Research on the Electrochemical Applications of Reduced Graphene Oxide
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Graphene Oxide Reduction Background and Objectives
Graphene oxide (GO) has emerged as a revolutionary material in the field of nanotechnology since its discovery in the early 2000s. As a derivative of graphene, GO possesses unique properties including large surface area, excellent mechanical strength, and remarkable electrical conductivity when reduced. The reduction of graphene oxide represents a critical process that transforms the oxygen-rich, electrically insulating GO into reduced graphene oxide (rGO), which exhibits properties closer to pristine graphene while maintaining some functional groups that enhance its versatility in electrochemical applications.
The evolution of graphene oxide reduction techniques has progressed significantly over the past decade. Initially, thermal reduction methods dominated the field, but these approaches often required high energy inputs and resulted in structural defects. Chemical reduction using hydrazine and other reducing agents subsequently gained popularity, offering more controlled processes at lower temperatures. Recent advancements have introduced environmentally friendly reduction methods including green reducing agents, electrochemical reduction, and photocatalytic approaches that minimize the use of hazardous chemicals while optimizing the reduction efficiency.
Current research trends indicate a growing interest in developing scalable, cost-effective, and environmentally sustainable reduction methods that preserve the structural integrity of graphene sheets while achieving high degrees of reduction. The fine-tuning of reduction parameters to control the type and density of remaining oxygen functional groups has become a focal point, as these characteristics directly influence the electrochemical performance of rGO in various applications.
The primary technical objective of this research is to comprehensively evaluate the relationship between different GO reduction methods and the resulting electrochemical properties of rGO. This includes investigating how various reduction techniques affect the surface chemistry, electrical conductivity, and electrochemical activity of rGO materials. Additionally, we aim to identify optimal reduction protocols for specific electrochemical applications, including energy storage devices, sensors, and electrocatalysts.
Further objectives include exploring novel hybrid reduction approaches that combine multiple reduction mechanisms to achieve synergistic effects, developing in-situ characterization techniques to monitor the reduction process in real-time, and establishing standardized protocols for evaluating the quality and performance of rGO materials in electrochemical systems. These efforts will contribute to the fundamental understanding of structure-property relationships in rGO and facilitate its integration into next-generation electrochemical technologies.
The ultimate goal is to bridge the gap between laboratory-scale production of high-quality rGO and industrial-scale manufacturing processes that can deliver consistent performance for commercial electrochemical applications. This requires addressing challenges related to batch-to-batch reproducibility, scalability of reduction methods, and long-term stability of rGO-based electrochemical systems under real-world operating conditions.
The evolution of graphene oxide reduction techniques has progressed significantly over the past decade. Initially, thermal reduction methods dominated the field, but these approaches often required high energy inputs and resulted in structural defects. Chemical reduction using hydrazine and other reducing agents subsequently gained popularity, offering more controlled processes at lower temperatures. Recent advancements have introduced environmentally friendly reduction methods including green reducing agents, electrochemical reduction, and photocatalytic approaches that minimize the use of hazardous chemicals while optimizing the reduction efficiency.
Current research trends indicate a growing interest in developing scalable, cost-effective, and environmentally sustainable reduction methods that preserve the structural integrity of graphene sheets while achieving high degrees of reduction. The fine-tuning of reduction parameters to control the type and density of remaining oxygen functional groups has become a focal point, as these characteristics directly influence the electrochemical performance of rGO in various applications.
The primary technical objective of this research is to comprehensively evaluate the relationship between different GO reduction methods and the resulting electrochemical properties of rGO. This includes investigating how various reduction techniques affect the surface chemistry, electrical conductivity, and electrochemical activity of rGO materials. Additionally, we aim to identify optimal reduction protocols for specific electrochemical applications, including energy storage devices, sensors, and electrocatalysts.
Further objectives include exploring novel hybrid reduction approaches that combine multiple reduction mechanisms to achieve synergistic effects, developing in-situ characterization techniques to monitor the reduction process in real-time, and establishing standardized protocols for evaluating the quality and performance of rGO materials in electrochemical systems. These efforts will contribute to the fundamental understanding of structure-property relationships in rGO and facilitate its integration into next-generation electrochemical technologies.
The ultimate goal is to bridge the gap between laboratory-scale production of high-quality rGO and industrial-scale manufacturing processes that can deliver consistent performance for commercial electrochemical applications. This requires addressing challenges related to batch-to-batch reproducibility, scalability of reduction methods, and long-term stability of rGO-based electrochemical systems under real-world operating conditions.
Electrochemical Market Demand Analysis
The electrochemical market for reduced graphene oxide (rGO) applications has witnessed substantial growth in recent years, driven by increasing demand for advanced energy storage solutions, sensors, and electrochemical catalysts. The global energy storage market, where rGO plays a significant role, was valued at approximately 210 billion USD in 2022 and is projected to grow at a compound annual growth rate of 8.5% through 2030, creating extensive opportunities for rGO-based technologies.
Supercapacitors represent one of the fastest-growing segments within the electrochemical applications of rGO. The supercapacitor market reached 3.5 billion USD in 2022, with expectations to double by 2028. This growth is primarily fueled by the automotive sector's shift toward electric vehicles and the need for rapid charging capabilities, where rGO-based supercapacitors offer superior power density and cycle stability compared to traditional energy storage technologies.
The lithium-ion battery market, another major application area for rGO, exceeded 50 billion USD in 2022. rGO's incorporation as electrode material enhances battery performance by improving conductivity, mechanical stability, and charge transfer kinetics. Consumer electronics manufacturers and electric vehicle producers are increasingly demanding batteries with higher energy density and faster charging capabilities, positioning rGO as a critical component in next-generation battery technologies.
Electrochemical sensors utilizing rGO have gained significant traction in healthcare, environmental monitoring, and industrial applications. The global electrochemical sensor market reached approximately 20 billion USD in 2022, with biosensors representing the largest segment. rGO-based sensors offer advantages in sensitivity, selectivity, and response time, driving their adoption in point-of-care diagnostics, food safety testing, and environmental pollutant detection.
The water treatment sector presents another substantial market opportunity for rGO electrochemical applications. With growing concerns about water scarcity and contamination, the global water treatment market exceeded 300 billion USD in 2022. rGO-based electrochemical systems for water purification and desalination demonstrate higher efficiency and lower energy consumption compared to conventional methods, addressing critical needs in both developed and developing regions.
Regional analysis indicates that Asia-Pacific dominates the market for rGO electrochemical applications, accounting for over 40% of global demand. This is attributed to the region's robust electronics manufacturing base, accelerating electric vehicle production, and significant investments in renewable energy infrastructure. North America and Europe follow, with increasing research funding and supportive regulatory frameworks for clean energy technologies driving market growth.
Supercapacitors represent one of the fastest-growing segments within the electrochemical applications of rGO. The supercapacitor market reached 3.5 billion USD in 2022, with expectations to double by 2028. This growth is primarily fueled by the automotive sector's shift toward electric vehicles and the need for rapid charging capabilities, where rGO-based supercapacitors offer superior power density and cycle stability compared to traditional energy storage technologies.
The lithium-ion battery market, another major application area for rGO, exceeded 50 billion USD in 2022. rGO's incorporation as electrode material enhances battery performance by improving conductivity, mechanical stability, and charge transfer kinetics. Consumer electronics manufacturers and electric vehicle producers are increasingly demanding batteries with higher energy density and faster charging capabilities, positioning rGO as a critical component in next-generation battery technologies.
Electrochemical sensors utilizing rGO have gained significant traction in healthcare, environmental monitoring, and industrial applications. The global electrochemical sensor market reached approximately 20 billion USD in 2022, with biosensors representing the largest segment. rGO-based sensors offer advantages in sensitivity, selectivity, and response time, driving their adoption in point-of-care diagnostics, food safety testing, and environmental pollutant detection.
The water treatment sector presents another substantial market opportunity for rGO electrochemical applications. With growing concerns about water scarcity and contamination, the global water treatment market exceeded 300 billion USD in 2022. rGO-based electrochemical systems for water purification and desalination demonstrate higher efficiency and lower energy consumption compared to conventional methods, addressing critical needs in both developed and developing regions.
Regional analysis indicates that Asia-Pacific dominates the market for rGO electrochemical applications, accounting for over 40% of global demand. This is attributed to the region's robust electronics manufacturing base, accelerating electric vehicle production, and significant investments in renewable energy infrastructure. North America and Europe follow, with increasing research funding and supportive regulatory frameworks for clean energy technologies driving market growth.
Current Status and Challenges in rGO Electrochemical Applications
The global research landscape for reduced graphene oxide (rGO) in electrochemical applications has witnessed significant growth over the past decade. Currently, rGO is being extensively investigated for applications in energy storage devices, sensors, electrocatalysis, and electrochemical biosensors. The material's exceptional electrical conductivity, large surface area, and excellent mechanical properties make it particularly attractive for these applications. However, despite promising advancements, several technical challenges persist that limit its widespread commercial adoption.
One major challenge facing rGO electrochemical applications is the inconsistency in production methods. Various reduction techniques—chemical, thermal, electrochemical, and microwave-assisted—yield rGO with different properties, making standardization difficult. This variability affects reproducibility in device performance and hinders large-scale manufacturing processes. Additionally, the reduction process often introduces defects in the graphene structure, which can negatively impact electrical conductivity and electrochemical performance.
The scalability of rGO production represents another significant hurdle. While laboratory-scale synthesis can produce high-quality rGO, translating these methods to industrial scale while maintaining quality and cost-effectiveness remains problematic. The environmental impact of certain reduction methods, particularly those using hazardous chemicals like hydrazine, also raises concerns about sustainability and regulatory compliance.
In energy storage applications, rGO-based electrodes face challenges related to long-term stability and cycling performance. The material often suffers from restacking of graphene sheets during cycling, which reduces the accessible surface area and consequently diminishes capacitance over time. This issue is particularly pronounced in supercapacitors and lithium-ion batteries, where maintaining high energy density over thousands of cycles is crucial.
For sensing applications, selectivity remains a persistent challenge. While rGO-based sensors demonstrate high sensitivity, they often lack specificity when exposed to complex sample matrices. This limitation restricts their practical utility in real-world environments where multiple analytes may be present simultaneously.
Geographically, research on rGO electrochemical applications is concentrated primarily in East Asia (particularly China and South Korea), North America, and Europe. China leads in publication output and patent filings, followed by the United States and South Korea. This distribution reflects significant regional variations in research focus and investment, with Asian countries generally emphasizing energy storage applications while Western research centers often focus more on sensing and biomedical applications.
Recent technological advancements have begun addressing some of these challenges through hybrid materials that combine rGO with metal oxides, conductive polymers, or other carbon nanomaterials. These composites often demonstrate synergistic effects that overcome individual material limitations, representing a promising direction for future development in the field.
One major challenge facing rGO electrochemical applications is the inconsistency in production methods. Various reduction techniques—chemical, thermal, electrochemical, and microwave-assisted—yield rGO with different properties, making standardization difficult. This variability affects reproducibility in device performance and hinders large-scale manufacturing processes. Additionally, the reduction process often introduces defects in the graphene structure, which can negatively impact electrical conductivity and electrochemical performance.
The scalability of rGO production represents another significant hurdle. While laboratory-scale synthesis can produce high-quality rGO, translating these methods to industrial scale while maintaining quality and cost-effectiveness remains problematic. The environmental impact of certain reduction methods, particularly those using hazardous chemicals like hydrazine, also raises concerns about sustainability and regulatory compliance.
In energy storage applications, rGO-based electrodes face challenges related to long-term stability and cycling performance. The material often suffers from restacking of graphene sheets during cycling, which reduces the accessible surface area and consequently diminishes capacitance over time. This issue is particularly pronounced in supercapacitors and lithium-ion batteries, where maintaining high energy density over thousands of cycles is crucial.
For sensing applications, selectivity remains a persistent challenge. While rGO-based sensors demonstrate high sensitivity, they often lack specificity when exposed to complex sample matrices. This limitation restricts their practical utility in real-world environments where multiple analytes may be present simultaneously.
Geographically, research on rGO electrochemical applications is concentrated primarily in East Asia (particularly China and South Korea), North America, and Europe. China leads in publication output and patent filings, followed by the United States and South Korea. This distribution reflects significant regional variations in research focus and investment, with Asian countries generally emphasizing energy storage applications while Western research centers often focus more on sensing and biomedical applications.
Recent technological advancements have begun addressing some of these challenges through hybrid materials that combine rGO with metal oxides, conductive polymers, or other carbon nanomaterials. These composites often demonstrate synergistic effects that overcome individual material limitations, representing a promising direction for future development in the field.
Current rGO Synthesis and Modification Approaches
01 Methods of producing reduced graphene oxide
Various methods can be employed to produce reduced graphene oxide, including chemical reduction, thermal reduction, and electrochemical reduction. These processes typically involve removing oxygen-containing functional groups from graphene oxide to restore the sp2 carbon network. The reduction methods can be optimized to control the degree of reduction, which affects the electrical, thermal, and mechanical properties of the resulting material.- Methods for producing reduced graphene oxide: Various methods can be employed to produce reduced graphene oxide (rGO) from graphene oxide. These methods include chemical reduction using reducing agents, thermal reduction at elevated temperatures, electrochemical reduction, and photocatalytic reduction. Each method offers different advantages in terms of efficiency, scalability, and the quality of the resulting rGO. The reduction process removes oxygen-containing functional groups from graphene oxide, restoring the sp2 carbon network and enhancing electrical conductivity.
- Applications of reduced graphene oxide in energy storage devices: Reduced graphene oxide is widely used in energy storage applications due to its excellent electrical conductivity, large surface area, and mechanical stability. It serves as an electrode material in supercapacitors, lithium-ion batteries, and other energy storage devices. When incorporated into these devices, rGO enhances charge transport, increases energy density, and improves cycling stability. The combination of rGO with other materials can create composite electrodes with superior performance characteristics.
- Reduced graphene oxide composites with metal/metal oxide nanoparticles: Hybrid materials combining reduced graphene oxide with metal or metal oxide nanoparticles exhibit enhanced properties for various applications. These composites leverage the synergistic effects between rGO and nanoparticles, resulting in improved catalytic activity, sensing capabilities, and electrical properties. The metal/metal oxide nanoparticles are typically anchored onto the rGO sheets, preventing aggregation and maximizing the available surface area. These hybrid materials find applications in catalysis, sensors, and environmental remediation.
- Reduced graphene oxide in sensing and detection applications: Reduced graphene oxide is utilized in various sensing platforms due to its electrical properties and large surface area. It can detect gases, biomolecules, heavy metals, and other analytes with high sensitivity and selectivity. The sensing mechanism typically involves changes in electrical conductivity upon analyte adsorption or interaction with functional groups on the rGO surface. These sensors can be further enhanced by functionalizing rGO with specific recognition elements or combining it with other nanomaterials to improve performance.
- Functionalization and modification of reduced graphene oxide: Reduced graphene oxide can be functionalized with various chemical groups or molecules to tailor its properties for specific applications. Functionalization can improve dispersibility in different solvents, enhance compatibility with polymer matrices, or introduce specific chemical reactivity. Methods for functionalization include covalent attachment of organic molecules, non-covalent interactions with surfactants or polymers, and doping with heteroatoms. These modifications expand the potential applications of rGO in areas such as composites, biomedical devices, and catalysis.
02 Applications in energy storage devices
Reduced graphene oxide is widely used in energy storage applications such as supercapacitors, lithium-ion batteries, and fuel cells. The material's high surface area, excellent electrical conductivity, and good mechanical properties make it an ideal candidate for electrode materials. When incorporated into energy storage devices, reduced graphene oxide can enhance charge storage capacity, improve cycling stability, and increase power density.Expand Specific Solutions03 Composite materials with reduced graphene oxide
Reduced graphene oxide can be combined with various materials including polymers, metals, and metal oxides to form composites with enhanced properties. These composites often exhibit improved mechanical strength, electrical conductivity, thermal stability, and barrier properties compared to the base materials. The synergistic effects between reduced graphene oxide and the matrix material enable applications in structural components, conductive films, and protective coatings.Expand Specific Solutions04 Sensing and detection applications
The unique electrical, optical, and surface properties of reduced graphene oxide make it suitable for various sensing applications. It can be used in gas sensors, biosensors, chemical sensors, and environmental monitoring devices. The material's high sensitivity to changes in its environment, large surface area for molecule adsorption, and tunable surface functionality enable the detection of various analytes with high sensitivity and selectivity.Expand Specific Solutions05 Functionalization of reduced graphene oxide
Reduced graphene oxide can be functionalized with various chemical groups to tailor its properties for specific applications. Functionalization can improve dispersibility in different solvents, enhance compatibility with other materials, and introduce specific chemical reactivity. Methods of functionalization include covalent attachment of organic molecules, doping with heteroatoms, and decoration with nanoparticles, resulting in materials with customized properties for applications in catalysis, biomedicine, and electronics.Expand Specific Solutions
Key Industry Players in rGO Electrochemical Research
The electrochemical applications of reduced graphene oxide (rGO) market is currently in a growth phase, with increasing adoption across energy storage, sensing, and electronics sectors. The global market size is expanding rapidly, projected to reach significant valuation due to rGO's exceptional conductivity and surface area properties. Leading players include Global Graphene Group, which specializes in commercializing graphene materials, and LG Electronics, integrating rGO into energy solutions. Academic-industrial partnerships are accelerating technology maturation, with institutions like Georgia Tech Research Corp, Nanyang Technological University, and University of Manchester collaborating with companies like Daejoo Electronic Materials and Tata Steel to develop practical applications. Korean entities (KERI, KAERI) are particularly active in advancing rGO electrochemical technologies, indicating regional leadership in this emerging field.
GLOBAL GRAPHENE GROUP INC
Technical Solution: Global Graphene Group has developed advanced reduced graphene oxide (rGO) materials specifically engineered for electrochemical applications. Their proprietary reduction processes create rGO with controlled oxygen content and defect density, optimizing electrical conductivity while maintaining high surface area. The company's technology includes a scalable manufacturing platform that produces high-quality rGO sheets with thickness control down to single-layer precision. Their materials are incorporated into next-generation energy storage devices, including supercapacitors with power densities exceeding 15 kW/kg and lithium-ion battery anodes with capacities approaching 1000 mAh/g. The company has also developed rGO-based electrochemical sensors capable of detecting analytes at concentrations below 1 ppb, utilizing the material's excellent electron transfer properties and high electroactive surface area.
Strengths: Proprietary scalable manufacturing process allows for consistent, high-volume production of rGO with tailored properties. Their materials demonstrate superior electrical conductivity and electrochemical performance compared to conventionally reduced graphene oxide. Weaknesses: Higher production costs compared to traditional carbon materials may limit adoption in price-sensitive applications. Some reduction methods may introduce contaminants that affect electrochemical performance in sensitive applications.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech Research Corporation has pioneered innovative approaches to reduced graphene oxide (rGO) synthesis and application in electrochemical systems. Their research focuses on developing environmentally friendly reduction methods using non-toxic reducing agents and green chemistry principles. The team has created a hydrothermal reduction process that maintains high surface area (>1500 m²/g) while achieving excellent electrical conductivity (>1000 S/m). Their rGO materials feature precisely engineered oxygen functional groups that serve as active sites for specific electrochemical reactions, enhancing catalytic activity. Georgia Tech has also developed composite materials combining rGO with metal nanoparticles and conductive polymers, creating synergistic effects that significantly improve electrochemical performance in energy storage and conversion applications. Their rGO-based electrodes have demonstrated exceptional stability, maintaining over 90% capacity after 10,000 charge-discharge cycles in supercapacitor applications.
Strengths: Environmentally sustainable reduction methods reduce toxic waste and environmental impact. Their approach to functional group engineering creates materials with superior selectivity and catalytic activity for specific electrochemical reactions. Weaknesses: Some green reduction methods may result in incomplete reduction, potentially limiting electrical conductivity compared to harsher chemical or thermal reduction processes. Scale-up challenges exist for transitioning from laboratory to industrial production.
Critical Patents and Breakthroughs in rGO Electrochemistry
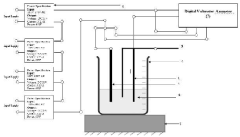
Electrochemical synthesis of graphene oxide (GO)/ reduced graphene oxide (RGO) by using externally applied high voltage (DC voltage)
PatentInactiveIN202011039288A
Innovation
- An electrochemical method involving an aqueous solution of sulfuric acid and DI water with real-time temperature monitoring, using circular graphite as the anode and a circular silver rod as the cathode, under the application of high DC voltage to synthesize Graphene Oxide/Reduced Graphene Oxide.
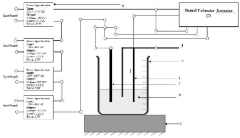
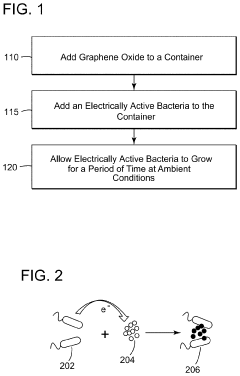
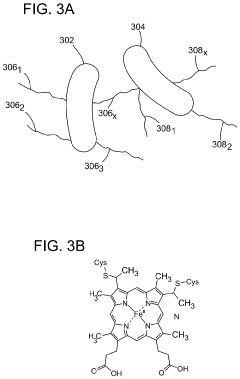
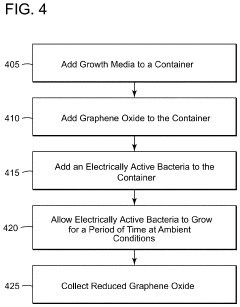
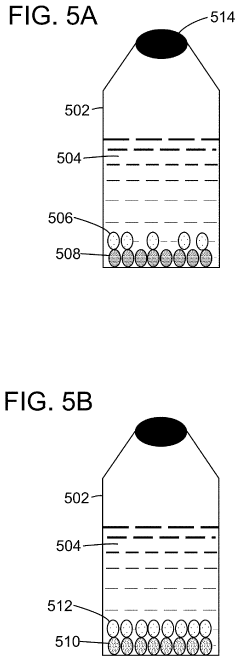
Method of producing electrically active bacteria reduced graphene oxide and electrically active bacteria reduced graphene oxide
PatentInactiveUS10876136B2
Innovation
- The method involves using electrically active bacteria, specifically Geobacter sulfurreducens, to interact with graphene oxide under ambient conditions, reducing it without the need for high temperatures or toxic chemicals and simultaneously doping the graphene oxide with elements present in the bacteria, thereby producing reduced graphene oxide with enhanced electrocatalytic and supercapacitance properties.
Environmental Impact and Sustainability Considerations
The environmental impact of reduced graphene oxide (rGO) in electrochemical applications represents a critical consideration as this technology advances toward widespread implementation. The production methods of rGO typically involve chemical reduction processes that utilize potentially harmful reducing agents such as hydrazine, sodium borohydride, or ascorbic acid. These processes generate hazardous waste streams that require proper treatment and disposal, presenting significant environmental challenges that must be addressed for sustainable scaling.
Water consumption during rGO synthesis and application development constitutes another environmental concern. The purification processes necessary to achieve high-quality rGO suitable for electrochemical applications demand substantial water resources, contributing to water stress in regions where manufacturing occurs. Additionally, energy requirements for rGO production—particularly during thermal reduction methods—result in considerable carbon footprints unless powered by renewable energy sources.
Life cycle assessment (LCA) studies of rGO-based electrochemical devices reveal mixed sustainability profiles. While these materials enable more efficient energy storage and conversion systems that could reduce overall environmental impacts during operation, their production phase often carries significant environmental burdens. Recent research indicates that green synthesis approaches utilizing plant extracts or microorganisms as reducing agents offer promising alternatives with substantially reduced environmental footprints.
The end-of-life management of rGO-containing devices presents both challenges and opportunities. The potential for graphene materials to persist in the environment raises concerns about long-term ecological impacts. However, emerging recycling technologies specifically designed for carbon nanomaterials show promise for recovering and reusing rGO from spent electrochemical devices, potentially creating closed-loop material systems that minimize waste.
Regulatory frameworks governing nanomaterials like rGO continue to evolve globally. The European Union's REACH regulations and similar initiatives in other regions increasingly require comprehensive environmental impact assessments before commercialization. Forward-thinking companies in this space are proactively developing green chemistry approaches and circular economy strategies to ensure compliance with current and anticipated regulations while simultaneously addressing stakeholder concerns about environmental stewardship.
The sustainability advantages of rGO electrochemical applications—including improved energy efficiency, reduced rare earth metal dependence, and extended device lifespans—must be carefully balanced against production impacts through holistic assessment methodologies. As the field matures, integrating environmental considerations into early-stage research and development will be essential for ensuring that rGO electrochemical technologies deliver genuine sustainability benefits across their complete life cycles.
Water consumption during rGO synthesis and application development constitutes another environmental concern. The purification processes necessary to achieve high-quality rGO suitable for electrochemical applications demand substantial water resources, contributing to water stress in regions where manufacturing occurs. Additionally, energy requirements for rGO production—particularly during thermal reduction methods—result in considerable carbon footprints unless powered by renewable energy sources.
Life cycle assessment (LCA) studies of rGO-based electrochemical devices reveal mixed sustainability profiles. While these materials enable more efficient energy storage and conversion systems that could reduce overall environmental impacts during operation, their production phase often carries significant environmental burdens. Recent research indicates that green synthesis approaches utilizing plant extracts or microorganisms as reducing agents offer promising alternatives with substantially reduced environmental footprints.
The end-of-life management of rGO-containing devices presents both challenges and opportunities. The potential for graphene materials to persist in the environment raises concerns about long-term ecological impacts. However, emerging recycling technologies specifically designed for carbon nanomaterials show promise for recovering and reusing rGO from spent electrochemical devices, potentially creating closed-loop material systems that minimize waste.
Regulatory frameworks governing nanomaterials like rGO continue to evolve globally. The European Union's REACH regulations and similar initiatives in other regions increasingly require comprehensive environmental impact assessments before commercialization. Forward-thinking companies in this space are proactively developing green chemistry approaches and circular economy strategies to ensure compliance with current and anticipated regulations while simultaneously addressing stakeholder concerns about environmental stewardship.
The sustainability advantages of rGO electrochemical applications—including improved energy efficiency, reduced rare earth metal dependence, and extended device lifespans—must be carefully balanced against production impacts through holistic assessment methodologies. As the field matures, integrating environmental considerations into early-stage research and development will be essential for ensuring that rGO electrochemical technologies deliver genuine sustainability benefits across their complete life cycles.
Scalability and Commercial Production Challenges
The transition from laboratory-scale production to industrial manufacturing of reduced graphene oxide (rGO) for electrochemical applications faces significant challenges. Current laboratory synthesis methods typically yield milligram to gram quantities, whereas commercial applications require kilogram to ton scale production. This scale-up gap represents one of the most pressing obstacles to widespread adoption of rGO in electrochemical devices such as batteries, supercapacitors, and sensors.
Production consistency presents another major hurdle. Batch-to-batch variations in reduction degree, defect density, and surface functionality significantly impact electrochemical performance. Industrial applications demand standardized materials with predictable properties, yet current manufacturing processes struggle to deliver this consistency. The heterogeneous nature of graphene oxide reduction processes contributes to this variability, with slight changes in temperature, reduction time, or reagent concentration yielding materials with markedly different electrochemical behaviors.
Cost considerations further complicate commercial viability. While graphite as a raw material is relatively inexpensive, the multi-step process to produce high-quality rGO involves costly chemicals, energy-intensive procedures, and specialized equipment. Current production costs range from $50-200 per gram for high-quality rGO, prohibitively expensive for mass-market applications. Economically viable electrochemical devices require production costs below $10 per gram, necessitating significant process optimization.
Environmental and safety concerns also impact scalability. Traditional reduction methods often employ hazardous chemicals like hydrazine or high-temperature thermal treatments. These approaches present significant challenges for industrial-scale implementation due to stringent environmental regulations and worker safety requirements. Green reduction methods using environmentally benign reducing agents show promise but typically yield rGO with inferior electrochemical performance compared to conventional methods.
Quality control and characterization at industrial scale represent additional challenges. Laboratory techniques like Raman spectroscopy, XPS, and electrochemical impedance spectroscopy are difficult to implement as in-line quality control measures for continuous production. The development of rapid, reliable quality assessment protocols suitable for manufacturing environments remains an unresolved challenge in the commercialization pathway.
Recent advances in continuous flow production methods and microwave-assisted reduction techniques show promise for addressing some scalability issues. Several companies, including Directa Plus, Sixth Element, and First Graphene, have developed proprietary processes for ton-scale production, though electrochemical-grade materials with tailored properties remain challenging to produce economically at industrial scale.
Production consistency presents another major hurdle. Batch-to-batch variations in reduction degree, defect density, and surface functionality significantly impact electrochemical performance. Industrial applications demand standardized materials with predictable properties, yet current manufacturing processes struggle to deliver this consistency. The heterogeneous nature of graphene oxide reduction processes contributes to this variability, with slight changes in temperature, reduction time, or reagent concentration yielding materials with markedly different electrochemical behaviors.
Cost considerations further complicate commercial viability. While graphite as a raw material is relatively inexpensive, the multi-step process to produce high-quality rGO involves costly chemicals, energy-intensive procedures, and specialized equipment. Current production costs range from $50-200 per gram for high-quality rGO, prohibitively expensive for mass-market applications. Economically viable electrochemical devices require production costs below $10 per gram, necessitating significant process optimization.
Environmental and safety concerns also impact scalability. Traditional reduction methods often employ hazardous chemicals like hydrazine or high-temperature thermal treatments. These approaches present significant challenges for industrial-scale implementation due to stringent environmental regulations and worker safety requirements. Green reduction methods using environmentally benign reducing agents show promise but typically yield rGO with inferior electrochemical performance compared to conventional methods.
Quality control and characterization at industrial scale represent additional challenges. Laboratory techniques like Raman spectroscopy, XPS, and electrochemical impedance spectroscopy are difficult to implement as in-line quality control measures for continuous production. The development of rapid, reliable quality assessment protocols suitable for manufacturing environments remains an unresolved challenge in the commercialization pathway.
Recent advances in continuous flow production methods and microwave-assisted reduction techniques show promise for addressing some scalability issues. Several companies, including Directa Plus, Sixth Element, and First Graphene, have developed proprietary processes for ton-scale production, though electrochemical-grade materials with tailored properties remain challenging to produce economically at industrial scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!