How Reduced Graphene Oxide Affects Electrode Kinetics
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
rGO Electrode Kinetics Background and Objectives
Reduced graphene oxide (rGO) has emerged as a revolutionary material in electrochemical applications over the past decade, transforming the landscape of energy storage, sensing technologies, and catalytic processes. The evolution of graphene-based materials began with the groundbreaking isolation of graphene in 2004, which subsequently led to extensive research into its derivatives, including graphene oxide (GO) and reduced graphene oxide. The reduction process of GO to rGO represents a critical step in tailoring the material's properties for specific electrochemical applications.
The technological trajectory of rGO has been characterized by continuous improvements in synthesis methods, from chemical reduction using hydrazine to environmentally friendly approaches utilizing green reducing agents. Thermal reduction techniques have also evolved significantly, enabling precise control over the degree of reduction and consequently the electrochemical properties of the resulting material. This progression has been driven by the need to optimize electrode kinetics for enhanced performance in various applications.
Electrode kinetics, fundamentally, refers to the rate at which electrochemical reactions occur at the electrode-electrolyte interface. The unique properties of rGO, including its high surface area, excellent electrical conductivity, and abundant functional groups, directly influence these kinetic parameters. Understanding how rGO affects electrode kinetics is essential for designing next-generation electrochemical systems with superior performance characteristics.
The primary technical objective of this investigation is to comprehensively analyze the relationship between rGO's structural and chemical properties and its impact on electrode kinetics. This includes examining how the degree of reduction, defect density, and remaining oxygen-containing functional groups influence charge transfer processes, reaction rates, and overall electrochemical performance.
Additionally, this research aims to establish quantitative correlations between rGO's synthesis parameters and the resulting kinetic properties, providing a framework for tailoring rGO-based electrodes for specific applications. By elucidating these relationships, we seek to develop predictive models that can guide the rational design of rGO electrodes with optimized kinetic performance.
The technological significance of this research extends beyond academic interest, as enhanced electrode kinetics directly translates to improved performance in practical applications such as supercapacitors, batteries, fuel cells, and electrochemical sensors. As global demand for high-performance energy storage and conversion devices continues to grow, understanding and optimizing rGO's influence on electrode kinetics becomes increasingly crucial for technological advancement in these fields.
Future technological trends in this domain are likely to focus on hybrid materials combining rGO with other functional components, precise control over defect engineering, and the development of scalable, environmentally sustainable production methods that maintain optimal kinetic properties.
The technological trajectory of rGO has been characterized by continuous improvements in synthesis methods, from chemical reduction using hydrazine to environmentally friendly approaches utilizing green reducing agents. Thermal reduction techniques have also evolved significantly, enabling precise control over the degree of reduction and consequently the electrochemical properties of the resulting material. This progression has been driven by the need to optimize electrode kinetics for enhanced performance in various applications.
Electrode kinetics, fundamentally, refers to the rate at which electrochemical reactions occur at the electrode-electrolyte interface. The unique properties of rGO, including its high surface area, excellent electrical conductivity, and abundant functional groups, directly influence these kinetic parameters. Understanding how rGO affects electrode kinetics is essential for designing next-generation electrochemical systems with superior performance characteristics.
The primary technical objective of this investigation is to comprehensively analyze the relationship between rGO's structural and chemical properties and its impact on electrode kinetics. This includes examining how the degree of reduction, defect density, and remaining oxygen-containing functional groups influence charge transfer processes, reaction rates, and overall electrochemical performance.
Additionally, this research aims to establish quantitative correlations between rGO's synthesis parameters and the resulting kinetic properties, providing a framework for tailoring rGO-based electrodes for specific applications. By elucidating these relationships, we seek to develop predictive models that can guide the rational design of rGO electrodes with optimized kinetic performance.
The technological significance of this research extends beyond academic interest, as enhanced electrode kinetics directly translates to improved performance in practical applications such as supercapacitors, batteries, fuel cells, and electrochemical sensors. As global demand for high-performance energy storage and conversion devices continues to grow, understanding and optimizing rGO's influence on electrode kinetics becomes increasingly crucial for technological advancement in these fields.
Future technological trends in this domain are likely to focus on hybrid materials combining rGO with other functional components, precise control over defect engineering, and the development of scalable, environmentally sustainable production methods that maintain optimal kinetic properties.
Market Applications and Demand Analysis for rGO Electrodes
The market for reduced graphene oxide (rGO) electrodes has witnessed substantial growth in recent years, driven primarily by the increasing demand for high-performance energy storage devices and electrochemical sensors. The global market for graphene-based electrodes was valued at approximately $45 million in 2022 and is projected to reach $120 million by 2028, representing a compound annual growth rate of 17.8% during the forecast period.
Energy storage applications constitute the largest market segment for rGO electrodes, accounting for nearly 40% of the total market share. This dominance is attributed to the superior electrode kinetics of rGO, which enables faster charge transfer and enhanced energy density in batteries and supercapacitors. The automotive industry, particularly the electric vehicle sector, has emerged as a significant consumer of rGO electrodes, with major manufacturers incorporating graphene-enhanced batteries to improve charging rates and overall vehicle performance.
The healthcare and biomedical sectors represent rapidly expanding markets for rGO electrodes, with applications in biosensors, drug delivery systems, and neural interfaces. The exceptional sensitivity and selectivity of rGO-based electrochemical sensors have positioned them as valuable tools for real-time monitoring of various biomarkers, creating a market segment expected to grow at 22% annually through 2027.
Environmental monitoring and remediation applications have also created substantial demand for rGO electrodes. Their ability to detect trace amounts of pollutants and facilitate electrochemical degradation of contaminants has led to increased adoption in water treatment systems and environmental sensing platforms, with this segment projected to reach $30 million by 2026.
Regionally, Asia-Pacific dominates the market, accounting for approximately 45% of global demand, followed by North America (30%) and Europe (20%). China and South Korea lead in production capacity, while significant research and development activities are concentrated in the United States and European Union.
Despite the promising market outlook, several factors constrain wider adoption of rGO electrodes. Cost remains a significant barrier, with high-quality rGO production still relatively expensive compared to traditional electrode materials. Additionally, challenges in scaling production while maintaining consistent electrode kinetic properties have limited penetration in mass-market applications.
Industry analysts predict that as manufacturing processes mature and economies of scale are achieved, production costs will decrease by 30-40% over the next five years, potentially catalyzing broader market adoption across multiple industries and applications.
Energy storage applications constitute the largest market segment for rGO electrodes, accounting for nearly 40% of the total market share. This dominance is attributed to the superior electrode kinetics of rGO, which enables faster charge transfer and enhanced energy density in batteries and supercapacitors. The automotive industry, particularly the electric vehicle sector, has emerged as a significant consumer of rGO electrodes, with major manufacturers incorporating graphene-enhanced batteries to improve charging rates and overall vehicle performance.
The healthcare and biomedical sectors represent rapidly expanding markets for rGO electrodes, with applications in biosensors, drug delivery systems, and neural interfaces. The exceptional sensitivity and selectivity of rGO-based electrochemical sensors have positioned them as valuable tools for real-time monitoring of various biomarkers, creating a market segment expected to grow at 22% annually through 2027.
Environmental monitoring and remediation applications have also created substantial demand for rGO electrodes. Their ability to detect trace amounts of pollutants and facilitate electrochemical degradation of contaminants has led to increased adoption in water treatment systems and environmental sensing platforms, with this segment projected to reach $30 million by 2026.
Regionally, Asia-Pacific dominates the market, accounting for approximately 45% of global demand, followed by North America (30%) and Europe (20%). China and South Korea lead in production capacity, while significant research and development activities are concentrated in the United States and European Union.
Despite the promising market outlook, several factors constrain wider adoption of rGO electrodes. Cost remains a significant barrier, with high-quality rGO production still relatively expensive compared to traditional electrode materials. Additionally, challenges in scaling production while maintaining consistent electrode kinetic properties have limited penetration in mass-market applications.
Industry analysts predict that as manufacturing processes mature and economies of scale are achieved, production costs will decrease by 30-40% over the next five years, potentially catalyzing broader market adoption across multiple industries and applications.
Current Status and Challenges in rGO Electrode Technology
Reduced graphene oxide (rGO) has emerged as a revolutionary material in electrode technology, offering exceptional electrical conductivity, large surface area, and mechanical flexibility. Currently, rGO-based electrodes are being extensively researched and developed across multiple industries, including energy storage, sensing technologies, and biomedical applications. The global market for graphene-based electrodes has shown significant growth, with an estimated market value exceeding $50 million in 2023 and projected to reach $120 million by 2028.
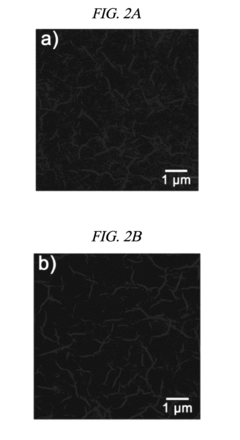
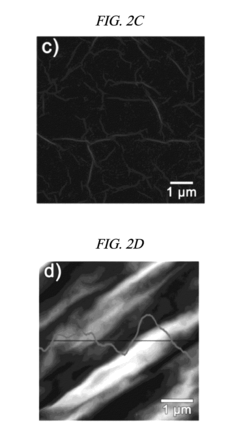
Despite these promising developments, several critical challenges persist in rGO electrode technology. The primary technical hurdle remains the scalable and reproducible production of high-quality rGO with consistent properties. Current reduction methods—thermal, chemical, and electrochemical—each present distinct limitations. Thermal reduction achieves high conductivity but often introduces structural defects. Chemical reduction methods, while more controllable, frequently involve environmentally hazardous reagents and leave residual functional groups that impede electron transfer kinetics.
The heterogeneity of rGO materials presents another significant challenge. Variations in reduction degree, defect density, and sheet size dramatically affect electrode performance, making standardization difficult. This inconsistency complicates both research comparisons and industrial scale-up efforts. Additionally, the restacking of graphene sheets during electrode fabrication reduces effective surface area and limits electrolyte accessibility, directly impacting electrode kinetics.
Interface engineering between rGO and current collectors represents another frontier challenge. Poor interfacial contact increases resistance and hampers electron transfer, while inadequate adhesion leads to mechanical instability during cycling. Researchers are exploring various binding strategies and surface treatments to optimize these interfaces, but a universally effective solution remains elusive.
Long-term stability issues also plague rGO electrodes, particularly in aqueous or biological environments. Gradual re-oxidation, mechanical degradation, and surface fouling can progressively deteriorate electrode performance. This is especially problematic in applications requiring extended operational lifetimes, such as implantable biomedical devices or grid-scale energy storage.
Geographically, research leadership in rGO electrode technology is distributed across several regions. East Asia, particularly China and South Korea, dominates in terms of patent filings and mass production capabilities. North America and Europe lead in fundamental research and high-precision applications. This global distribution has created competitive innovation ecosystems but also challenges in standardization and intellectual property management.
Regulatory hurdles further complicate advancement, with inconsistent safety guidelines for nanomaterials across different jurisdictions. Environmental concerns regarding production processes and end-of-life disposal of rGO-based devices remain inadequately addressed, potentially limiting widespread commercial adoption.
Despite these promising developments, several critical challenges persist in rGO electrode technology. The primary technical hurdle remains the scalable and reproducible production of high-quality rGO with consistent properties. Current reduction methods—thermal, chemical, and electrochemical—each present distinct limitations. Thermal reduction achieves high conductivity but often introduces structural defects. Chemical reduction methods, while more controllable, frequently involve environmentally hazardous reagents and leave residual functional groups that impede electron transfer kinetics.
The heterogeneity of rGO materials presents another significant challenge. Variations in reduction degree, defect density, and sheet size dramatically affect electrode performance, making standardization difficult. This inconsistency complicates both research comparisons and industrial scale-up efforts. Additionally, the restacking of graphene sheets during electrode fabrication reduces effective surface area and limits electrolyte accessibility, directly impacting electrode kinetics.
Interface engineering between rGO and current collectors represents another frontier challenge. Poor interfacial contact increases resistance and hampers electron transfer, while inadequate adhesion leads to mechanical instability during cycling. Researchers are exploring various binding strategies and surface treatments to optimize these interfaces, but a universally effective solution remains elusive.
Long-term stability issues also plague rGO electrodes, particularly in aqueous or biological environments. Gradual re-oxidation, mechanical degradation, and surface fouling can progressively deteriorate electrode performance. This is especially problematic in applications requiring extended operational lifetimes, such as implantable biomedical devices or grid-scale energy storage.
Geographically, research leadership in rGO electrode technology is distributed across several regions. East Asia, particularly China and South Korea, dominates in terms of patent filings and mass production capabilities. North America and Europe lead in fundamental research and high-precision applications. This global distribution has created competitive innovation ecosystems but also challenges in standardization and intellectual property management.
Regulatory hurdles further complicate advancement, with inconsistent safety guidelines for nanomaterials across different jurisdictions. Environmental concerns regarding production processes and end-of-life disposal of rGO-based devices remain inadequately addressed, potentially limiting widespread commercial adoption.
Current Methods for Enhancing rGO Electrode Kinetics
01 Synthesis and preparation methods of reduced graphene oxide electrodes
Various methods for synthesizing and preparing reduced graphene oxide (rGO) electrodes with enhanced electrode kinetics. These methods include chemical reduction, thermal reduction, and electrochemical reduction techniques that effectively remove oxygen-containing functional groups from graphene oxide to restore the sp2 carbon network. The preparation methods significantly influence the electrical conductivity, surface area, and electrochemical performance of the resulting rGO electrodes.- Synthesis methods for reduced graphene oxide electrodes: Various methods can be employed to synthesize reduced graphene oxide (rGO) for electrode applications, including chemical reduction, thermal reduction, and electrochemical reduction. These synthesis methods significantly influence the resulting electrode kinetics by controlling the degree of reduction, defect density, and functional group content. Optimized synthesis protocols can enhance electron transfer rates and improve overall electrochemical performance.
- Surface modification of reduced graphene oxide for enhanced electrode kinetics: Surface modification techniques can be applied to reduced graphene oxide electrodes to improve their kinetic properties. These modifications include doping with heteroatoms (such as nitrogen, boron, or sulfur), functionalization with specific chemical groups, and creation of defects or active sites. Such modifications can significantly enhance electron transfer rates, catalytic activity, and overall electrochemical performance by altering the electronic structure and surface properties of the material.
- Composite materials with reduced graphene oxide for improved electrode performance: Combining reduced graphene oxide with other materials to form composites can significantly enhance electrode kinetics. These composites may incorporate metal nanoparticles, metal oxides, conductive polymers, or other carbon nanomaterials. The synergistic effects between rGO and these materials can improve conductivity, provide additional active sites, and enhance mass transport, resulting in superior electrode kinetics for various electrochemical applications.
- Structural engineering of reduced graphene oxide for optimized electrode kinetics: The structural properties of reduced graphene oxide, including its porosity, layer stacking, and three-dimensional architecture, significantly impact electrode kinetics. Engineering these structural features through techniques such as template-assisted synthesis, freeze-drying, or controlled assembly can create optimized pathways for electron transfer and ion diffusion. These structurally engineered rGO materials demonstrate enhanced kinetic performance in various electrochemical applications.
- Application-specific optimization of reduced graphene oxide electrode kinetics: Reduced graphene oxide electrode kinetics can be specifically optimized for different applications such as energy storage devices, sensors, catalysts, and bioelectronics. This optimization involves tailoring the reduction degree, defect concentration, functional groups, and composite formulation to meet the specific requirements of each application. Understanding the relationship between rGO properties and electrode kinetics in different electrochemical environments enables the development of high-performance application-specific electrodes.
02 Doping and functionalization of reduced graphene oxide for improved electrode kinetics
Doping and functionalization strategies to enhance the electrode kinetics of reduced graphene oxide. Incorporating heteroatoms such as nitrogen, boron, or sulfur into the graphene lattice creates active sites that facilitate faster electron transfer. Surface functionalization with metal nanoparticles or conductive polymers can also improve the electrochemical performance by increasing the number of reaction sites and enhancing charge transfer rates at the electrode-electrolyte interface.Expand Specific Solutions03 Reduced graphene oxide composites for energy storage applications
Development of reduced graphene oxide composite materials for energy storage applications with improved electrode kinetics. These composites combine rGO with metal oxides, conductive polymers, or other carbon materials to create synergistic effects that enhance electron transport, ion diffusion, and overall electrochemical performance. The composite electrodes demonstrate higher specific capacitance, better rate capability, and improved cycling stability compared to pure rGO electrodes.Expand Specific Solutions04 Structural engineering of reduced graphene oxide for enhanced electrode kinetics
Structural engineering approaches to optimize the electrode kinetics of reduced graphene oxide. These include creating 3D architectures, controlling pore size distribution, introducing defects, and manipulating the interlayer spacing of rGO sheets. Such structural modifications increase the accessible surface area, facilitate ion transport, and reduce diffusion resistance, resulting in improved electrochemical performance for various applications including supercapacitors, batteries, and sensors.Expand Specific Solutions05 Characterization and measurement of electrode kinetics in reduced graphene oxide
Methods and techniques for characterizing and measuring electrode kinetics in reduced graphene oxide materials. These include electrochemical impedance spectroscopy, cyclic voltammetry, rotating disk electrode measurements, and various spectroscopic techniques. These analytical approaches help quantify key parameters such as electron transfer rates, charge transfer resistance, and exchange current density, which are crucial for understanding and optimizing the electrochemical performance of rGO-based electrodes.Expand Specific Solutions
Leading Research Groups and Companies in rGO Technology
The reduced graphene oxide (rGO) electrode kinetics market is in a growth phase, characterized by increasing research intensity and commercial applications. The global market for graphene-based electrodes is expanding rapidly, driven by energy storage, electronics, and sensing applications. Key players like Korea Electrotechnology Research Institute, LG Energy Solution, and Dongjin Semichem are leading commercial development, while research institutions such as South China University of Technology and CSIC are advancing fundamental understanding. The technology is approaching maturity in certain applications, with companies like SVOLT Energy and Morion NanoTech commercializing rGO-enhanced batteries. Academic-industrial collaborations between institutions like Paul Scherrer Institut and commercial entities are accelerating technology transfer and application development.
Korea Electrotechnology Research Institute
Technical Solution: Korea Electrotechnology Research Institute (KERI) has developed sophisticated approaches to utilizing reduced graphene oxide (rGO) for enhancing electrode kinetics across various electrochemical systems. Their research focuses on precise control of rGO's reduction degree to optimize the balance between electronic conductivity and electrochemically active sites. KERI's proprietary thermal-chemical reduction process creates rGO with tailored oxygen functional groups (typically 5-15% oxygen content) that serve as reaction sites while maintaining excellent conductivity. Their studies have demonstrated that strategically incorporating rGO into electrode structures can decrease charge transfer resistance by up to 70% compared to conventional carbon additives. KERI has pioneered a hierarchical electrode architecture where rGO forms a conductive backbone network throughout the electrode, creating efficient electron transport pathways while preserving open channels for ion diffusion. This structure has been shown to significantly enhance reaction kinetics, particularly in energy storage applications where rapid charge/discharge capabilities are critical. Their research has revealed that rGO's two-dimensional structure facilitates faster electron transfer across electrode interfaces by reducing tortuosity in electron transport paths. Additionally, KERI has developed specialized surface functionalization techniques for rGO that enhance its interaction with electrolytes, further improving charge transfer at the electrode-electrolyte interface.
Strengths: Exceptional control over rGO's electronic properties through precise reduction processes; superior integration capabilities with various active materials; excellent stability under electrochemical cycling conditions. Weaknesses: Complex synthesis procedures requiring specialized equipment; challenges in achieving uniform rGO quality at scale; higher cost compared to traditional carbon materials limiting commercial implementation.
South China University of Technology
Technical Solution: South China University of Technology has conducted extensive research on reduced graphene oxide (rGO) and its effects on electrode kinetics across various electrochemical systems. Their approach focuses on developing novel synthesis methods that produce rGO with optimized properties specifically tailored for enhancing electrode performance. The university's research team has pioneered a controlled reduction process that preserves specific oxygen-containing functional groups (typically 7-12% oxygen content) while restoring the sp² carbon network, creating rGO with both high conductivity and abundant active sites. Their studies have demonstrated that incorporating rGO into electrode materials can decrease charge transfer resistance by up to 65% compared to conventional carbon additives. The university has developed a unique "layer-by-layer" electrode fabrication technique where rGO sheets are strategically positioned between active material layers, creating efficient electron transport highways throughout the electrode structure. This configuration has been shown to significantly enhance reaction kinetics by providing direct conductive pathways while maintaining open channels for ion diffusion. Their research has revealed that rGO's two-dimensional structure with high specific surface area (typically >1200 m²/g) provides numerous interfacial contact points with active materials and electrolytes, facilitating rapid charge transfer processes. Additionally, they've demonstrated that rGO's mechanical flexibility allows electrodes to maintain structural integrity during cycling, preserving reaction kinetics over extended operation.
Strengths: Exceptional ability to form conductive networks at low loading percentages (typically 1-3 wt%); excellent compatibility with various electrode materials; superior stability under electrochemical conditions. Weaknesses: Challenges in preventing rGO agglomeration during electrode fabrication; difficulty in achieving consistent quality across different batches; potential environmental concerns related to some reduction methods using hazardous chemicals.
Key Scientific Breakthroughs in rGO Electrode Mechanisms
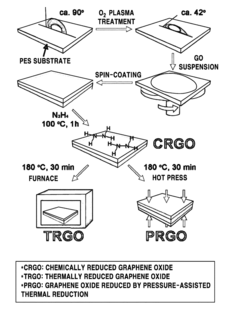
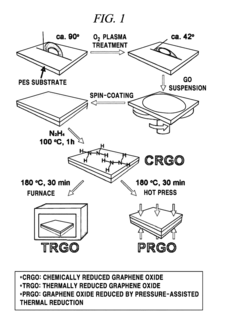
Preparing method of reduced graphene oxide film using a chemical reduction method and a pressure-assisted thermal reduction method, reduced graphene oxide film prepared by the same, and graphene electrode including the reduced graphene oxide film
PatentActiveUS9236156B2
Innovation
- A method involving the coating of a graphene oxide-dispersed solution on a substrate followed by a combination of chemical reduction and pressure-assisted thermal reduction to form a reduced graphene oxide film, allowing for efficient production at low temperatures and achieving low surface resistance and high conductivity.
Reduced graphene oxide for secondary battery, preparation method therefor, and electrode and secondary battery which use same
PatentWO2023080290A1
Innovation
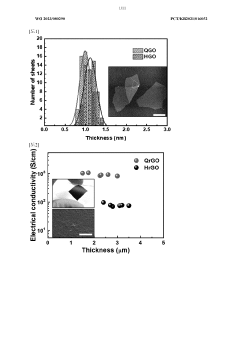
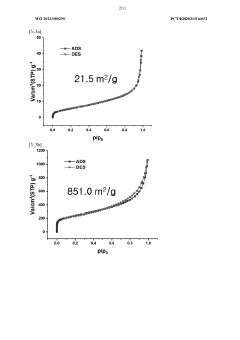
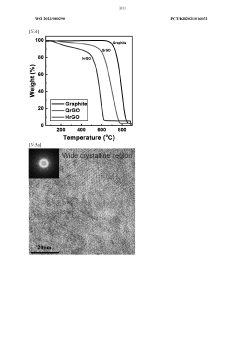
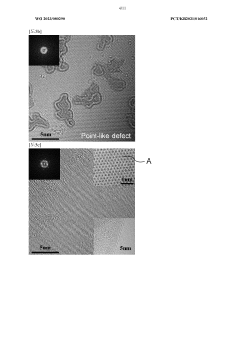
- A method to produce reduced graphene oxide with a thickness of 1 to 5 nm, electrical conductivity of 100 to 1,000 S/cm, and specific surface area of 1 to 100 m2/g, achieved by synthesizing graphite oxide flakes, exfoliating and dispersing them, and spray drying to form a crystalline powder with a two-dimensional structure, reducing defects and enhancing crystallinity.
Scalability and Manufacturing Considerations for rGO Electrodes
The scalability of reduced graphene oxide (rGO) electrode manufacturing represents a critical factor in determining the commercial viability of rGO-based electrochemical systems. Current laboratory-scale production methods, while effective for research purposes, face significant challenges when transitioning to industrial-scale manufacturing. These challenges include maintaining consistent quality, ensuring reproducibility of electrode kinetic properties, and managing production costs.
Batch-to-batch variation remains one of the most pressing issues in rGO electrode manufacturing. The reduction process of graphene oxide significantly influences the resulting electrochemical properties, with variations in reduction parameters leading to inconsistent electrode kinetics. Industrial-scale production requires standardized protocols that can deliver uniform rGO materials with predictable electrochemical performance across large production volumes.
Several manufacturing approaches show promise for scaling rGO electrode production. Roll-to-roll processing enables continuous production of rGO films with controlled thickness and surface properties, potentially allowing for high-throughput manufacturing of electrodes with consistent kinetic behavior. Spray coating and inkjet printing technologies offer alternative approaches for depositing rGO onto various substrates with precise control over electrode morphology, which directly impacts electrode kinetics.
The economic considerations of rGO electrode manufacturing cannot be overlooked. While graphene-based materials initially carried high production costs, recent advancements in manufacturing techniques have significantly reduced expenses. Cost-benefit analyses indicate that the enhanced electrode kinetics and resulting performance improvements may justify the additional production costs compared to traditional carbon electrodes in many applications.
Environmental and safety considerations also play crucial roles in scaling rGO production. The chemical reduction processes often involve hazardous reagents that require careful handling and waste management. Greener reduction methods, such as thermal, photocatalytic, or electrochemical approaches, are gaining attention for their potential to minimize environmental impact while maintaining the desired enhancement of electrode kinetics.
Quality control systems must evolve alongside manufacturing scale-up. Advanced characterization techniques, including in-line Raman spectroscopy and electrical conductivity measurements, can provide real-time feedback on rGO quality during production, ensuring consistent electrode kinetic properties across manufacturing batches.
Batch-to-batch variation remains one of the most pressing issues in rGO electrode manufacturing. The reduction process of graphene oxide significantly influences the resulting electrochemical properties, with variations in reduction parameters leading to inconsistent electrode kinetics. Industrial-scale production requires standardized protocols that can deliver uniform rGO materials with predictable electrochemical performance across large production volumes.
Several manufacturing approaches show promise for scaling rGO electrode production. Roll-to-roll processing enables continuous production of rGO films with controlled thickness and surface properties, potentially allowing for high-throughput manufacturing of electrodes with consistent kinetic behavior. Spray coating and inkjet printing technologies offer alternative approaches for depositing rGO onto various substrates with precise control over electrode morphology, which directly impacts electrode kinetics.
The economic considerations of rGO electrode manufacturing cannot be overlooked. While graphene-based materials initially carried high production costs, recent advancements in manufacturing techniques have significantly reduced expenses. Cost-benefit analyses indicate that the enhanced electrode kinetics and resulting performance improvements may justify the additional production costs compared to traditional carbon electrodes in many applications.
Environmental and safety considerations also play crucial roles in scaling rGO production. The chemical reduction processes often involve hazardous reagents that require careful handling and waste management. Greener reduction methods, such as thermal, photocatalytic, or electrochemical approaches, are gaining attention for their potential to minimize environmental impact while maintaining the desired enhancement of electrode kinetics.
Quality control systems must evolve alongside manufacturing scale-up. Advanced characterization techniques, including in-line Raman spectroscopy and electrical conductivity measurements, can provide real-time feedback on rGO quality during production, ensuring consistent electrode kinetic properties across manufacturing batches.
Environmental Impact and Sustainability of rGO Production
The production of reduced graphene oxide (rGO) presents significant environmental considerations that must be addressed as this material gains prominence in electrode applications. Traditional methods of rGO production often involve chemical reduction processes using hydrazine, sodium borohydride, or other hazardous reducing agents that pose substantial environmental and health risks. These chemicals can lead to water pollution, soil contamination, and potential harm to aquatic ecosystems if not properly managed during manufacturing processes.
Energy consumption represents another critical environmental concern in rGO production. The thermal reduction methods commonly employed require high temperatures (often exceeding 1000°C), resulting in considerable carbon footprints. This energy-intensive process contradicts the sustainability goals that many advanced materials aim to support, particularly when considering applications in green energy technologies.
Waste generation during rGO synthesis presents additional challenges. The purification steps generate acidic and toxic waste streams that require specialized treatment before disposal. Studies indicate that for every kilogram of rGO produced, approximately 5-10 kilograms of waste may be generated, highlighting the inefficiency of current production methods from a sustainability perspective.
Recent advancements have focused on developing more environmentally benign reduction methods. Green reduction approaches utilizing plant extracts, ascorbic acid, or microbial reduction have shown promise in laboratory settings. These bio-based reduction methods can significantly reduce the environmental impact while maintaining the desirable electrochemical properties of rGO for electrode applications. For instance, reduction using tea polyphenols has demonstrated up to 80% lower environmental impact compared to hydrazine-based methods.
Life cycle assessment (LCA) studies of rGO production reveal that the environmental footprint varies dramatically depending on the synthesis route. Electrochemical reduction methods show particular promise, with up to 60% lower global warming potential compared to chemical reduction approaches. These methods also typically consume less water and generate fewer hazardous byproducts.
Scaling considerations present additional sustainability challenges. While laboratory-scale production may demonstrate favorable environmental profiles, industrial-scale production introduces new variables including transportation impacts, storage requirements, and quality control measures that may affect the overall sustainability profile. Developing closed-loop systems for rGO production, where solvents and reagents are recovered and reused, represents a promising direction for improving the sustainability of large-scale manufacturing operations.
Energy consumption represents another critical environmental concern in rGO production. The thermal reduction methods commonly employed require high temperatures (often exceeding 1000°C), resulting in considerable carbon footprints. This energy-intensive process contradicts the sustainability goals that many advanced materials aim to support, particularly when considering applications in green energy technologies.
Waste generation during rGO synthesis presents additional challenges. The purification steps generate acidic and toxic waste streams that require specialized treatment before disposal. Studies indicate that for every kilogram of rGO produced, approximately 5-10 kilograms of waste may be generated, highlighting the inefficiency of current production methods from a sustainability perspective.
Recent advancements have focused on developing more environmentally benign reduction methods. Green reduction approaches utilizing plant extracts, ascorbic acid, or microbial reduction have shown promise in laboratory settings. These bio-based reduction methods can significantly reduce the environmental impact while maintaining the desirable electrochemical properties of rGO for electrode applications. For instance, reduction using tea polyphenols has demonstrated up to 80% lower environmental impact compared to hydrazine-based methods.
Life cycle assessment (LCA) studies of rGO production reveal that the environmental footprint varies dramatically depending on the synthesis route. Electrochemical reduction methods show particular promise, with up to 60% lower global warming potential compared to chemical reduction approaches. These methods also typically consume less water and generate fewer hazardous byproducts.
Scaling considerations present additional sustainability challenges. While laboratory-scale production may demonstrate favorable environmental profiles, industrial-scale production introduces new variables including transportation impacts, storage requirements, and quality control measures that may affect the overall sustainability profile. Developing closed-loop systems for rGO production, where solvents and reagents are recovered and reused, represents a promising direction for improving the sustainability of large-scale manufacturing operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!