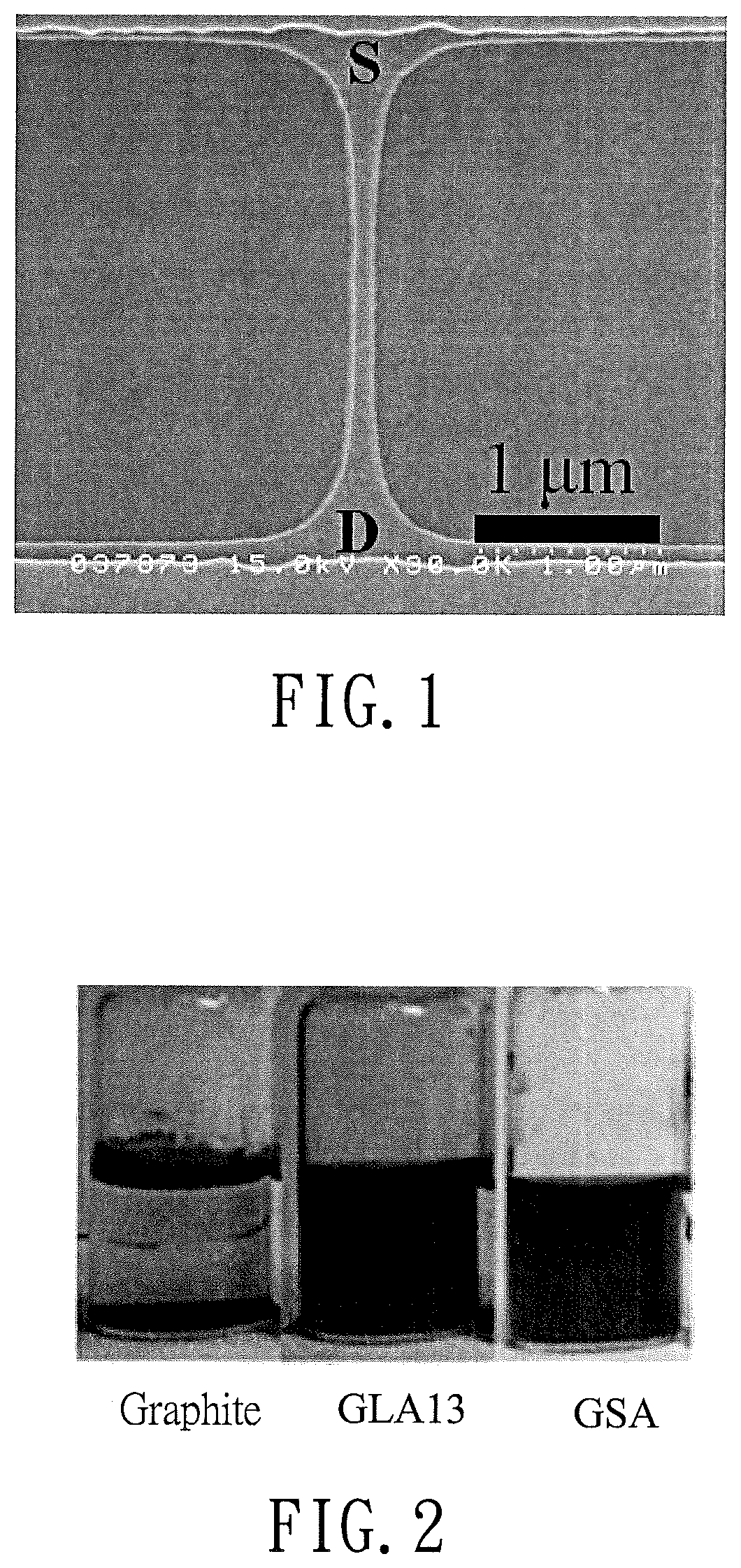
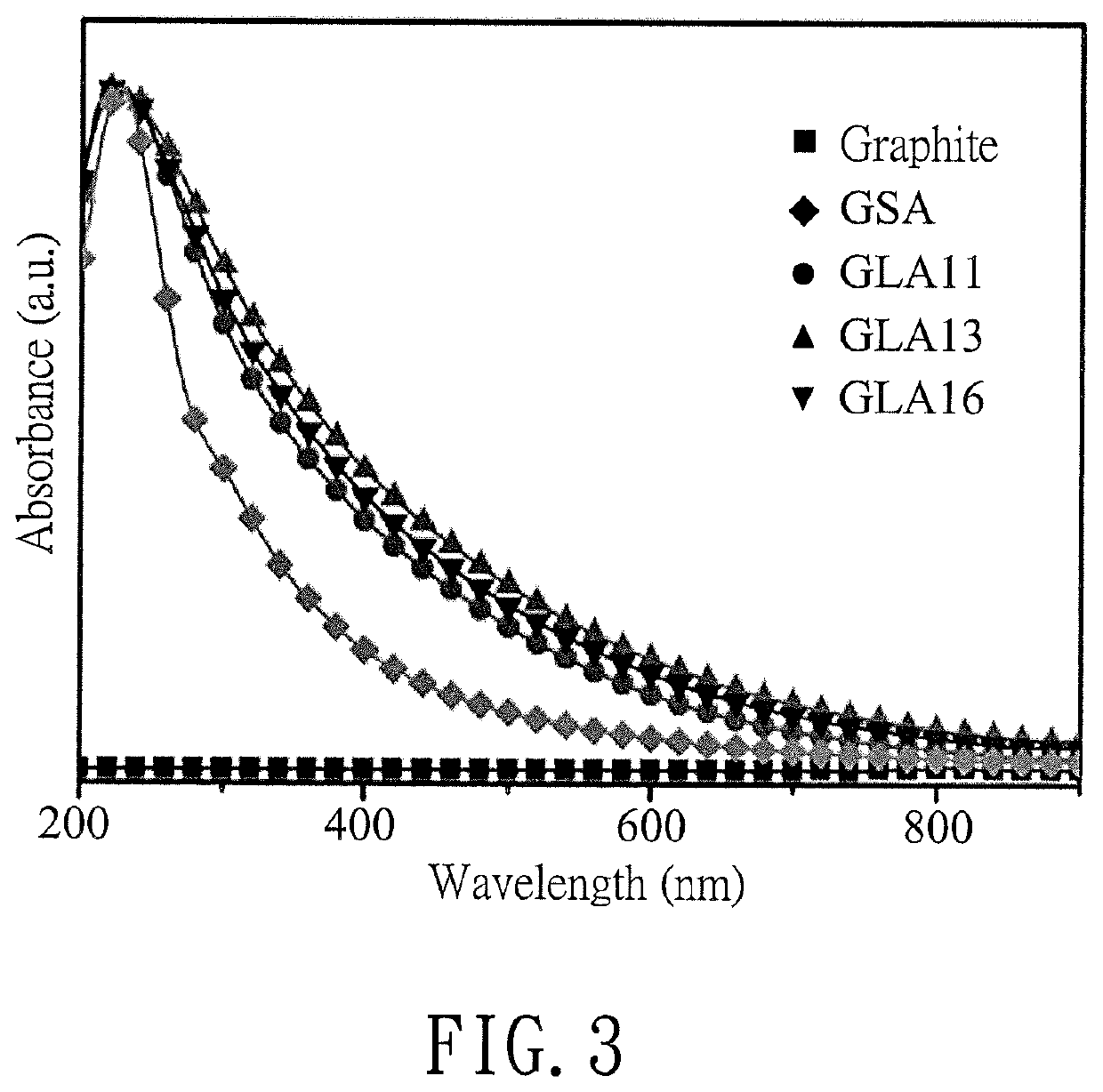
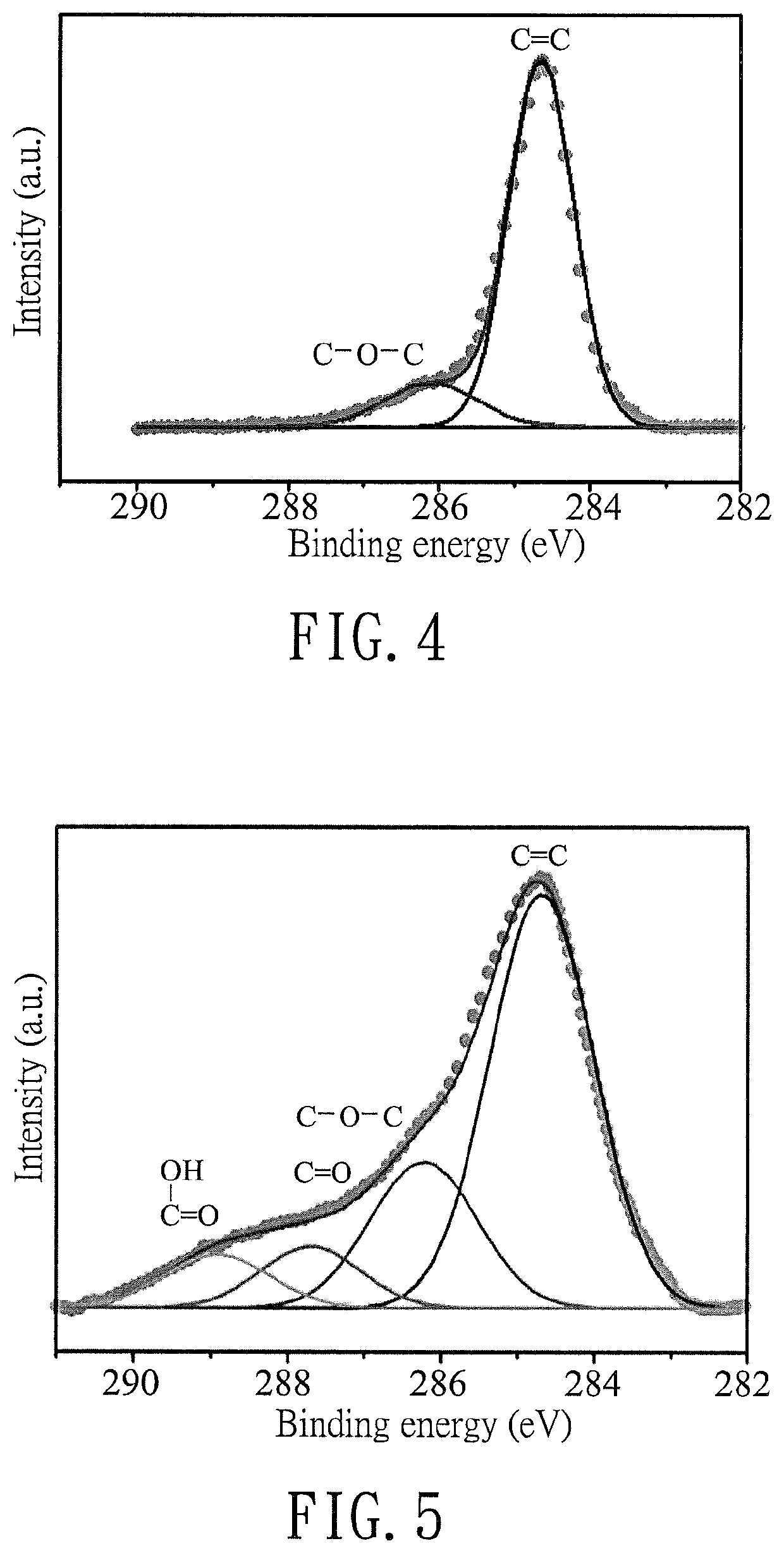
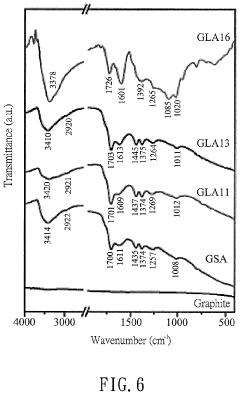
Comparing Reduced Graphene Oxide with Graphite Oxide in Sensors
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Graphene-Based Sensing Technology Background and Objectives
Graphene-based sensing technology has evolved significantly since the groundbreaking isolation of graphene by Geim and Novoselov in 2004, which earned them the Nobel Prize in Physics in 2010. This two-dimensional carbon material, with its exceptional electrical, thermal, and mechanical properties, has revolutionized multiple technological domains, particularly sensing applications. The journey from graphite to graphene oxide (GO) and subsequently to reduced graphene oxide (rGO) represents a critical evolutionary path in materials science that has enabled unprecedented sensing capabilities.
Historically, graphite oxide was first prepared by Brodie in 1859, later improved by Hummers in 1958, establishing what is now known as the Hummers method. The reduction of graphite oxide to graphene oxide emerged as a scalable approach to produce graphene-like materials, addressing the mass production limitations of mechanical exfoliation methods. This technological progression has been driven by the quest for enhanced sensitivity, selectivity, and response time in sensing applications.
The fundamental difference between graphite oxide and reduced graphene oxide lies in their structural and electronic properties. Graphite oxide maintains a layered structure with oxygen-containing functional groups, while reduced graphene oxide partially restores the sp² hybridization network of pristine graphene, significantly improving electrical conductivity. This distinction is crucial for sensing applications where electron transport mechanisms directly influence device performance.
Current research trends focus on optimizing reduction methods to achieve rGO with properties closer to pristine graphene while maintaining processability. Chemical, thermal, microwave, and photocatalytic reduction techniques represent diverse approaches with varying impacts on the resulting material's properties and sensing capabilities. The controlled functionalization of GO and rGO surfaces further expands their application spectrum in selective sensing of gases, biomolecules, and environmental pollutants.
The primary technical objective in comparing rGO with GO in sensing applications is to establish quantitative relationships between material properties and sensing performance metrics. This includes investigating how reduction degree affects sensitivity thresholds, response/recovery times, and long-term stability. Additionally, understanding the interaction mechanisms between target analytes and different oxygen-containing functional groups on GO versus rGO surfaces remains a critical research goal.
Looking forward, the field aims to develop standardized fabrication protocols that ensure reproducible sensor performance, address current limitations in selectivity through strategic functionalization approaches, and integrate these materials with complementary technologies such as MEMS/NEMS platforms. The ultimate goal is to transition from laboratory demonstrations to commercially viable sensing devices that leverage the unique properties of both GO and rGO in targeted applications ranging from healthcare diagnostics to environmental monitoring and industrial process control.
Historically, graphite oxide was first prepared by Brodie in 1859, later improved by Hummers in 1958, establishing what is now known as the Hummers method. The reduction of graphite oxide to graphene oxide emerged as a scalable approach to produce graphene-like materials, addressing the mass production limitations of mechanical exfoliation methods. This technological progression has been driven by the quest for enhanced sensitivity, selectivity, and response time in sensing applications.
The fundamental difference between graphite oxide and reduced graphene oxide lies in their structural and electronic properties. Graphite oxide maintains a layered structure with oxygen-containing functional groups, while reduced graphene oxide partially restores the sp² hybridization network of pristine graphene, significantly improving electrical conductivity. This distinction is crucial for sensing applications where electron transport mechanisms directly influence device performance.
Current research trends focus on optimizing reduction methods to achieve rGO with properties closer to pristine graphene while maintaining processability. Chemical, thermal, microwave, and photocatalytic reduction techniques represent diverse approaches with varying impacts on the resulting material's properties and sensing capabilities. The controlled functionalization of GO and rGO surfaces further expands their application spectrum in selective sensing of gases, biomolecules, and environmental pollutants.
The primary technical objective in comparing rGO with GO in sensing applications is to establish quantitative relationships between material properties and sensing performance metrics. This includes investigating how reduction degree affects sensitivity thresholds, response/recovery times, and long-term stability. Additionally, understanding the interaction mechanisms between target analytes and different oxygen-containing functional groups on GO versus rGO surfaces remains a critical research goal.
Looking forward, the field aims to develop standardized fabrication protocols that ensure reproducible sensor performance, address current limitations in selectivity through strategic functionalization approaches, and integrate these materials with complementary technologies such as MEMS/NEMS platforms. The ultimate goal is to transition from laboratory demonstrations to commercially viable sensing devices that leverage the unique properties of both GO and rGO in targeted applications ranging from healthcare diagnostics to environmental monitoring and industrial process control.
Market Analysis for Graphene Oxide Sensor Applications
The graphene oxide (GO) and reduced graphene oxide (rGO) sensor market has witnessed substantial growth in recent years, driven by their exceptional properties and versatile applications. The global market for graphene-based sensors was valued at approximately $52 million in 2021 and is projected to reach $230 million by 2026, representing a compound annual growth rate (CAGR) of 34.7%. This remarkable growth trajectory underscores the increasing adoption of these materials across various industries.
Healthcare and biomedical applications currently dominate the market landscape, accounting for nearly 35% of the total market share. The superior sensitivity and selectivity of GO and rGO-based biosensors for detecting biomarkers, pathogens, and pharmaceutical compounds have positioned them as preferred alternatives to conventional sensing technologies. The healthcare segment is expected to maintain its leading position, with projected growth exceeding 40% annually through 2028.
Environmental monitoring represents another significant market segment, constituting approximately 28% of the current market. The ability of GO and rGO sensors to detect trace amounts of pollutants, heavy metals, and toxic gases has driven their adoption in environmental applications. This segment is anticipated to grow at a CAGR of 32% over the next five years, fueled by increasingly stringent environmental regulations worldwide.
The electronics and semiconductor industry accounts for roughly 20% of the market, where these materials are utilized in developing next-generation electronic devices, including flexible electronics and wearable technology. The automotive sector represents a smaller but rapidly growing segment at 12%, with applications in fuel cells, batteries, and various sensing systems.
Regionally, North America leads the market with a 38% share, followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 39% annually, primarily driven by increasing investments in research and development activities in China, Japan, and South Korea.
A notable market trend is the shift from graphite oxide toward reduced graphene oxide in sensor applications. While graphite oxide sensors held approximately 60% market share in 2020, this figure is projected to decrease to 45% by 2025, with rGO sensors gaining prominence due to their enhanced electrical conductivity and sensing capabilities. This transition is particularly evident in applications requiring higher sensitivity and faster response times.
The market is characterized by intense competition among key players including Graphenea, ACS Material, Abalonyx, and Garmor. Strategic collaborations between material manufacturers and sensor developers have become increasingly common, aimed at accelerating commercialization efforts and expanding application portfolios.
Healthcare and biomedical applications currently dominate the market landscape, accounting for nearly 35% of the total market share. The superior sensitivity and selectivity of GO and rGO-based biosensors for detecting biomarkers, pathogens, and pharmaceutical compounds have positioned them as preferred alternatives to conventional sensing technologies. The healthcare segment is expected to maintain its leading position, with projected growth exceeding 40% annually through 2028.
Environmental monitoring represents another significant market segment, constituting approximately 28% of the current market. The ability of GO and rGO sensors to detect trace amounts of pollutants, heavy metals, and toxic gases has driven their adoption in environmental applications. This segment is anticipated to grow at a CAGR of 32% over the next five years, fueled by increasingly stringent environmental regulations worldwide.
The electronics and semiconductor industry accounts for roughly 20% of the market, where these materials are utilized in developing next-generation electronic devices, including flexible electronics and wearable technology. The automotive sector represents a smaller but rapidly growing segment at 12%, with applications in fuel cells, batteries, and various sensing systems.
Regionally, North America leads the market with a 38% share, followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 39% annually, primarily driven by increasing investments in research and development activities in China, Japan, and South Korea.
A notable market trend is the shift from graphite oxide toward reduced graphene oxide in sensor applications. While graphite oxide sensors held approximately 60% market share in 2020, this figure is projected to decrease to 45% by 2025, with rGO sensors gaining prominence due to their enhanced electrical conductivity and sensing capabilities. This transition is particularly evident in applications requiring higher sensitivity and faster response times.
The market is characterized by intense competition among key players including Graphenea, ACS Material, Abalonyx, and Garmor. Strategic collaborations between material manufacturers and sensor developers have become increasingly common, aimed at accelerating commercialization efforts and expanding application portfolios.
Current Status and Challenges in rGO vs GO Sensor Development
The global research landscape for graphene-based sensors has witnessed significant growth in the past decade, with reduced graphene oxide (rGO) and graphene oxide (GO) emerging as prominent materials. Currently, both materials are being extensively investigated for sensing applications across multiple domains including environmental monitoring, healthcare diagnostics, and industrial quality control. The development status varies considerably between regions, with Asia-Pacific countries, particularly China and South Korea, leading in patent filings and commercial applications, while North America and Europe focus more on fundamental research and high-precision sensing technologies.
Despite promising advancements, several technical challenges persist in the development of rGO and GO sensors. The primary challenge involves achieving consistent quality and reproducibility in material synthesis. GO production typically employs modified Hummers' method, which often results in batch-to-batch variations affecting sensor performance. Similarly, the reduction process for converting GO to rGO lacks standardization, leading to inconsistent electrical properties and sensing capabilities across different production batches.
Stability represents another significant hurdle, particularly for rGO-based sensors which tend to experience performance degradation over time due to re-oxidation or structural changes when exposed to ambient conditions. GO sensors, while more stable chemically, suffer from limited electrical conductivity that restricts their application in certain sensing platforms requiring higher signal transduction efficiency.
Selectivity remains a critical challenge for both materials. Current GO and rGO sensors often exhibit cross-sensitivity to multiple analytes, limiting their effectiveness in complex real-world environments where multiple interferents may be present. This issue is particularly pronounced in biological sensing applications where sample matrices are inherently complex.
The scalable manufacturing of these sensors constitutes another major constraint. Laboratory-scale production methods often fail to translate effectively to industrial-scale manufacturing, creating a significant gap between research prototypes and commercially viable products. The integration of GO and rGO with conventional electronics also presents compatibility issues, particularly regarding interface engineering and signal processing.
From a commercial perspective, cost factors continue to impede widespread adoption. While GO production costs have decreased, high-quality rGO still requires expensive reduction processes. Additionally, the environmental impact of chemical reduction methods used for rGO production raises sustainability concerns that must be addressed for long-term viability.
Recent research has begun focusing on hybrid systems combining GO or rGO with other nanomaterials to overcome these limitations, though such approaches introduce additional complexity to the manufacturing process and may affect long-term stability.
Despite promising advancements, several technical challenges persist in the development of rGO and GO sensors. The primary challenge involves achieving consistent quality and reproducibility in material synthesis. GO production typically employs modified Hummers' method, which often results in batch-to-batch variations affecting sensor performance. Similarly, the reduction process for converting GO to rGO lacks standardization, leading to inconsistent electrical properties and sensing capabilities across different production batches.
Stability represents another significant hurdle, particularly for rGO-based sensors which tend to experience performance degradation over time due to re-oxidation or structural changes when exposed to ambient conditions. GO sensors, while more stable chemically, suffer from limited electrical conductivity that restricts their application in certain sensing platforms requiring higher signal transduction efficiency.
Selectivity remains a critical challenge for both materials. Current GO and rGO sensors often exhibit cross-sensitivity to multiple analytes, limiting their effectiveness in complex real-world environments where multiple interferents may be present. This issue is particularly pronounced in biological sensing applications where sample matrices are inherently complex.
The scalable manufacturing of these sensors constitutes another major constraint. Laboratory-scale production methods often fail to translate effectively to industrial-scale manufacturing, creating a significant gap between research prototypes and commercially viable products. The integration of GO and rGO with conventional electronics also presents compatibility issues, particularly regarding interface engineering and signal processing.
From a commercial perspective, cost factors continue to impede widespread adoption. While GO production costs have decreased, high-quality rGO still requires expensive reduction processes. Additionally, the environmental impact of chemical reduction methods used for rGO production raises sustainability concerns that must be addressed for long-term viability.
Recent research has begun focusing on hybrid systems combining GO or rGO with other nanomaterials to overcome these limitations, though such approaches introduce additional complexity to the manufacturing process and may affect long-term stability.
Comparative Analysis of rGO and GO Sensor Solutions
01 Synthesis methods for reduced graphene oxide
Various methods can be employed to synthesize reduced graphene oxide (rGO) from graphite oxide. These include chemical reduction using reducing agents, thermal reduction at elevated temperatures, electrochemical reduction, and photocatalytic reduction. Each method offers different advantages in terms of efficiency, scalability, and the quality of the resulting rGO. The reduction process removes oxygen-containing functional groups from graphite oxide, restoring the sp² carbon network and electrical conductivity.- Synthesis methods for reduced graphene oxide: Various methods can be employed to synthesize reduced graphene oxide from graphite oxide, including chemical reduction, thermal reduction, and electrochemical reduction. These processes remove oxygen-containing functional groups from graphite oxide to restore the sp2 carbon network. The reduction methods significantly affect the properties of the resulting reduced graphene oxide, such as electrical conductivity, surface area, and mechanical strength.
- Applications of reduced graphene oxide in energy storage: Reduced graphene oxide has significant applications in energy storage devices such as supercapacitors, batteries, and fuel cells. Its high surface area, excellent electrical conductivity, and mechanical flexibility make it an ideal material for electrode fabrication. When incorporated into energy storage systems, reduced graphene oxide enhances charge storage capacity, improves cycling stability, and facilitates faster charge/discharge rates compared to conventional materials.
- Functionalization of graphene oxide and reduced graphene oxide: Functionalization of graphene oxide and reduced graphene oxide involves attaching various chemical groups to their surfaces to enhance specific properties or compatibility with other materials. This can be achieved through covalent bonding with organic molecules, polymers, or nanoparticles, or through non-covalent interactions. Functionalized graphene materials exhibit improved dispersibility in various solvents, enhanced compatibility with polymer matrices, and tailored properties for specific applications.
- Characterization techniques for graphene oxide and reduced graphene oxide: Various analytical techniques are employed to characterize the structural, chemical, and physical properties of graphene oxide and reduced graphene oxide. These include spectroscopic methods like Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and Fourier-transform infrared spectroscopy (FTIR), as well as microscopic techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM). These characterization methods help determine the degree of oxidation, reduction efficiency, layer thickness, and surface morphology.
- Environmental and biomedical applications of reduced graphene oxide: Reduced graphene oxide finds applications in environmental remediation and biomedical fields due to its unique properties. In environmental applications, it serves as an adsorbent for removing pollutants from water, as a photocatalyst for degrading organic contaminants, and as a component in sensors for detecting environmental pollutants. In biomedical applications, reduced graphene oxide is used in drug delivery systems, tissue engineering scaffolds, biosensors, and antimicrobial coatings, leveraging its large surface area, biocompatibility, and functionalization potential.
02 Applications of reduced graphene oxide in energy storage
Reduced graphene oxide has significant applications in energy storage devices due to its excellent electrical conductivity, large surface area, and mechanical strength. It is used in the development of high-performance electrodes for batteries, supercapacitors, and fuel cells. When incorporated into these devices, rGO enhances charge transport, increases energy density, and improves cycling stability. The combination of rGO with other materials creates composite electrodes with superior electrochemical properties.Expand Specific Solutions03 Production of graphite oxide and its characteristics
Graphite oxide is typically produced through the oxidation of graphite using strong oxidizing agents such as potassium permanganate in acidic conditions, known as modified Hummers method. The resulting material contains various oxygen-containing functional groups including hydroxyl, epoxy, and carboxyl groups on its basal planes and edges. These functional groups make graphite oxide hydrophilic and easily dispersible in water, forming stable colloidal suspensions. The degree of oxidation can be controlled to tailor the properties of the resulting material.Expand Specific Solutions04 Functionalization of reduced graphene oxide
Reduced graphene oxide can be functionalized with various chemical groups or molecules to enhance its properties or impart new functionalities. Functionalization can be achieved through covalent bonding to residual oxygen groups or through non-covalent interactions such as π-π stacking. This process allows for the creation of specialized materials for specific applications including sensors, catalysts, and biomedical devices. Functionalized rGO often shows improved dispersibility, biocompatibility, or selective binding capabilities.Expand Specific Solutions05 Environmental and industrial applications of graphene-based materials
Reduced graphene oxide and graphite oxide have numerous environmental and industrial applications. They are used in water purification systems for the removal of heavy metals and organic pollutants due to their high adsorption capacity. In industrial settings, these materials serve as additives in polymers and composites to enhance mechanical, thermal, and electrical properties. They also find applications in catalysis, sensors, and protective coatings. The scalable production of these materials enables their widespread use in various technological fields.Expand Specific Solutions
Key Industry Players in Graphene-Based Sensor Manufacturing
The graphene oxide sensor market is currently in a growth phase, characterized by increasing research activities and commercial applications. The competition between reduced graphene oxide (rGO) and graphite oxide in sensor technologies reflects the industry's evolution toward more efficient nanomaterials. The market is expanding rapidly, driven by demand for high-performance sensing devices across healthcare, environmental monitoring, and industrial sectors. Academic institutions like Tianjin University, Indian Institute of Science, and University of Electronic Science & Technology of China are advancing fundamental research, while companies such as Global Graphene Group, LG Electronics, and Dioxide Materials are commercializing these technologies. The technical maturity varies significantly, with rGO demonstrating superior electrical conductivity and sensing capabilities compared to graphite oxide, though manufacturing scalability remains a challenge for widespread industrial adoption.
GLOBAL GRAPHENE GROUP INC
Technical Solution: Global Graphene Group has developed advanced sensor technologies utilizing reduced graphene oxide (rGO) with superior performance compared to graphite oxide (GO). Their proprietary reduction processes create rGO with higher electrical conductivity (>1000 S/m compared to GO's <10 S/m) while maintaining excellent surface functionality[1]. Their sensors incorporate rGO-based nanocomposites that demonstrate enhanced sensitivity to various analytes including gases, biomolecules, and environmental pollutants. The company's patented manufacturing techniques allow for scalable production of rGO with consistent quality, achieving sheet resistance below 100 Ω/sq[3]. Their sensors exhibit faster response times (<5 seconds) and lower detection limits (parts-per-billion range) compared to traditional GO-based sensors, making them suitable for real-time monitoring applications in healthcare, environmental protection, and industrial safety[5].
Strengths: Superior electrical conductivity enabling higher sensitivity; scalable manufacturing process; faster response times; lower detection limits. Weaknesses: Higher production costs compared to GO-based sensors; potential for reduced shelf life due to rGO's reactivity with environmental factors; requires more complex integration into existing sensing platforms.
Suzhou Institute of Nano-Tech & Nano-Bionics (SINANO)
Technical Solution: SINANO has pioneered innovative approaches in comparing and optimizing rGO and GO for sensing applications. Their research demonstrates that rGO-based sensors exhibit significantly higher sensitivity (up to 20 times greater) and faster response times compared to GO counterparts for detecting various gases and biomolecules[2]. SINANO's proprietary controlled reduction process preserves critical oxygen functional groups while restoring sp² carbon networks, achieving an optimal balance between conductivity and selectivity. Their sensors incorporate hierarchical nanostructures combining rGO with metal oxide nanoparticles, creating synergistic effects that enhance sensing performance. SINANO has developed flexible rGO-based sensor arrays with multi-analyte detection capabilities operating at room temperature, consuming minimal power (μW range), and demonstrating remarkable stability over extended periods (>6 months)[4]. Their comparative studies have established quantitative structure-property relationships between reduction degree and sensing parameters across different target analytes.
Strengths: Exceptional balance between conductivity and selectivity; room temperature operation; low power consumption; multi-analyte detection capabilities; long-term stability. Weaknesses: Complex fabrication processes requiring precise control of reduction parameters; potential batch-to-batch variations affecting reproducibility; higher material costs compared to conventional sensing materials.
Critical Patents and Research on Graphene Oxide Sensor Technologies
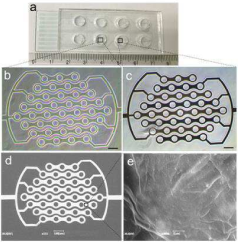
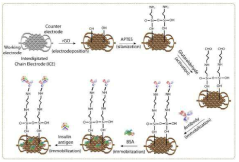
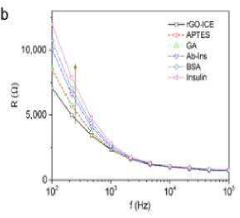
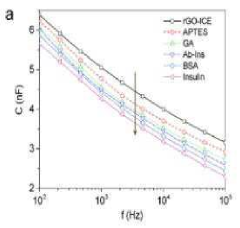
Reduced Graphene Oxide Modified Interdigitated Chain Electrode, method for preparing thereof, the rGO-ICE based Insulin Sensor, and method for preparing thereof
PatentInactiveKR1020170085320A
Innovation
- The development of an rGO-modified Interdigitated Chain Electrode (ICE) with rounded corners and wrinkled graphene oxide sheets, which reduces the corner concentration effect and enhances sensitivity across the entire electrode surface.
Reduced graphene oxide-based biosensor and use thereof
PatentActiveUS10598658B2
Innovation
- A reduced graphene oxide-based biosensor with a linking moiety of —(C═O)—X—COOH, where X represents a C1-C3 alkenylene or alkylene group, is developed to enhance receptor immobilization and bioactivity by increasing the chain length and space for analyte targeting.
Environmental Impact and Sustainability of Graphene Oxide Production
The production of graphene oxide (GO) and reduced graphene oxide (rGO) presents significant environmental considerations that must be evaluated when comparing their applications in sensor technologies. The synthesis of GO typically involves the oxidation of graphite using strong oxidizing agents such as potassium permanganate in concentrated sulfuric acid, known as the modified Hummers method. This process generates hazardous waste streams containing heavy metals and strong acids that require careful treatment and disposal.
Reduced graphene oxide production introduces additional environmental challenges. The reduction process often employs hydrazine, sodium borohydride, or thermal treatments, all of which have varying environmental footprints. Hydrazine, while effective, is highly toxic and carcinogenic, posing risks to both human health and aquatic ecosystems if improperly managed. Thermal reduction methods, though avoiding toxic chemicals, consume significant energy, contributing to carbon emissions when non-renewable energy sources are used.
Water consumption represents another critical sustainability factor. GO production requires substantial volumes of water for washing and purification steps to remove residual acids and oxidizing agents. This creates potential for water pollution if wastewater treatment is inadequate. Comparatively, rGO production may require less water during the reduction phase but still inherits the water footprint from its GO precursor.
Recent advancements have focused on developing greener synthesis routes. Eco-friendly reducing agents such as ascorbic acid, green tea extracts, and bacterial reduction have emerged as promising alternatives for rGO production. These approaches significantly reduce toxicity concerns while maintaining acceptable electrical and mechanical properties for sensor applications.
Life cycle assessment (LCA) studies indicate that the environmental impact of graphene-based materials varies significantly depending on production scale and methodology. Small-scale laboratory production typically shows higher environmental costs per gram than industrial-scale operations, which benefit from process optimization and energy efficiency improvements.
Recycling and circular economy principles are increasingly being applied to graphene oxide production. Research demonstrates that spent graphene oxide from certain sensor applications can be recovered, regenerated, and reused, potentially reducing the overall environmental footprint of these materials in sensing technologies.
The sustainability comparison between GO and rGO in sensor applications must also consider performance longevity. While rGO-based sensors may require more resource-intensive production, their enhanced stability and longer functional lifespan could offset initial environmental costs compared to less durable GO-based alternatives, presenting a complex sustainability equation that extends beyond production impacts alone.
Reduced graphene oxide production introduces additional environmental challenges. The reduction process often employs hydrazine, sodium borohydride, or thermal treatments, all of which have varying environmental footprints. Hydrazine, while effective, is highly toxic and carcinogenic, posing risks to both human health and aquatic ecosystems if improperly managed. Thermal reduction methods, though avoiding toxic chemicals, consume significant energy, contributing to carbon emissions when non-renewable energy sources are used.
Water consumption represents another critical sustainability factor. GO production requires substantial volumes of water for washing and purification steps to remove residual acids and oxidizing agents. This creates potential for water pollution if wastewater treatment is inadequate. Comparatively, rGO production may require less water during the reduction phase but still inherits the water footprint from its GO precursor.
Recent advancements have focused on developing greener synthesis routes. Eco-friendly reducing agents such as ascorbic acid, green tea extracts, and bacterial reduction have emerged as promising alternatives for rGO production. These approaches significantly reduce toxicity concerns while maintaining acceptable electrical and mechanical properties for sensor applications.
Life cycle assessment (LCA) studies indicate that the environmental impact of graphene-based materials varies significantly depending on production scale and methodology. Small-scale laboratory production typically shows higher environmental costs per gram than industrial-scale operations, which benefit from process optimization and energy efficiency improvements.
Recycling and circular economy principles are increasingly being applied to graphene oxide production. Research demonstrates that spent graphene oxide from certain sensor applications can be recovered, regenerated, and reused, potentially reducing the overall environmental footprint of these materials in sensing technologies.
The sustainability comparison between GO and rGO in sensor applications must also consider performance longevity. While rGO-based sensors may require more resource-intensive production, their enhanced stability and longer functional lifespan could offset initial environmental costs compared to less durable GO-based alternatives, presenting a complex sustainability equation that extends beyond production impacts alone.
Standardization and Quality Control in Graphene-Based Sensors
Standardization and quality control are critical aspects in the development and commercialization of graphene-based sensors, particularly when comparing reduced graphene oxide (rGO) with graphite oxide (GO). The lack of universally accepted standards has been a significant barrier to consistent performance evaluation and reliable comparison between different sensor technologies.
Current standardization efforts primarily focus on establishing protocols for material characterization, including sheet resistance, layer thickness, defect density, and functional group distribution. For rGO-based sensors, standardization must address the variable reduction processes that significantly impact sensing performance. Thermal, chemical, and electrochemical reduction methods produce rGO with different properties, necessitating standardized reduction protocols to ensure reproducibility.
In contrast, GO standardization focuses on oxidation degree, lateral dimensions, and functional group composition. The Hummers method and its modifications remain the most common GO synthesis approaches, yet variations in production parameters lead to inconsistent sensor performance. Organizations such as ISO/TC 229 and IEC/TC 113 are developing standards specifically for graphene materials in sensing applications.
Quality control measures for graphene-based sensors include rigorous testing protocols for sensitivity, selectivity, response time, and long-term stability. Raman spectroscopy serves as a primary quality control tool, with the ID/IG ratio providing critical information about defect density in both GO and rGO. X-ray photoelectron spectroscopy (XPS) enables quantitative assessment of oxygen-containing functional groups, essential for understanding the chemical properties affecting sensor performance.
Electrical characterization through four-point probe measurements provides standardized sheet resistance values, while atomic force microscopy (AFM) and scanning electron microscopy (SEM) offer morphological information critical for sensor quality assessment. Interlaboratory comparisons have revealed significant variations in reported sensor parameters, highlighting the urgent need for reference materials and calibration standards.
Recent advancements include the development of certified reference materials for GO and rGO, enabling more reliable benchmarking of sensor performance. Statistical process control methods are increasingly implemented in manufacturing environments to monitor key quality parameters throughout production. Machine learning algorithms are being integrated into quality control systems to predict sensor performance based on material characteristics, potentially revolutionizing quality assurance in graphene sensor production.
The establishment of comprehensive standardization and quality control frameworks will accelerate the commercialization of graphene-based sensors by ensuring consistent performance, facilitating meaningful comparisons between different sensor technologies, and building consumer confidence in these emerging sensing platforms.
Current standardization efforts primarily focus on establishing protocols for material characterization, including sheet resistance, layer thickness, defect density, and functional group distribution. For rGO-based sensors, standardization must address the variable reduction processes that significantly impact sensing performance. Thermal, chemical, and electrochemical reduction methods produce rGO with different properties, necessitating standardized reduction protocols to ensure reproducibility.
In contrast, GO standardization focuses on oxidation degree, lateral dimensions, and functional group composition. The Hummers method and its modifications remain the most common GO synthesis approaches, yet variations in production parameters lead to inconsistent sensor performance. Organizations such as ISO/TC 229 and IEC/TC 113 are developing standards specifically for graphene materials in sensing applications.
Quality control measures for graphene-based sensors include rigorous testing protocols for sensitivity, selectivity, response time, and long-term stability. Raman spectroscopy serves as a primary quality control tool, with the ID/IG ratio providing critical information about defect density in both GO and rGO. X-ray photoelectron spectroscopy (XPS) enables quantitative assessment of oxygen-containing functional groups, essential for understanding the chemical properties affecting sensor performance.
Electrical characterization through four-point probe measurements provides standardized sheet resistance values, while atomic force microscopy (AFM) and scanning electron microscopy (SEM) offer morphological information critical for sensor quality assessment. Interlaboratory comparisons have revealed significant variations in reported sensor parameters, highlighting the urgent need for reference materials and calibration standards.
Recent advancements include the development of certified reference materials for GO and rGO, enabling more reliable benchmarking of sensor performance. Statistical process control methods are increasingly implemented in manufacturing environments to monitor key quality parameters throughout production. Machine learning algorithms are being integrated into quality control systems to predict sensor performance based on material characteristics, potentially revolutionizing quality assurance in graphene sensor production.
The establishment of comprehensive standardization and quality control frameworks will accelerate the commercialization of graphene-based sensors by ensuring consistent performance, facilitating meaningful comparisons between different sensor technologies, and building consumer confidence in these emerging sensing platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!