Comparing Fluidic Architectures in Microfluidic Chip Design
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Chip Design Evolution and Objectives
Microfluidic technology has evolved significantly since its inception in the early 1990s, transforming from simple channel designs to sophisticated integrated systems capable of performing complex laboratory functions on a chip. The evolution began with the development of capillary electrophoresis chips, followed by the introduction of continuous-flow microfluidics that leveraged laminar flow properties for precise fluid manipulation. This progression established the foundation for modern microfluidic architectures.
The mid-2000s witnessed a paradigm shift with the emergence of droplet-based microfluidics, enabling compartmentalization of reactions in discrete volumes and significantly enhancing throughput capabilities. Concurrently, paper-based microfluidics gained traction as a cost-effective alternative for point-of-care diagnostics in resource-limited settings. Digital microfluidics, utilizing electrowetting principles for droplet manipulation, emerged as another architectural approach offering unique advantages in certain applications.
Recent years have seen the integration of multiple architectural paradigms within single platforms, creating hybrid systems that capitalize on the strengths of different approaches. The incorporation of advanced materials such as hydrogels, stimuli-responsive polymers, and nanomaterials has further expanded design possibilities, enabling novel functionalities and improved performance characteristics across various fluidic architectures.
The technological trajectory indicates a clear trend toward greater integration, automation, and functionality. Microfluidic platforms are increasingly incorporating sensing elements, actuation mechanisms, and computational components, moving toward truly autonomous lab-on-a-chip systems. This integration is facilitated by advances in microfabrication techniques, including 3D printing, which has democratized prototyping and accelerated innovation cycles in chip design.
The primary objective in comparing fluidic architectures is to establish a comprehensive framework for evaluating different approaches based on application-specific requirements. This includes assessing parameters such as throughput, precision, scalability, ease of fabrication, operational complexity, and compatibility with biological samples. Understanding the fundamental trade-offs between different architectural paradigms is essential for informed design decisions.
Additionally, this comparative analysis aims to identify opportunities for architectural innovation by recognizing gaps in current approaches and potential synergies between different paradigms. The ultimate goal is to advance microfluidic technology toward more versatile, efficient, and accessible platforms that can address emerging challenges in healthcare, environmental monitoring, and fundamental research, while establishing design principles that can guide future development efforts in this rapidly evolving field.
The mid-2000s witnessed a paradigm shift with the emergence of droplet-based microfluidics, enabling compartmentalization of reactions in discrete volumes and significantly enhancing throughput capabilities. Concurrently, paper-based microfluidics gained traction as a cost-effective alternative for point-of-care diagnostics in resource-limited settings. Digital microfluidics, utilizing electrowetting principles for droplet manipulation, emerged as another architectural approach offering unique advantages in certain applications.
Recent years have seen the integration of multiple architectural paradigms within single platforms, creating hybrid systems that capitalize on the strengths of different approaches. The incorporation of advanced materials such as hydrogels, stimuli-responsive polymers, and nanomaterials has further expanded design possibilities, enabling novel functionalities and improved performance characteristics across various fluidic architectures.
The technological trajectory indicates a clear trend toward greater integration, automation, and functionality. Microfluidic platforms are increasingly incorporating sensing elements, actuation mechanisms, and computational components, moving toward truly autonomous lab-on-a-chip systems. This integration is facilitated by advances in microfabrication techniques, including 3D printing, which has democratized prototyping and accelerated innovation cycles in chip design.
The primary objective in comparing fluidic architectures is to establish a comprehensive framework for evaluating different approaches based on application-specific requirements. This includes assessing parameters such as throughput, precision, scalability, ease of fabrication, operational complexity, and compatibility with biological samples. Understanding the fundamental trade-offs between different architectural paradigms is essential for informed design decisions.
Additionally, this comparative analysis aims to identify opportunities for architectural innovation by recognizing gaps in current approaches and potential synergies between different paradigms. The ultimate goal is to advance microfluidic technology toward more versatile, efficient, and accessible platforms that can address emerging challenges in healthcare, environmental monitoring, and fundamental research, while establishing design principles that can guide future development efforts in this rapidly evolving field.
Market Applications and Demand Analysis for Microfluidic Technologies
The microfluidic technology market has experienced significant growth over the past decade, driven by increasing applications across healthcare, pharmaceuticals, and life sciences. The global microfluidics market was valued at approximately 20 billion USD in 2022 and is projected to reach 42 billion USD by 2027, growing at a CAGR of 16%. This robust growth reflects the expanding demand for point-of-care diagnostics, drug delivery systems, and analytical devices that leverage various microfluidic architectures.
Healthcare applications represent the largest market segment for microfluidic technologies, accounting for nearly 45% of the total market share. Within this segment, point-of-care diagnostics has emerged as a primary driver, with demand accelerating during the COVID-19 pandemic. Different fluidic architectures, particularly pressure-driven and capillary-driven systems, have found widespread adoption in rapid diagnostic tests and portable analyzers.
Pharmaceutical research and drug discovery constitute another significant market segment, valued at approximately 6 billion USD. The demand for high-throughput screening platforms and organ-on-chip systems has created opportunities for digital microfluidics and droplet-based architectures. These technologies enable precise manipulation of small fluid volumes, reducing reagent consumption and accelerating drug development timelines.
The life sciences research sector demonstrates growing interest in microfluidic technologies for single-cell analysis, genomics, and proteomics. This market segment is expected to grow at 18% annually through 2027, with academic and research institutions increasingly adopting centrifugal microfluidics and electrokinetic systems for specialized applications.
Regional analysis reveals that North America dominates the microfluidics market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is witnessing the fastest growth rate at 20% annually, driven by increasing healthcare infrastructure investments and expanding biotechnology sectors in China, Japan, and South Korea.
End-user preferences regarding fluidic architectures vary significantly across applications. Continuous-flow microfluidics remains dominant in analytical applications, while digital microfluidics is gaining traction in diagnostic platforms. Market surveys indicate that 65% of end-users prioritize reliability and reproducibility when selecting microfluidic architectures, while 55% emphasize ease of integration with existing workflows.
Emerging market trends include increasing demand for integrated microfluidic systems that combine multiple fluidic architectures to achieve complex functionalities. Additionally, there is growing interest in sustainable microfluidic solutions, with 70% of surveyed organizations expressing preference for environmentally friendly materials and manufacturing processes.
Healthcare applications represent the largest market segment for microfluidic technologies, accounting for nearly 45% of the total market share. Within this segment, point-of-care diagnostics has emerged as a primary driver, with demand accelerating during the COVID-19 pandemic. Different fluidic architectures, particularly pressure-driven and capillary-driven systems, have found widespread adoption in rapid diagnostic tests and portable analyzers.
Pharmaceutical research and drug discovery constitute another significant market segment, valued at approximately 6 billion USD. The demand for high-throughput screening platforms and organ-on-chip systems has created opportunities for digital microfluidics and droplet-based architectures. These technologies enable precise manipulation of small fluid volumes, reducing reagent consumption and accelerating drug development timelines.
The life sciences research sector demonstrates growing interest in microfluidic technologies for single-cell analysis, genomics, and proteomics. This market segment is expected to grow at 18% annually through 2027, with academic and research institutions increasingly adopting centrifugal microfluidics and electrokinetic systems for specialized applications.
Regional analysis reveals that North America dominates the microfluidics market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is witnessing the fastest growth rate at 20% annually, driven by increasing healthcare infrastructure investments and expanding biotechnology sectors in China, Japan, and South Korea.
End-user preferences regarding fluidic architectures vary significantly across applications. Continuous-flow microfluidics remains dominant in analytical applications, while digital microfluidics is gaining traction in diagnostic platforms. Market surveys indicate that 65% of end-users prioritize reliability and reproducibility when selecting microfluidic architectures, while 55% emphasize ease of integration with existing workflows.
Emerging market trends include increasing demand for integrated microfluidic systems that combine multiple fluidic architectures to achieve complex functionalities. Additionally, there is growing interest in sustainable microfluidic solutions, with 70% of surveyed organizations expressing preference for environmentally friendly materials and manufacturing processes.
Current Fluidic Architectures and Technical Challenges
Microfluidic chip design has evolved significantly over the past two decades, with several distinct fluidic architectures emerging as dominant paradigms. Currently, the field is primarily divided between continuous flow, droplet-based, digital microfluidics, and paper-based architectures, each with unique advantages and limitations for specific applications.
Continuous flow systems represent the most established architecture, utilizing pressure-driven or electrokinetic forces to move fluids through microchannel networks. While offering precise control over fluid dynamics and excellent reproducibility, these systems face challenges in scaling and parallelization. The requirement for external pumping equipment also limits their portability and increases system complexity.
Droplet-based microfluidics has gained significant traction for high-throughput applications, enabling compartmentalization of reactions in discrete droplets. This architecture excels in applications requiring massive parallelization, such as single-cell analysis and digital PCR. However, technical challenges persist in achieving consistent droplet generation, preventing cross-contamination between droplets, and developing robust methods for droplet manipulation and analysis.
Digital microfluidics (DMF), based on electrowetting principles, offers unprecedented flexibility in fluid handling without fixed channels. By manipulating discrete droplets on an array of electrodes, DMF systems can reconfigure fluid pathways on demand. Despite these advantages, DMF faces significant challenges in surface fouling, electrode degradation, and limited compatibility with certain biological samples. The complexity of control systems and manufacturing processes also presents barriers to widespread adoption.
Paper-based microfluidics has emerged as a cost-effective alternative for point-of-care diagnostics, leveraging capillary action to eliminate the need for external pumps. While offering exceptional affordability and simplicity, these systems struggle with precise fluid control, limited sensitivity, and challenges in implementing complex multi-step assays.
Integration of multiple architectures into hybrid systems represents a growing trend, aiming to combine the strengths of different approaches. However, these hybrid designs introduce new challenges in interface management and system integration.
From a geographical perspective, research leadership in microfluidic architectures shows distinct patterns. North American institutions dominate in fundamental research and commercialization of droplet-based systems, while European research centers excel in continuous flow innovations. Asian institutions, particularly in China, Singapore, and Japan, have made significant advances in paper-based microfluidics and manufacturing technologies.
The field currently faces several cross-cutting technical challenges, including standardization of fabrication methods, development of universal interconnects between components, and creation of user-friendly interfaces that don't require specialized expertise. Material compatibility issues also persist, particularly for applications involving biological samples or harsh chemicals.
Continuous flow systems represent the most established architecture, utilizing pressure-driven or electrokinetic forces to move fluids through microchannel networks. While offering precise control over fluid dynamics and excellent reproducibility, these systems face challenges in scaling and parallelization. The requirement for external pumping equipment also limits their portability and increases system complexity.
Droplet-based microfluidics has gained significant traction for high-throughput applications, enabling compartmentalization of reactions in discrete droplets. This architecture excels in applications requiring massive parallelization, such as single-cell analysis and digital PCR. However, technical challenges persist in achieving consistent droplet generation, preventing cross-contamination between droplets, and developing robust methods for droplet manipulation and analysis.
Digital microfluidics (DMF), based on electrowetting principles, offers unprecedented flexibility in fluid handling without fixed channels. By manipulating discrete droplets on an array of electrodes, DMF systems can reconfigure fluid pathways on demand. Despite these advantages, DMF faces significant challenges in surface fouling, electrode degradation, and limited compatibility with certain biological samples. The complexity of control systems and manufacturing processes also presents barriers to widespread adoption.
Paper-based microfluidics has emerged as a cost-effective alternative for point-of-care diagnostics, leveraging capillary action to eliminate the need for external pumps. While offering exceptional affordability and simplicity, these systems struggle with precise fluid control, limited sensitivity, and challenges in implementing complex multi-step assays.
Integration of multiple architectures into hybrid systems represents a growing trend, aiming to combine the strengths of different approaches. However, these hybrid designs introduce new challenges in interface management and system integration.
From a geographical perspective, research leadership in microfluidic architectures shows distinct patterns. North American institutions dominate in fundamental research and commercialization of droplet-based systems, while European research centers excel in continuous flow innovations. Asian institutions, particularly in China, Singapore, and Japan, have made significant advances in paper-based microfluidics and manufacturing technologies.
The field currently faces several cross-cutting technical challenges, including standardization of fabrication methods, development of universal interconnects between components, and creation of user-friendly interfaces that don't require specialized expertise. Material compatibility issues also persist, particularly for applications involving biological samples or harsh chemicals.
Comparative Analysis of Existing Fluidic Architecture Solutions
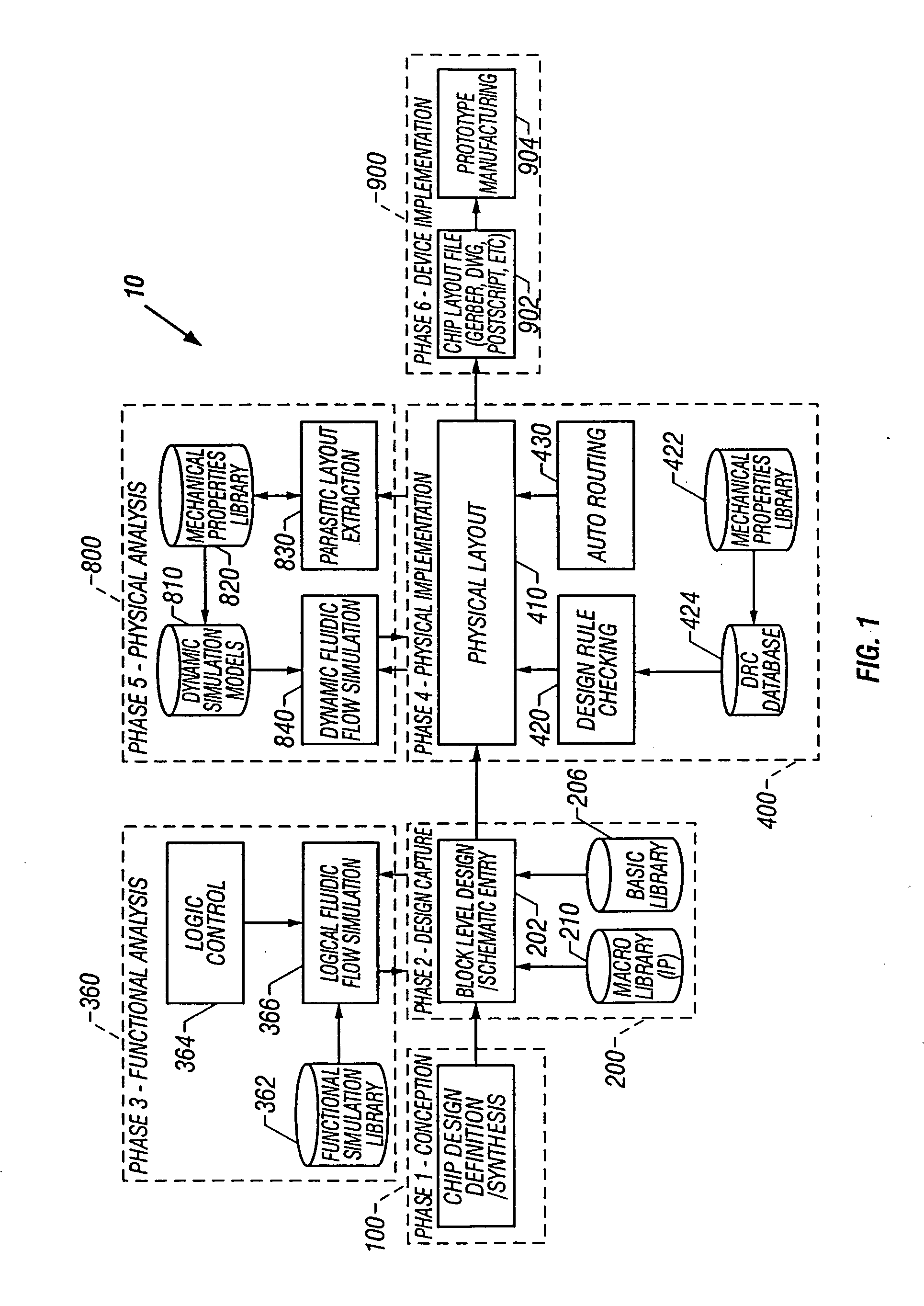
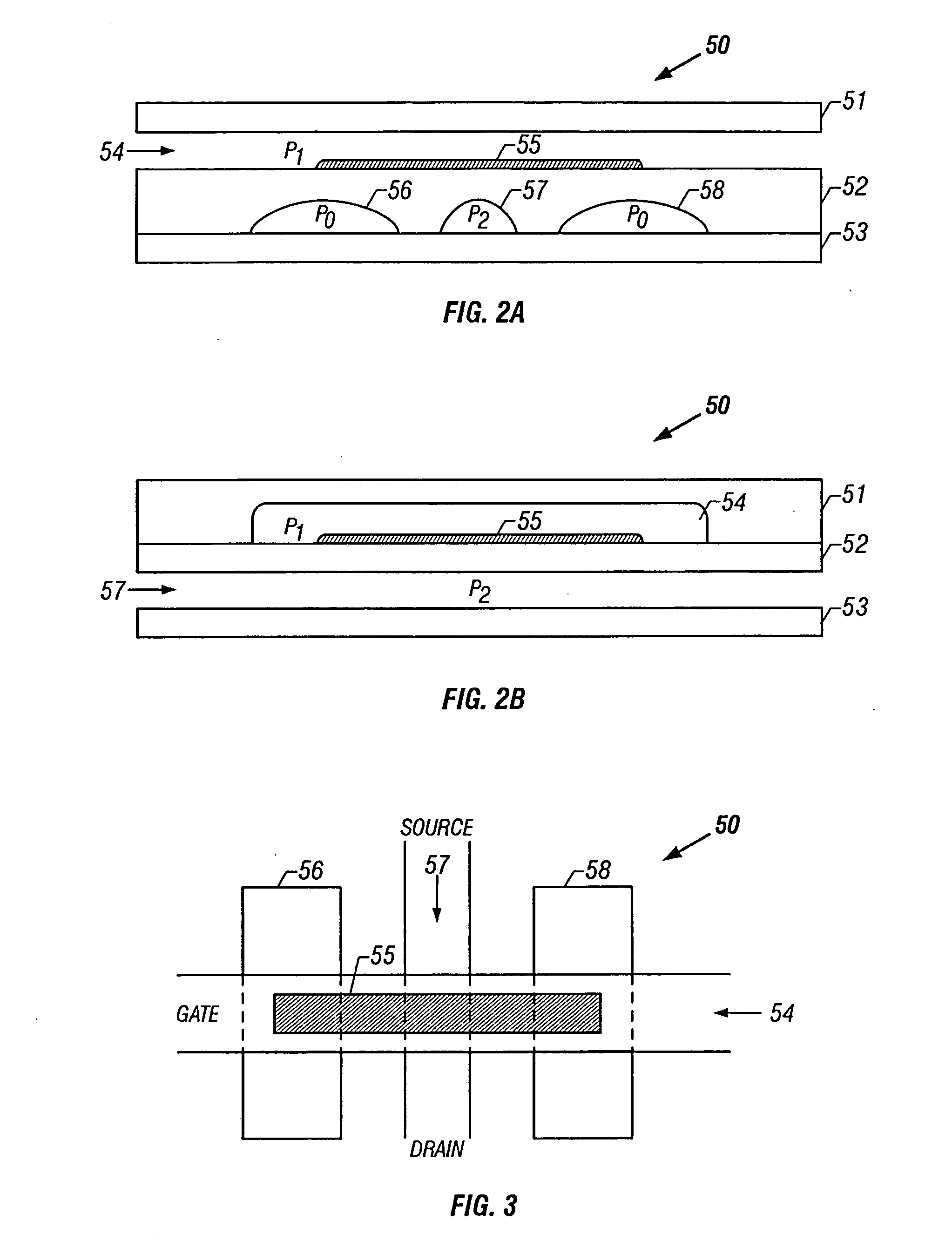
01 Channel design and fluid flow control
Microfluidic chips incorporate specialized channel designs to control fluid flow within the device. These designs include various geometries, dimensions, and surface treatments that influence flow characteristics. Advanced channel architectures enable precise manipulation of fluids, allowing for controlled mixing, separation, and reaction processes. The channel design can incorporate features such as valves, pumps, and flow restrictors to regulate fluid movement throughout the microfluidic network.- Microfluidic channel design and fabrication: Microfluidic chips can be designed with various channel architectures to control fluid flow. These designs include straight channels, serpentine patterns, and branching networks that can be optimized for specific applications. Advanced fabrication techniques allow for precise control over channel dimensions, surface properties, and integration of functional elements. The channel geometry significantly impacts flow characteristics, mixing efficiency, and overall chip performance.
- Fluid control and manipulation systems: Microfluidic architectures incorporate various mechanisms for controlling and manipulating fluids within the chip. These include valves, pumps, mixers, and flow regulators that enable precise control over fluid movement, direction, and interaction. Advanced designs may feature pressure-driven systems, electrokinetic flow control, or capillary-based passive flow mechanisms. These control elements are essential for complex operations such as sample preparation, reagent mixing, and sequential processing.
- Integration of detection and analysis components: Modern microfluidic chip designs integrate various detection and analysis components directly into the fluidic architecture. These may include optical detection zones, electrochemical sensors, or spectroscopic elements that enable real-time monitoring of reactions and processes. The strategic placement of these components within the flow path optimizes sensitivity and accuracy while minimizing sample volume requirements. This integration is crucial for applications in diagnostics, environmental monitoring, and analytical chemistry.
- Droplet-based microfluidic architectures: Droplet-based microfluidic systems represent a specialized architecture where discrete droplets serve as individual reaction vessels. These designs incorporate droplet generation structures, fusion chambers, and sorting mechanisms to enable high-throughput operations. The architecture typically features T-junctions or flow-focusing geometries for controlled droplet formation, along with specialized channels for droplet manipulation. This approach allows for massive parallelization of reactions while minimizing cross-contamination and reagent consumption.
- 3D and multilayer microfluidic architectures: Advanced microfluidic chip designs utilize three-dimensional and multilayer architectures to increase functionality within a compact footprint. These designs feature vertical connections between layers, allowing for complex routing of fluids in three dimensions. The multilayer approach enables the separation of different functional elements while facilitating their interconnection through carefully designed vias and bridges. This architecture is particularly valuable for complex analytical processes requiring multiple sequential or parallel operations.
02 Integration of functional components
Modern microfluidic chip designs integrate various functional components to enhance performance and capabilities. These components include sensors, actuators, electrodes, and optical elements that enable detection, analysis, and manipulation of samples. The integration of these elements requires careful consideration of spatial arrangement, material compatibility, and fabrication techniques. This approach allows for the development of complete lab-on-a-chip systems that can perform multiple analytical functions in a single device.Expand Specific Solutions03 Multilayer and 3D fluidic architectures
Advanced microfluidic designs utilize multilayer and three-dimensional architectures to increase functionality and efficiency. These structures allow for complex fluid routing, increased integration density, and improved performance. By stacking multiple functional layers, designers can create sophisticated networks that enable parallel processing and reduce the overall footprint of the device. 3D architectures also facilitate the implementation of vertical fluid transport and interconnections between different functional regions of the chip.Expand Specific Solutions04 Modular and reconfigurable designs
Modular microfluidic architectures allow for flexible and reconfigurable systems that can be adapted for different applications. These designs incorporate standardized interfaces and connection methods that enable the assembly of various functional modules into customized systems. Reconfigurable platforms provide versatility in research and development settings, allowing researchers to modify chip configurations without complete redesign. This approach reduces development time and costs while increasing the adaptability of microfluidic systems.Expand Specific Solutions05 Novel fabrication techniques for complex architectures
Innovative fabrication methods enable the creation of increasingly complex microfluidic architectures. These techniques include advanced lithography, 3D printing, laser ablation, and soft lithography approaches that allow for precise control over feature dimensions and surface properties. Novel materials and manufacturing processes facilitate the integration of different functional elements and improve the overall performance of microfluidic devices. These fabrication advances have expanded the design possibilities for microfluidic chips, enabling previously unattainable geometries and functionalities.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidic chip design market is currently in a growth phase, with increasing adoption across healthcare, diagnostics, and research sectors. The global market size is estimated to reach several billion dollars by 2025, driven by applications in point-of-care testing and personalized medicine. Regarding technical maturity, fluidic architectures show varying levels of development: established players like Agilent Technologies and IBM have advanced commercial platforms, while academic institutions (Caltech, Tsinghua University, Cornell) focus on fundamental innovations. Companies like Rheonix, Curiosity Diagnostics, and microTEC are developing specialized architectures for specific applications, with Fraunhofer-Gesellschaft bridging research-to-market gaps. Asian manufacturers including Shanghai Tianma and BOE Technology are scaling production capabilities, while healthcare-focused firms like Siemens Healthcare and AB Sciex integrate microfluidics into broader diagnostic ecosystems.
Agilent Technologies, Inc.
Technical Solution: Agilent has pioneered advanced microfluidic architectures through their lab-on-a-chip platform that integrates multiple fluidic architectures within a single system. Their technology employs pressure-driven flow systems with integrated electrokinetic components for precise sample manipulation. Agilent's microfluidic chips feature hierarchical channel networks with varying cross-sections to optimize fluid dynamics across different functional regions. Their proprietary High Sensitivity Flow Cell architecture incorporates laminar flow control with minimal dead volume, achieving separation efficiencies exceeding 100,000 theoretical plates. The company has developed hybrid architectures combining pressure-driven and electrokinetic flows, allowing dynamic switching between flow regimes to optimize separation performance for different analyte classes.
Strengths: Superior manufacturing precision with channel tolerances below 2μm; integrated optical detection systems with sub-femtoliter detection volumes; comprehensive software integration for automated workflow control. Weaknesses: Higher production costs compared to simpler designs; requires specialized equipment for operation; more complex user interface with steeper learning curve for new operators.
International Business Machines Corp.
Technical Solution: IBM has developed a distinctive microfluidic architecture called "microfluidic multiplexing technology" that enables high-throughput analysis on a single chip. Their design incorporates a network of microchannels with programmable valves that can be dynamically reconfigured to create different fluidic pathways. IBM's architecture employs a hierarchical approach with primary distribution channels feeding into secondary processing chambers, all controlled through pneumatic membrane valves. Their chips feature integrated electronic sensors for real-time monitoring of fluid flow and composition. IBM has pioneered digital microfluidics using electrowetting principles, allowing discrete droplet manipulation without physical channels. Their most advanced systems incorporate machine learning algorithms that adaptively control flow parameters based on feedback from integrated sensors, optimizing separation efficiency in real-time.
Strengths: Exceptional reconfigurability allowing multiple assay types on a single platform; advanced integration with electronic components; superior automation capabilities with minimal user intervention. Weaknesses: Complex manufacturing process requiring specialized facilities; higher power consumption compared to passive systems; requires sophisticated control systems that increase overall system cost.
Key Patents and Innovations in Microfluidic Chip Design
Microfluidic design automation method and system
PatentInactiveUS20050065735A1
Innovation
- A microfluidic computer-aided design (CAD) system that provides tools for designing, analyzing, and implementing customized microfluidic systems using building block components, including multilayered structures, with features like drawing constraints, I/O port connectivity, and easy layout manipulation, allowing for quick and efficient creation of complex networks for various applications.
Micro-fluidic chip
PatentActiveUS12251698B2
Innovation
- A modular microfluidic chip design comprising multiple units with operation and transition regions, allowing for flexible combination and local repair or replacement of units, with second sub-electrodes in transition regions enabling droplet movement between units.
Manufacturing Processes and Materials for Microfluidic Chips
The manufacturing of microfluidic chips requires specialized processes and materials to achieve the precision necessary for effective fluidic architectures. Traditional fabrication methods include photolithography, soft lithography, and etching techniques. Photolithography, borrowed from semiconductor manufacturing, enables high-resolution patterning but often requires clean room facilities and specialized equipment. Soft lithography, particularly PDMS (polydimethylsiloxane) molding, has become popular due to its accessibility and rapid prototyping capabilities, making it ideal for comparing different fluidic architectures during development phases.
Material selection significantly impacts the performance characteristics of different fluidic architectures. PDMS remains the most widely used material due to its optical transparency, gas permeability, and ease of fabrication. However, its hydrophobicity can cause non-specific adsorption issues in certain biological applications. Glass substrates offer excellent chemical resistance and optical properties but present challenges in complex channel fabrication. Thermoplastics such as PMMA (polymethyl methacrylate), COC (cyclic olefin copolymer), and PC (polycarbonate) have gained traction for mass production scenarios due to their compatibility with injection molding and hot embossing techniques.
Advanced manufacturing approaches like 3D printing are revolutionizing microfluidic chip production, allowing for rapid iteration between different fluidic architectures. Stereolithography (SLA) and digital light processing (DLP) techniques can achieve resolutions suitable for microfluidic features, while two-photon polymerization enables even finer structures. These additive manufacturing methods facilitate the creation of complex 3D channel networks that would be challenging to produce with traditional planar fabrication techniques.
Surface modification processes play a crucial role in optimizing different fluidic architectures. Plasma treatment, chemical functionalization, and coating techniques can alter surface properties to control wettability, prevent fouling, or enable specific biochemical interactions. These modifications are particularly important when comparing pressure-driven versus capillary-driven fluidic architectures, as surface chemistry directly impacts fluid behavior at the microscale.
The integration of functional materials presents another manufacturing consideration when comparing fluidic architectures. Conductive materials for electrodes, magnetically responsive elements, or stimuli-responsive polymers can be incorporated to enable active control over fluid manipulation. The manufacturing complexity increases with the integration of these functional components, requiring multi-step fabrication processes and precise alignment techniques.
Scale-up considerations vary significantly between different fluidic architectures. While some designs may be optimized for research purposes using low-throughput fabrication methods, transitioning to high-volume production requires consideration of manufacturing scalability. Injection molding and roll-to-roll processing offer pathways to mass production but may impose constraints on architectural complexity compared to laboratory prototyping methods.
Material selection significantly impacts the performance characteristics of different fluidic architectures. PDMS remains the most widely used material due to its optical transparency, gas permeability, and ease of fabrication. However, its hydrophobicity can cause non-specific adsorption issues in certain biological applications. Glass substrates offer excellent chemical resistance and optical properties but present challenges in complex channel fabrication. Thermoplastics such as PMMA (polymethyl methacrylate), COC (cyclic olefin copolymer), and PC (polycarbonate) have gained traction for mass production scenarios due to their compatibility with injection molding and hot embossing techniques.
Advanced manufacturing approaches like 3D printing are revolutionizing microfluidic chip production, allowing for rapid iteration between different fluidic architectures. Stereolithography (SLA) and digital light processing (DLP) techniques can achieve resolutions suitable for microfluidic features, while two-photon polymerization enables even finer structures. These additive manufacturing methods facilitate the creation of complex 3D channel networks that would be challenging to produce with traditional planar fabrication techniques.
Surface modification processes play a crucial role in optimizing different fluidic architectures. Plasma treatment, chemical functionalization, and coating techniques can alter surface properties to control wettability, prevent fouling, or enable specific biochemical interactions. These modifications are particularly important when comparing pressure-driven versus capillary-driven fluidic architectures, as surface chemistry directly impacts fluid behavior at the microscale.
The integration of functional materials presents another manufacturing consideration when comparing fluidic architectures. Conductive materials for electrodes, magnetically responsive elements, or stimuli-responsive polymers can be incorporated to enable active control over fluid manipulation. The manufacturing complexity increases with the integration of these functional components, requiring multi-step fabrication processes and precise alignment techniques.
Scale-up considerations vary significantly between different fluidic architectures. While some designs may be optimized for research purposes using low-throughput fabrication methods, transitioning to high-volume production requires consideration of manufacturing scalability. Injection molding and roll-to-roll processing offer pathways to mass production but may impose constraints on architectural complexity compared to laboratory prototyping methods.
Standardization and Integration Challenges in Microfluidic Systems
The standardization of microfluidic systems remains one of the most significant challenges in advancing microfluidic chip design across different fluidic architectures. Unlike the electronics industry, which benefits from well-established standards, the microfluidics field suffers from fragmentation in design approaches, manufacturing processes, and component interfaces. This lack of standardization creates substantial barriers to integration, scalability, and widespread adoption of microfluidic technologies.
When comparing different fluidic architectures—such as pressure-driven flow, electrokinetic systems, centrifugal platforms, and digital microfluidics—the absence of common standards becomes particularly problematic. Each architecture employs distinct operational principles, materials, and control mechanisms, making cross-platform integration exceptionally difficult. For instance, connecting a pressure-driven microfluidic chip to an electrokinetic system often requires custom interface solutions that are neither reusable nor scalable.
Material compatibility presents another critical integration challenge. Various fluidic architectures demand specific material properties: PDMS dominates in pressure-driven systems due to its elasticity and optical transparency, while glass or silicon may be preferred for electrokinetic applications due to surface charge characteristics. These material differences complicate the development of standardized fabrication processes and interconnection technologies that work across multiple architectural paradigms.
The interface between microfluidic chips and external equipment—pumps, controllers, sensors, and analytical instruments—further exemplifies the integration challenge. Each fluidic architecture typically requires specialized connection ports, control systems, and detection methods. The resulting "world-to-chip" interface problem creates significant barriers to plug-and-play functionality that would otherwise accelerate development and adoption.
Data integration represents yet another dimension of the standardization challenge. Different fluidic architectures generate and process data in architecture-specific formats, making data sharing, analysis, and interpretation across platforms exceedingly difficult. The absence of standardized data protocols hampers collaborative research and slows the accumulation of comparable results across different microfluidic approaches.
Industry efforts toward standardization have emerged, including initiatives like the Microfluidics Consortium and ISO Technical Committee 48. However, these efforts remain fragmented and have not yet produced widely adopted standards that address the full spectrum of integration challenges across different fluidic architectures. The development of universal connection interfaces, standardized design tools, and cross-platform control systems represents a critical frontier for advancing the field beyond its current siloed state.
When comparing different fluidic architectures—such as pressure-driven flow, electrokinetic systems, centrifugal platforms, and digital microfluidics—the absence of common standards becomes particularly problematic. Each architecture employs distinct operational principles, materials, and control mechanisms, making cross-platform integration exceptionally difficult. For instance, connecting a pressure-driven microfluidic chip to an electrokinetic system often requires custom interface solutions that are neither reusable nor scalable.
Material compatibility presents another critical integration challenge. Various fluidic architectures demand specific material properties: PDMS dominates in pressure-driven systems due to its elasticity and optical transparency, while glass or silicon may be preferred for electrokinetic applications due to surface charge characteristics. These material differences complicate the development of standardized fabrication processes and interconnection technologies that work across multiple architectural paradigms.
The interface between microfluidic chips and external equipment—pumps, controllers, sensors, and analytical instruments—further exemplifies the integration challenge. Each fluidic architecture typically requires specialized connection ports, control systems, and detection methods. The resulting "world-to-chip" interface problem creates significant barriers to plug-and-play functionality that would otherwise accelerate development and adoption.
Data integration represents yet another dimension of the standardization challenge. Different fluidic architectures generate and process data in architecture-specific formats, making data sharing, analysis, and interpretation across platforms exceedingly difficult. The absence of standardized data protocols hampers collaborative research and slows the accumulation of comparable results across different microfluidic approaches.
Industry efforts toward standardization have emerged, including initiatives like the Microfluidics Consortium and ISO Technical Committee 48. However, these efforts remain fragmented and have not yet produced widely adopted standards that address the full spectrum of integration challenges across different fluidic architectures. The development of universal connection interfaces, standardized design tools, and cross-platform control systems represents a critical frontier for advancing the field beyond its current siloed state.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!