manufacturing techniques are employed to produce high-quality graphitic materials for NMC batteries. These processes include controlled pyrolysis of carbon precursors, mechanical or chemical exfoliation of graphite, and precision milling to achieve desired particle morphologies. Advanced manufacturing methods also focus on creating hierarchical porous structures, optimizing particle size distribution, and ensuring uniform dispersion of graphite in electrode formulations to maximize electrochemical performance and production efficiency of NMC battery systems.Expand Specific Solutions
Key Industry Players in NMC Battery Technology
The NMC battery graphitic composition optimization market is currently in a growth phase, with an estimated market size exceeding $15 billion and projected to expand at a CAGR of 12-15% through 2030. The competitive landscape features established players like CATL, LG Chem, and Panasonic leading commercial deployment, while QuantumScape and Echion Technologies drive innovation in next-generation compositions. Research institutions including UT-Battelle and Argonne National Laboratory are advancing fundamental understanding of graphite-NMC interactions. The technology maturity varies across applications, with automotive implementations (supported by Ford and Renault) reaching commercial scale, while advanced compositions for ultra-fast charging and extended cycle life remain in development phases. Major battery manufacturers are increasingly focusing on optimizing silicon-graphite composite anodes to enhance energy density.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an advanced NMC battery optimization approach focusing on precise graphitic composition control in their cell designs. Their technology involves a multi-layer gradient graphite structure where different carbon particle sizes and morphologies are strategically arranged to maximize electron transport pathways while maintaining structural stability. CATL employs a proprietary surface modification technique for graphite particles that creates functional groups to enhance the solid electrolyte interphase (SEI) formation and stability. Their manufacturing process includes precise temperature-controlled graphitization that achieves optimal crystallinity levels of 94-96%, significantly improving conductivity while maintaining appropriate porosity for lithium-ion intercalation. This balanced approach results in NMC cells with energy densities exceeding 300 Wh/kg while maintaining excellent cycle life.
Strengths: Superior energy density optimization through precise graphite engineering; excellent manufacturing scalability; proven reliability in commercial applications. Weaknesses: Higher production costs compared to standard graphite formulations; requires specialized manufacturing equipment; performance advantages diminish at extremely high discharge rates.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a cutting-edge scientific approach to graphitic composition optimization for NMC batteries through their "Structural Engineering at Atomic Scale" (SEAS) technology. Their research focuses on precise control of graphite's crystallographic properties, including interlayer spacing (optimized to 3.35-3.38Å) and domain size to maximize lithium intercalation capacity while minimizing structural degradation. Argonne employs advanced characterization techniques including in-situ XRD and neutron diffraction to understand graphite structural evolution during cycling, enabling them to design optimal particle morphologies. Their technology includes a novel edge-plane passivation treatment that selectively functionalizes reactive graphite edge sites while preserving the high-conductivity basal planes. Argonne has pioneered computational modeling approaches that predict graphite-electrolyte interactions at the molecular level, allowing for tailored electrolyte formulations that enhance SEI stability. This science-driven approach has demonstrated NMC cells with exceptional capacity retention (>90% after 1000 cycles) and significantly reduced gas generation during extended cycling.
Strengths: Scientifically rigorous approach based on fundamental materials science; excellent long-term stability and reduced aging effects; superior gas generation control. Weaknesses: Currently at lower technology readiness level for mass production; requires specialized analytical equipment for quality control; higher initial development costs compared to conventional approaches.
Critical Patents in NMC-Graphite Interface Engineering
Lithium ion secondary cell
PatentWO2015190481A1
Innovation
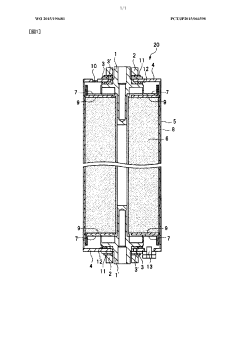
- A lithium-ion battery design featuring a wound structure with a positive electrode mixture containing layered lithium-nickel-manganese-cobalt composite oxide (NMC) as the active material, coated on both sides of a current collector, and a negative electrode mixture with graphitizable carbon, optimized for high capacity and safety, with specific composition and coating amounts to enhance input/output characteristics and energy density.
Lithium nickel manganese cobalt composite oxide used as cathode active material for lithium rechargeable battery, manufacturing method thereof, and lithium rechargeable battery
PatentActiveUS7838148B2
Innovation
- A lithium nickel manganese cobalt composite oxide with an average particle size of 5-40 μm, a BET ratio surface area of 5-25 m2/g, and a tap density of at least 1.70 g/ml, obtained by sintering a mixture of lithium nickel manganese cobalt composite carbonate and a lithium compound at 650-850°C, is used as a cathode active material, enhancing battery performance.
Sustainability Aspects of NMC Battery Production
The sustainability of NMC (Nickel Manganese Cobalt) battery production has become increasingly critical as electric vehicle adoption accelerates globally. When optimizing graphitic composition in NMC cell designs, environmental considerations must be integrated throughout the entire value chain, from raw material extraction to end-of-life management.
The carbon footprint of graphite production varies significantly between synthetic and natural sources. Synthetic graphite production is energy-intensive, requiring temperatures exceeding 2800°C, resulting in approximately 5-10 kg CO2 equivalent per kg of material produced. Natural graphite mining, while less energy-intensive, presents concerns regarding habitat disruption and water contamination in extraction regions, particularly in major producing countries like China, Brazil, and Mozambique.
Water usage represents another significant sustainability challenge. The processing of both graphite and NMC cathode materials requires substantial water resources, with estimates suggesting 50-100 liters per kWh of battery capacity. Optimizing graphitic composition can reduce water requirements through improved processing techniques and recycled water systems, potentially decreasing consumption by 20-30%.
Chemical leaching during graphite purification introduces additional environmental concerns. Traditional methods using hydrofluoric acid present severe ecological risks. Recent advancements in environmentally friendly leaching agents and mechanical purification techniques have demonstrated promising results, reducing hazardous waste by up to 40% while maintaining performance specifications for battery applications.
The recyclability of graphite components significantly impacts the overall sustainability profile of NMC batteries. Current recycling processes focus primarily on recovering valuable metals like nickel and cobalt, often neglecting graphite components. Emerging technologies utilizing hydrometallurgical processes have shown potential for recovering up to 95% of graphite materials, substantially extending the resource lifecycle.
Energy density optimization through graphitic composition adjustments offers sustainability benefits beyond production. Higher energy density formulations reduce material requirements per kWh of storage capacity, decreasing resource intensity by 15-25% compared to first-generation NMC formulations. This translates to lower environmental impact across transportation, manufacturing, and deployment phases.
Regulatory frameworks increasingly influence sustainability requirements for battery production. The European Battery Directive and similar emerging regulations in North America and Asia establish recycled content requirements and carbon footprint limitations that directly impact graphitic composition decisions in cell design. Forward-looking manufacturers are proactively developing compositions that anticipate these regulatory trends, focusing on reduced cobalt content and improved recyclability profiles.
The carbon footprint of graphite production varies significantly between synthetic and natural sources. Synthetic graphite production is energy-intensive, requiring temperatures exceeding 2800°C, resulting in approximately 5-10 kg CO2 equivalent per kg of material produced. Natural graphite mining, while less energy-intensive, presents concerns regarding habitat disruption and water contamination in extraction regions, particularly in major producing countries like China, Brazil, and Mozambique.
Water usage represents another significant sustainability challenge. The processing of both graphite and NMC cathode materials requires substantial water resources, with estimates suggesting 50-100 liters per kWh of battery capacity. Optimizing graphitic composition can reduce water requirements through improved processing techniques and recycled water systems, potentially decreasing consumption by 20-30%.
Chemical leaching during graphite purification introduces additional environmental concerns. Traditional methods using hydrofluoric acid present severe ecological risks. Recent advancements in environmentally friendly leaching agents and mechanical purification techniques have demonstrated promising results, reducing hazardous waste by up to 40% while maintaining performance specifications for battery applications.
The recyclability of graphite components significantly impacts the overall sustainability profile of NMC batteries. Current recycling processes focus primarily on recovering valuable metals like nickel and cobalt, often neglecting graphite components. Emerging technologies utilizing hydrometallurgical processes have shown potential for recovering up to 95% of graphite materials, substantially extending the resource lifecycle.
Energy density optimization through graphitic composition adjustments offers sustainability benefits beyond production. Higher energy density formulations reduce material requirements per kWh of storage capacity, decreasing resource intensity by 15-25% compared to first-generation NMC formulations. This translates to lower environmental impact across transportation, manufacturing, and deployment phases.
Regulatory frameworks increasingly influence sustainability requirements for battery production. The European Battery Directive and similar emerging regulations in North America and Asia establish recycled content requirements and carbon footprint limitations that directly impact graphitic composition decisions in cell design. Forward-looking manufacturers are proactively developing compositions that anticipate these regulatory trends, focusing on reduced cobalt content and improved recyclability profiles.
Safety Standards for High-Energy Density NMC Cells
The safety standards for high-energy density NMC (Nickel Manganese Cobalt) cells have evolved significantly in response to increasing energy densities and the associated safety risks. These standards are critical for ensuring that optimized graphitic compositions in NMC battery cell designs meet rigorous safety requirements across various applications.
International safety standards such as IEC 62133, UL 1642, and UN 38.3 establish baseline requirements for lithium-ion batteries including NMC chemistries. However, high-energy density NMC cells with optimized graphitic compositions require additional safety considerations due to their enhanced energy storage capabilities and potential thermal instability.
Thermal runaway prevention represents a primary focus of safety standards for these advanced cells. The standards typically mandate specific temperature thresholds during operation, charging, and discharging processes. For NMC cells with optimized graphite content, these thresholds are particularly stringent due to the exothermic reactions that can occur between nickel-rich cathodes and graphite anodes under abuse conditions.
Mechanical integrity testing forms another crucial component of safety standards. High-energy density NMC cells must withstand crush tests, impact tests, and vibration tests without experiencing catastrophic failure. The interface between the graphitic anode and the NMC cathode must maintain stability under mechanical stress, with standards specifying maximum allowable deformation and minimum structural integrity requirements.
Electrical safety standards address overcharge protection, short circuit prevention, and voltage management. For optimized graphitic compositions in NMC cells, these standards typically specify maximum charging voltages (often 4.2-4.3V per cell), minimum discharge voltages, and mandatory protection circuitry. The standards recognize that graphite intercalation compounds can become unstable at high voltages, necessitating strict upper voltage limits.
Cell venting mechanisms are mandated by most safety standards to prevent explosive failures. High-energy density NMC cells must incorporate pressure relief mechanisms that activate before catastrophic pressure buildup occurs. The standards specify maximum internal pressure thresholds and minimum venting capacity requirements based on the cell's energy density and graphitic content.
Transportation safety regulations impose additional requirements on high-energy density NMC cells. UN 38.3 testing includes altitude simulation, thermal cycling, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. Cells with optimized graphitic compositions must pass all these tests before being approved for air, sea, or land transport.
Manufacturing quality control standards are increasingly incorporated into safety frameworks for advanced battery technologies. These standards mandate traceability of materials, consistency in graphite particle size distribution, electrode coating uniformity, and electrolyte purity to ensure consistent safety performance across production batches.
International safety standards such as IEC 62133, UL 1642, and UN 38.3 establish baseline requirements for lithium-ion batteries including NMC chemistries. However, high-energy density NMC cells with optimized graphitic compositions require additional safety considerations due to their enhanced energy storage capabilities and potential thermal instability.
Thermal runaway prevention represents a primary focus of safety standards for these advanced cells. The standards typically mandate specific temperature thresholds during operation, charging, and discharging processes. For NMC cells with optimized graphite content, these thresholds are particularly stringent due to the exothermic reactions that can occur between nickel-rich cathodes and graphite anodes under abuse conditions.
Mechanical integrity testing forms another crucial component of safety standards. High-energy density NMC cells must withstand crush tests, impact tests, and vibration tests without experiencing catastrophic failure. The interface between the graphitic anode and the NMC cathode must maintain stability under mechanical stress, with standards specifying maximum allowable deformation and minimum structural integrity requirements.
Electrical safety standards address overcharge protection, short circuit prevention, and voltage management. For optimized graphitic compositions in NMC cells, these standards typically specify maximum charging voltages (often 4.2-4.3V per cell), minimum discharge voltages, and mandatory protection circuitry. The standards recognize that graphite intercalation compounds can become unstable at high voltages, necessitating strict upper voltage limits.
Cell venting mechanisms are mandated by most safety standards to prevent explosive failures. High-energy density NMC cells must incorporate pressure relief mechanisms that activate before catastrophic pressure buildup occurs. The standards specify maximum internal pressure thresholds and minimum venting capacity requirements based on the cell's energy density and graphitic content.
Transportation safety regulations impose additional requirements on high-energy density NMC cells. UN 38.3 testing includes altitude simulation, thermal cycling, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. Cells with optimized graphitic compositions must pass all these tests before being approved for air, sea, or land transport.
Manufacturing quality control standards are increasingly incorporated into safety frameworks for advanced battery technologies. These standards mandate traceability of materials, consistency in graphite particle size distribution, electrode coating uniformity, and electrolyte purity to ensure consistent safety performance across production batches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!