Regulatory Insights for Reduced Graphene Oxide in Medical Devices
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
rGO Medical Device Regulatory Background and Objectives
Reduced Graphene Oxide (rGO) has emerged as a revolutionary material in the medical device industry over the past decade. This carbon-based nanomaterial, derived from graphene oxide through reduction processes, exhibits exceptional electrical conductivity, mechanical strength, and biocompatibility properties that make it particularly attractive for medical applications. The evolution of rGO technology began with graphene's discovery in 2004, followed by significant advancements in reduction methods that have enabled its practical application in healthcare settings.
The regulatory landscape for nanomaterials in medical devices has developed concurrently, though often lagging behind technological innovation. Initially, regulatory frameworks were not specifically designed to address the unique properties and potential risks associated with nanomaterials like rGO. This gap has gradually narrowed as regulatory bodies worldwide have recognized the need for specialized guidance for these novel materials.
Current regulatory trends indicate increasing scrutiny of nanomaterials in medical devices, with particular attention to their long-term safety profiles, potential for migration or degradation in the body, and biological interactions at the cellular level. The FDA, EMA, and other global regulatory authorities have been developing more specific guidelines for nanomaterial characterization, safety assessment, and quality control in medical devices.
The primary objective of this technical research is to provide a comprehensive analysis of the current regulatory requirements and challenges specific to rGO incorporation in medical devices. This includes identifying regulatory pathways across major markets, understanding testing requirements for safety and efficacy demonstration, and anticipating future regulatory developments that may impact product development timelines.
Additionally, this research aims to establish a clear understanding of how rGO's unique physicochemical properties influence its regulatory classification and assessment. The variable characteristics of rGO based on production methods, functionalization, and integration techniques create a complex regulatory landscape that requires careful navigation.
The technical evolution trajectory suggests that rGO applications in medical devices will continue to expand, necessitating more sophisticated regulatory approaches. As applications evolve from external sensors to implantable devices and drug delivery systems, regulatory requirements will likely become more stringent, requiring manufacturers to develop robust data packages demonstrating both short and long-term safety.
This research will serve as a foundation for developing regulatory strategies that can accelerate the translation of rGO-based medical technologies from laboratory concepts to commercially viable and compliant medical devices, ultimately benefiting patients while ensuring appropriate safety standards are maintained.
The regulatory landscape for nanomaterials in medical devices has developed concurrently, though often lagging behind technological innovation. Initially, regulatory frameworks were not specifically designed to address the unique properties and potential risks associated with nanomaterials like rGO. This gap has gradually narrowed as regulatory bodies worldwide have recognized the need for specialized guidance for these novel materials.
Current regulatory trends indicate increasing scrutiny of nanomaterials in medical devices, with particular attention to their long-term safety profiles, potential for migration or degradation in the body, and biological interactions at the cellular level. The FDA, EMA, and other global regulatory authorities have been developing more specific guidelines for nanomaterial characterization, safety assessment, and quality control in medical devices.
The primary objective of this technical research is to provide a comprehensive analysis of the current regulatory requirements and challenges specific to rGO incorporation in medical devices. This includes identifying regulatory pathways across major markets, understanding testing requirements for safety and efficacy demonstration, and anticipating future regulatory developments that may impact product development timelines.
Additionally, this research aims to establish a clear understanding of how rGO's unique physicochemical properties influence its regulatory classification and assessment. The variable characteristics of rGO based on production methods, functionalization, and integration techniques create a complex regulatory landscape that requires careful navigation.
The technical evolution trajectory suggests that rGO applications in medical devices will continue to expand, necessitating more sophisticated regulatory approaches. As applications evolve from external sensors to implantable devices and drug delivery systems, regulatory requirements will likely become more stringent, requiring manufacturers to develop robust data packages demonstrating both short and long-term safety.
This research will serve as a foundation for developing regulatory strategies that can accelerate the translation of rGO-based medical technologies from laboratory concepts to commercially viable and compliant medical devices, ultimately benefiting patients while ensuring appropriate safety standards are maintained.
Market Demand Analysis for rGO-based Medical Devices
The global market for reduced graphene oxide (rGO) in medical devices is experiencing significant growth, driven by the material's exceptional properties and versatility in healthcare applications. Current market analysis indicates that the medical device sector represents one of the fastest-growing application areas for rGO, with a compound annual growth rate projected to exceed the overall graphene market growth rate through 2030.
Healthcare providers are increasingly seeking advanced materials that can enhance device performance while maintaining biocompatibility and safety profiles. This demand is particularly evident in implantable devices, biosensors, and diagnostic equipment where rGO's electrical conductivity, mechanical strength, and surface functionalization capabilities offer substantial advantages over conventional materials.
The biosensor segment demonstrates particularly strong demand, as healthcare systems worldwide shift toward point-of-care diagnostics and continuous monitoring solutions. rGO-based biosensors enable higher sensitivity, faster response times, and more reliable detection of biomarkers compared to traditional sensor technologies. This aligns with the growing emphasis on preventive healthcare and early disease detection protocols in developed healthcare markets.
Tissue engineering and regenerative medicine represent another high-growth application area. The demand for scaffolds and substrates that can promote cell adhesion, proliferation, and differentiation is driving interest in rGO-based materials. Research indicates that healthcare providers are seeking solutions that can accelerate wound healing, improve tissue regeneration, and enhance integration of medical implants.
Antimicrobial applications constitute a rapidly expanding market segment for rGO-based medical devices. With healthcare-associated infections remaining a significant challenge globally, there is substantial demand for materials that can inhibit bacterial colonization on device surfaces. rGO's demonstrated antimicrobial properties address this critical need, particularly in implantable and indwelling devices.
Regional analysis reveals that North America currently leads in adoption of rGO-based medical technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to demonstrate the highest growth rate, driven by expanding healthcare infrastructure, increasing medical device manufacturing capacity, and growing investment in advanced materials research.
Market barriers include concerns about long-term biocompatibility, regulatory uncertainty, and manufacturing scalability. Healthcare providers and device manufacturers express particular interest in products with established regulatory pathways and clear safety profiles. This highlights the critical importance of regulatory insights and standardization in accelerating market adoption of rGO-based medical devices.
Healthcare providers are increasingly seeking advanced materials that can enhance device performance while maintaining biocompatibility and safety profiles. This demand is particularly evident in implantable devices, biosensors, and diagnostic equipment where rGO's electrical conductivity, mechanical strength, and surface functionalization capabilities offer substantial advantages over conventional materials.
The biosensor segment demonstrates particularly strong demand, as healthcare systems worldwide shift toward point-of-care diagnostics and continuous monitoring solutions. rGO-based biosensors enable higher sensitivity, faster response times, and more reliable detection of biomarkers compared to traditional sensor technologies. This aligns with the growing emphasis on preventive healthcare and early disease detection protocols in developed healthcare markets.
Tissue engineering and regenerative medicine represent another high-growth application area. The demand for scaffolds and substrates that can promote cell adhesion, proliferation, and differentiation is driving interest in rGO-based materials. Research indicates that healthcare providers are seeking solutions that can accelerate wound healing, improve tissue regeneration, and enhance integration of medical implants.
Antimicrobial applications constitute a rapidly expanding market segment for rGO-based medical devices. With healthcare-associated infections remaining a significant challenge globally, there is substantial demand for materials that can inhibit bacterial colonization on device surfaces. rGO's demonstrated antimicrobial properties address this critical need, particularly in implantable and indwelling devices.
Regional analysis reveals that North America currently leads in adoption of rGO-based medical technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to demonstrate the highest growth rate, driven by expanding healthcare infrastructure, increasing medical device manufacturing capacity, and growing investment in advanced materials research.
Market barriers include concerns about long-term biocompatibility, regulatory uncertainty, and manufacturing scalability. Healthcare providers and device manufacturers express particular interest in products with established regulatory pathways and clear safety profiles. This highlights the critical importance of regulatory insights and standardization in accelerating market adoption of rGO-based medical devices.
Global Regulatory Status and Challenges for rGO Materials
The regulatory landscape for reduced graphene oxide (rGO) in medical devices remains fragmented globally, with significant variations across different jurisdictions. In the United States, the FDA has not established specific regulatory pathways for nanomaterials like rGO, instead evaluating them within existing frameworks for medical devices. This creates uncertainty for manufacturers as rGO-based devices often fall into regulatory gray areas between device classifications or between drug-device combinations.
The European Union has implemented more structured approaches through the Medical Device Regulation (MDR) and the General Product Safety Directive, which include provisions for nanomaterials. However, rGO specifically lacks clear classification guidelines, leading to case-by-case evaluations that extend development timelines and increase regulatory compliance costs. The EU's REACH regulation adds another layer of complexity for rGO materials.
In Asia, regulatory frameworks show considerable diversity. Japan's PMDA has developed advanced guidelines for nanomaterials in medical applications, while China's NMPA is rapidly evolving its approach but still lacks specific provisions for graphene-based materials. South Korea has established a relatively progressive regulatory environment for nanomedicine, potentially offering instructive models for other jurisdictions.
A significant challenge across all regions is the lack of standardized testing protocols for rGO materials. This absence creates inconsistencies in safety and efficacy evaluations, complicating cross-border approvals and technology transfer. The physicochemical properties of rGO, including variable oxidation states and surface functionalization, further complicate regulatory categorization.
Biocompatibility assessment presents another major hurdle, as traditional testing frameworks may not adequately capture the unique biological interactions of rGO. Long-term safety data remains limited, creating regulatory hesitancy around permanent implantable devices incorporating these materials.
International harmonization efforts through organizations like the International Medical Device Regulators Forum (IMDRF) and ISO technical committees are underway but progress slowly. The development of ISO standards specific to graphene materials represents a positive step, though implementation in regulatory frameworks lags behind scientific advancements.
For medical device manufacturers, these regulatory challenges translate to extended development cycles, increased compliance costs, and market access barriers. Companies must navigate different requirements across regions, often conducting redundant testing to satisfy various authorities, which significantly impacts commercialization timelines for innovative rGO-based medical technologies.
The European Union has implemented more structured approaches through the Medical Device Regulation (MDR) and the General Product Safety Directive, which include provisions for nanomaterials. However, rGO specifically lacks clear classification guidelines, leading to case-by-case evaluations that extend development timelines and increase regulatory compliance costs. The EU's REACH regulation adds another layer of complexity for rGO materials.
In Asia, regulatory frameworks show considerable diversity. Japan's PMDA has developed advanced guidelines for nanomaterials in medical applications, while China's NMPA is rapidly evolving its approach but still lacks specific provisions for graphene-based materials. South Korea has established a relatively progressive regulatory environment for nanomedicine, potentially offering instructive models for other jurisdictions.
A significant challenge across all regions is the lack of standardized testing protocols for rGO materials. This absence creates inconsistencies in safety and efficacy evaluations, complicating cross-border approvals and technology transfer. The physicochemical properties of rGO, including variable oxidation states and surface functionalization, further complicate regulatory categorization.
Biocompatibility assessment presents another major hurdle, as traditional testing frameworks may not adequately capture the unique biological interactions of rGO. Long-term safety data remains limited, creating regulatory hesitancy around permanent implantable devices incorporating these materials.
International harmonization efforts through organizations like the International Medical Device Regulators Forum (IMDRF) and ISO technical committees are underway but progress slowly. The development of ISO standards specific to graphene materials represents a positive step, though implementation in regulatory frameworks lags behind scientific advancements.
For medical device manufacturers, these regulatory challenges translate to extended development cycles, increased compliance costs, and market access barriers. Companies must navigate different requirements across regions, often conducting redundant testing to satisfy various authorities, which significantly impacts commercialization timelines for innovative rGO-based medical technologies.
Current Regulatory Frameworks for Graphene-Based Medical Products
01 Methods for producing reduced graphene oxide
Various methods can be employed to produce reduced graphene oxide, including chemical reduction, thermal reduction, and electrochemical reduction. These processes typically involve removing oxygen-containing functional groups from graphene oxide to restore the sp2 carbon network. The reduction methods can be optimized to control the degree of reduction, which affects the electrical, thermal, and mechanical properties of the resulting material.- Methods of producing reduced graphene oxide: Various methods can be employed to produce reduced graphene oxide, including chemical reduction, thermal reduction, and electrochemical reduction. These processes typically involve removing oxygen-containing functional groups from graphene oxide to restore the sp2 carbon network. The reduction methods can be optimized to control the degree of reduction, which affects the electrical, thermal, and mechanical properties of the resulting material.
- Applications in energy storage devices: Reduced graphene oxide is widely used in energy storage applications due to its high surface area, excellent electrical conductivity, and mechanical stability. It serves as an electrode material in supercapacitors, lithium-ion batteries, and other energy storage devices. The incorporation of reduced graphene oxide can enhance charge transfer, increase energy density, and improve cycling stability of these devices.
- Composite materials with reduced graphene oxide: Reduced graphene oxide can be combined with various materials to form composites with enhanced properties. These composites may incorporate polymers, metals, metal oxides, or other nanomaterials. The resulting materials often exhibit improved mechanical strength, electrical conductivity, thermal stability, and other functional properties compared to the individual components, making them suitable for a wide range of applications.
- Sensing and detection applications: Reduced graphene oxide is utilized in various sensing and detection systems due to its large surface area and excellent electrical properties. It can be functionalized or combined with other materials to create sensors for detecting gases, biomolecules, heavy metals, and other analytes. These sensors often demonstrate high sensitivity, selectivity, and fast response times, making them valuable for environmental monitoring, medical diagnostics, and industrial applications.
- Environmental applications and water treatment: Reduced graphene oxide materials are employed in environmental remediation and water treatment processes. Their high surface area and tunable surface chemistry make them effective adsorbents for removing pollutants, heavy metals, and organic contaminants from water. Additionally, they can be incorporated into membranes or filters to enhance separation efficiency and anti-fouling properties, contributing to more sustainable water purification technologies.
02 Applications in energy storage devices
Reduced graphene oxide is widely used in energy storage applications such as supercapacitors, lithium-ion batteries, and fuel cells. Its high surface area, excellent electrical conductivity, and mechanical stability make it an ideal material for electrode fabrication. When incorporated into energy storage devices, reduced graphene oxide can enhance charge transfer, increase energy density, and improve cycling stability.Expand Specific Solutions03 Composite materials with reduced graphene oxide
Reduced graphene oxide can be combined with various materials including polymers, metals, and metal oxides to create composite materials with enhanced properties. These composites often exhibit improved mechanical strength, electrical conductivity, thermal stability, and barrier properties compared to the base materials. The synergistic effects between reduced graphene oxide and other components make these composites suitable for a wide range of applications.Expand Specific Solutions04 Sensing and detection applications
Reduced graphene oxide is utilized in various sensing and detection applications due to its large surface area and excellent electrical properties. It can be functionalized or combined with other materials to create sensors for detecting gases, biomolecules, heavy metals, and other analytes. These sensors typically offer high sensitivity, fast response times, and good selectivity, making them valuable for environmental monitoring, medical diagnostics, and industrial quality control.Expand Specific Solutions05 Environmental remediation applications
Reduced graphene oxide can be employed in environmental remediation processes such as water purification and waste treatment. Its high adsorption capacity allows it to effectively remove various pollutants including heavy metals, organic contaminants, and dyes from water. Additionally, reduced graphene oxide can be modified or combined with catalytic materials to enhance the degradation of environmental pollutants through advanced oxidation processes.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The regulatory landscape for reduced graphene oxide (rGO) in medical devices is evolving within an emerging market characterized by significant growth potential but still developing technical standards. The field is in its early commercialization phase, with market size expanding as applications in biosensors, drug delivery, and tissue engineering gain traction. Technical maturity varies considerably among key players, with research institutions like MIT, Indian Institute of Science, and King Abdullah University leading fundamental research, while companies like Global Graphene Group and LG Electronics focus on commercial applications. Academic-industry partnerships, exemplified by collaborations between Semiconductor Energy Laboratory and universities, are accelerating regulatory pathway development. The fragmented competitive landscape includes specialized materials companies alongside large electronics manufacturers, all navigating complex regulatory requirements across different jurisdictions.
GLOBAL GRAPHENE GROUP INC
Technical Solution: Global Graphene Group (G3) has developed proprietary technologies for manufacturing reduced graphene oxide (rGO) specifically for medical device applications. Their approach focuses on creating biocompatible rGO materials that meet FDA regulatory requirements through controlled reduction processes that minimize residual functional groups and contaminants. G3's regulatory strategy includes comprehensive toxicological profiling of their rGO materials, with extensive in vitro and in vivo studies demonstrating biocompatibility according to ISO 10993 standards. They've established a quality management system compliant with ISO 13485 for medical device manufacturing, implementing rigorous process controls to ensure batch-to-batch consistency in rGO production. G3 has also developed specialized surface functionalization techniques that enhance biocompatibility while maintaining regulatory compliance, allowing their rGO to be used in implantable devices, wound dressings, and drug delivery systems with appropriate safety documentation.
Strengths: Comprehensive regulatory documentation and established quality management system specifically for medical-grade rGO; extensive toxicological data supporting safety claims. Weaknesses: Potential challenges with scaling production while maintaining consistent quality parameters required for medical applications; higher manufacturing costs compared to non-medical grade graphene materials.
Sanford Burnham Prebys Medical Discovery Institute
Technical Solution: Sanford Burnham Prebys has developed a specialized regulatory approach for reduced graphene oxide (rGO) in medical devices focused on immunological safety assessment. Their technical solution centers on comprehensive immunotoxicity profiling that goes beyond standard regulatory requirements to address specific concerns about nanomaterial-immune system interactions. They've established in vitro and ex vivo testing platforms using human immune cells to evaluate rGO's effects on immune activation, complement system, and cytokine responses, providing data packages that satisfy FDA requirements for novel biomaterials. Their regulatory strategy includes detailed protocols for evaluating protein corona formation on rGO surfaces in physiological environments and how these interactions influence biocompatibility outcomes. The Institute has developed specialized histopathological assessment methods for evaluating tissue responses to rGO-containing implants, with quantitative image analysis techniques that provide objective measures of biocompatibility. They've also created a database correlating different rGO manufacturing methods with immunological safety profiles, helping manufacturers select production processes most likely to yield regulatory-compliant materials for specific medical applications.
Strengths: Specialized expertise in immunological assessment of nanomaterials that addresses a key regulatory concern for rGO; established relationships with regulatory toxicologists. Weaknesses: Primary focus on biological aspects may require partnering with materials science experts for complete regulatory packages; limited experience with large-scale manufacturing compliance issues.
Critical Regulatory Guidelines and Scientific Literature
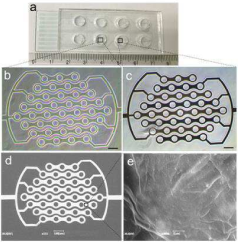
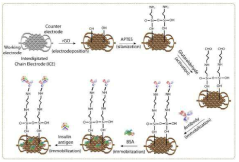
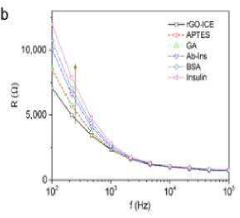
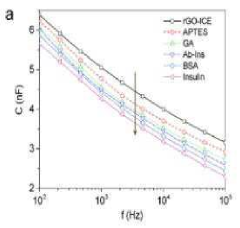
Reduced Graphene Oxide Modified Interdigitated Chain Electrode, method for preparing thereof, the rGO-ICE based Insulin Sensor, and method for preparing thereof
PatentInactiveKR1020170085320A
Innovation
- The development of an rGO-modified Interdigitated Chain Electrode (ICE) with rounded corners and wrinkled graphene oxide sheets, which reduces the corner concentration effect and enhances sensitivity across the entire electrode surface.
Reduced graphene oxide with improved antibacterial properties and production method therefor
PatentWO2024071514A1
Innovation
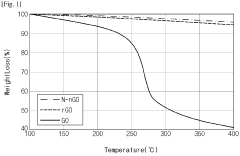
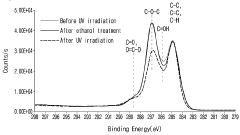
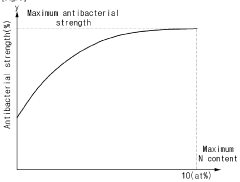
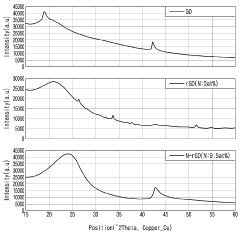
- Nitrogen-doped reduced graphene oxide (N-rGO) is produced through hydrothermal synthesis with urea and hydrazine, followed by washing with metallic antibacterial additives like Ag, maintaining high thermal and UV stability while enhancing antibacterial properties.
Biocompatibility and Safety Assessment Requirements
The biocompatibility and safety assessment of reduced graphene oxide (rGO) in medical devices requires rigorous evaluation under established regulatory frameworks. Medical device manufacturers must comply with ISO 10993 series standards, particularly ISO 10993-1, which outlines the biological evaluation of medical devices. For rGO-containing devices, this evaluation becomes particularly critical due to the novel nature of the material and its unique physicochemical properties.
The FDA and European Medical Device Regulation (MDR) require comprehensive testing protocols for nanomaterials like rGO. These include cytotoxicity assessments to determine potential cellular damage, genotoxicity testing to evaluate DNA damage potential, and sensitization studies to assess allergic response risks. Long-term implantation studies are mandatory for devices intended for extended contact with human tissues, with particular attention to potential degradation products of rGO.
Hemocompatibility testing represents another crucial requirement for blood-contacting devices incorporating rGO. This includes evaluations of hemolysis, thrombogenicity, and complement activation to ensure the material does not adversely affect blood components or trigger coagulation cascades. The unique surface properties of rGO necessitate specialized testing protocols beyond standard approaches.
Material characterization requirements for rGO are particularly stringent, demanding detailed analysis of purity levels, residual chemical contaminants from the reduction process, and precise particle size distribution. Regulatory bodies increasingly require manufacturers to demonstrate batch-to-batch consistency in these parameters to ensure reproducible safety profiles.
Risk assessment frameworks for rGO-based medical devices must address both known and theoretical risks. These include potential long-term accumulation in organs, translocation across biological barriers, and interaction with the immune system. The FDA's guidance on nanotechnology recommends applying a case-by-case approach, with safety margins that account for the current limitations in scientific understanding of nanomaterial-biological interactions.
International harmonization efforts are underway to standardize testing requirements for graphene-based materials in medical applications. The International Medical Device Regulators Forum (IMDRF) has established working groups focused on developing consistent approaches to nanomaterial safety assessment, though specific guidance for rGO remains in development. Manufacturers are advised to engage in early consultation with regulatory authorities to establish appropriate testing strategies for their specific rGO-containing devices.
The FDA and European Medical Device Regulation (MDR) require comprehensive testing protocols for nanomaterials like rGO. These include cytotoxicity assessments to determine potential cellular damage, genotoxicity testing to evaluate DNA damage potential, and sensitization studies to assess allergic response risks. Long-term implantation studies are mandatory for devices intended for extended contact with human tissues, with particular attention to potential degradation products of rGO.
Hemocompatibility testing represents another crucial requirement for blood-contacting devices incorporating rGO. This includes evaluations of hemolysis, thrombogenicity, and complement activation to ensure the material does not adversely affect blood components or trigger coagulation cascades. The unique surface properties of rGO necessitate specialized testing protocols beyond standard approaches.
Material characterization requirements for rGO are particularly stringent, demanding detailed analysis of purity levels, residual chemical contaminants from the reduction process, and precise particle size distribution. Regulatory bodies increasingly require manufacturers to demonstrate batch-to-batch consistency in these parameters to ensure reproducible safety profiles.
Risk assessment frameworks for rGO-based medical devices must address both known and theoretical risks. These include potential long-term accumulation in organs, translocation across biological barriers, and interaction with the immune system. The FDA's guidance on nanotechnology recommends applying a case-by-case approach, with safety margins that account for the current limitations in scientific understanding of nanomaterial-biological interactions.
International harmonization efforts are underway to standardize testing requirements for graphene-based materials in medical applications. The International Medical Device Regulators Forum (IMDRF) has established working groups focused on developing consistent approaches to nanomaterial safety assessment, though specific guidance for rGO remains in development. Manufacturers are advised to engage in early consultation with regulatory authorities to establish appropriate testing strategies for their specific rGO-containing devices.
International Harmonization Efforts for Nanomaterial Regulation
The global landscape of nanomaterial regulation presents significant challenges due to varying approaches across different jurisdictions. In response, several international initiatives have emerged to harmonize regulatory frameworks for nanomaterials, including reduced graphene oxide (rGO) used in medical devices. The Organization for Economic Co-operation and Development (OECD) has established the Working Party on Manufactured Nanomaterials (WPMN), which develops standardized testing methods and risk assessment frameworks applicable to graphene-based materials in healthcare applications.
The International Organization for Standardization (ISO) Technical Committee 229 has developed crucial standards for nanomaterial characterization, terminology, and safety assessment that directly impact rGO regulation in medical devices. These standards provide a common language and methodological approach that facilitates international regulatory convergence and reduces technical barriers to trade.
The International Council on Nanotechnology (ICON) serves as a global platform for knowledge sharing among regulatory bodies, industry stakeholders, and academic institutions. Their collaborative efforts have resulted in best practice guidelines for nanomaterial safety assessment that are increasingly being adopted across regulatory frameworks, creating more predictable pathways for rGO-based medical device approvals.
Regional harmonization efforts are also noteworthy, with the European Union's REACH regulation and the U.S. FDA's approach to nanomaterials showing signs of convergence through mutual recognition agreements and shared scientific consultations. The EU-US Regulatory Cooperation initiative specifically addresses nanomaterials in medical applications, establishing common principles for safety evaluation while acknowledging region-specific regulatory requirements.
The World Health Organization has established an expert committee on nanomedicine regulation that provides guidance documents specifically addressing nanomaterials in healthcare applications. Their framework for benefit-risk assessment of nanomaterials like rGO in medical devices has been adopted by several national regulatory authorities, particularly in developing economies seeking to establish regulatory frameworks.
Bilateral agreements between major markets have further advanced harmonization efforts. The Australia-Canada-EU-USA Regulatory Cooperation on nanomaterials has established mutual acceptance of toxicological data and characterization methods for graphene-based materials, significantly reducing redundant testing requirements and accelerating approval processes for innovative medical devices incorporating rGO.
Despite these advances, challenges remain in achieving full international harmonization. Differences in risk tolerance, socioeconomic factors, and existing regulatory infrastructures continue to create divergent approaches. The International Medical Device Regulators Forum (IMDRF) is addressing these challenges through its Nanomaterials Working Group, which focuses specifically on creating convergent pathways for novel nanomaterials like rGO in medical applications.
The International Organization for Standardization (ISO) Technical Committee 229 has developed crucial standards for nanomaterial characterization, terminology, and safety assessment that directly impact rGO regulation in medical devices. These standards provide a common language and methodological approach that facilitates international regulatory convergence and reduces technical barriers to trade.
The International Council on Nanotechnology (ICON) serves as a global platform for knowledge sharing among regulatory bodies, industry stakeholders, and academic institutions. Their collaborative efforts have resulted in best practice guidelines for nanomaterial safety assessment that are increasingly being adopted across regulatory frameworks, creating more predictable pathways for rGO-based medical device approvals.
Regional harmonization efforts are also noteworthy, with the European Union's REACH regulation and the U.S. FDA's approach to nanomaterials showing signs of convergence through mutual recognition agreements and shared scientific consultations. The EU-US Regulatory Cooperation initiative specifically addresses nanomaterials in medical applications, establishing common principles for safety evaluation while acknowledging region-specific regulatory requirements.
The World Health Organization has established an expert committee on nanomedicine regulation that provides guidance documents specifically addressing nanomaterials in healthcare applications. Their framework for benefit-risk assessment of nanomaterials like rGO in medical devices has been adopted by several national regulatory authorities, particularly in developing economies seeking to establish regulatory frameworks.
Bilateral agreements between major markets have further advanced harmonization efforts. The Australia-Canada-EU-USA Regulatory Cooperation on nanomaterials has established mutual acceptance of toxicological data and characterization methods for graphene-based materials, significantly reducing redundant testing requirements and accelerating approval processes for innovative medical devices incorporating rGO.
Despite these advances, challenges remain in achieving full international harmonization. Differences in risk tolerance, socioeconomic factors, and existing regulatory infrastructures continue to create divergent approaches. The International Medical Device Regulators Forum (IMDRF) is addressing these challenges through its Nanomaterials Working Group, which focuses specifically on creating convergent pathways for novel nanomaterials like rGO in medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!