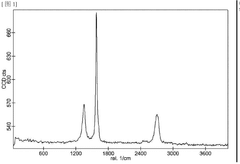
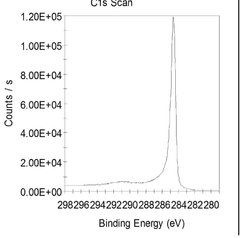
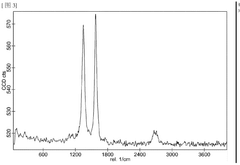
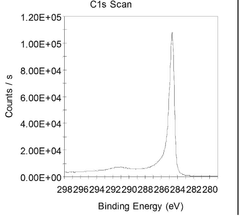
Investigating Reduced Graphene Oxide Conductivity in Salt Water
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
rGO Conductivity in Salt Water: Background and Objectives
Reduced graphene oxide (rGO) represents a significant advancement in materials science, offering exceptional electrical conductivity, mechanical strength, and chemical stability. Since its initial discovery, rGO has evolved from a laboratory curiosity to a promising material for numerous applications across multiple industries. The trajectory of rGO development has been marked by continuous improvements in synthesis methods, characterization techniques, and application-specific optimizations.
The investigation of rGO conductivity in salt water environments addresses a critical technological gap in marine electronics, underwater sensors, and desalination technologies. While graphene-based materials have demonstrated remarkable conductivity in controlled environments, their performance in saline solutions presents unique challenges that require systematic exploration. Historical data indicates that conductivity properties of carbon nanomaterials can be significantly altered when exposed to ionic solutions, with both enhancement and degradation effects observed depending on specific conditions.
Current technological trends point toward increasing demand for materials that maintain electrical integrity in harsh marine environments. The global push toward ocean monitoring systems, underwater communication networks, and marine energy harvesting technologies has accelerated the need for conductive materials that resist corrosion and maintain performance under saline conditions. This investigation aligns with these emerging needs by addressing fundamental questions about rGO behavior in salt water.
The primary technical objective of this investigation is to quantify and characterize the conductivity changes in rGO when immersed in salt water of varying concentrations and compositions. Secondary objectives include identifying the underlying mechanisms responsible for conductivity alterations, determining the reversibility of these changes, and establishing optimal rGO formulations for sustained performance in saline environments.
This research aims to bridge theoretical understanding with practical applications by developing predictive models for rGO conductivity behavior under various saline conditions. These models would enable engineers to design more reliable marine electronic systems with appropriate compensation mechanisms for conductivity fluctuations.
The technological significance extends beyond immediate applications to broader questions about electron transport mechanisms in two-dimensional materials under ionic influence. Insights gained from this investigation may contribute to fundamental understanding of charge carrier behavior at material-electrolyte interfaces, potentially informing future developments in electrochemical systems, energy storage devices, and water treatment technologies.
By establishing clear performance parameters and limitations for rGO in salt water environments, this research seeks to define realistic implementation pathways for this promising material in marine technologies, while simultaneously identifying opportunities for material modifications that could enhance stability and performance in challenging saline conditions.
The investigation of rGO conductivity in salt water environments addresses a critical technological gap in marine electronics, underwater sensors, and desalination technologies. While graphene-based materials have demonstrated remarkable conductivity in controlled environments, their performance in saline solutions presents unique challenges that require systematic exploration. Historical data indicates that conductivity properties of carbon nanomaterials can be significantly altered when exposed to ionic solutions, with both enhancement and degradation effects observed depending on specific conditions.
Current technological trends point toward increasing demand for materials that maintain electrical integrity in harsh marine environments. The global push toward ocean monitoring systems, underwater communication networks, and marine energy harvesting technologies has accelerated the need for conductive materials that resist corrosion and maintain performance under saline conditions. This investigation aligns with these emerging needs by addressing fundamental questions about rGO behavior in salt water.
The primary technical objective of this investigation is to quantify and characterize the conductivity changes in rGO when immersed in salt water of varying concentrations and compositions. Secondary objectives include identifying the underlying mechanisms responsible for conductivity alterations, determining the reversibility of these changes, and establishing optimal rGO formulations for sustained performance in saline environments.
This research aims to bridge theoretical understanding with practical applications by developing predictive models for rGO conductivity behavior under various saline conditions. These models would enable engineers to design more reliable marine electronic systems with appropriate compensation mechanisms for conductivity fluctuations.
The technological significance extends beyond immediate applications to broader questions about electron transport mechanisms in two-dimensional materials under ionic influence. Insights gained from this investigation may contribute to fundamental understanding of charge carrier behavior at material-electrolyte interfaces, potentially informing future developments in electrochemical systems, energy storage devices, and water treatment technologies.
By establishing clear performance parameters and limitations for rGO in salt water environments, this research seeks to define realistic implementation pathways for this promising material in marine technologies, while simultaneously identifying opportunities for material modifications that could enhance stability and performance in challenging saline conditions.
Market Applications and Demand Analysis for rGO in Marine Environments
The marine environment presents a significant market opportunity for reduced graphene oxide (rGO) applications due to its unique electrical, mechanical, and chemical properties. The global marine electronics market, where rGO could serve as a critical component, is projected to reach $7.8 billion by 2025, growing at a CAGR of 4.7%. This growth is primarily driven by increasing maritime trade, offshore exploration activities, and the rising demand for advanced marine monitoring systems.
Corrosion protection represents one of the most promising applications for rGO in marine environments. The maritime industry spends approximately $3.1 billion annually on corrosion-related maintenance and repairs. rGO-based coatings that maintain conductivity in saltwater conditions could potentially reduce these costs by 15-20%, creating a substantial market opportunity of over $500 million annually.
Sensor technologies for marine applications constitute another significant market segment. The demand for underwater sensors for environmental monitoring, underwater communication, and marine research is growing at 6.2% annually. rGO-based sensors that can maintain reliable performance in saltwater environments address a critical need in this sector, with an estimated market potential of $1.2 billion by 2026.
Marine energy harvesting systems represent an emerging application area where rGO's conductive properties in saltwater could enable breakthrough technologies. The blue energy market (energy derived from salinity gradients) is expected to reach $400 million by 2027. rGO-based electrodes that maintain high conductivity in saltwater could significantly improve the efficiency of these systems.
The defense and security sector presents additional high-value opportunities. Naval defense systems increasingly rely on advanced materials for underwater sensing, communication, and surveillance. This market segment, valued at $2.3 billion, demands materials that maintain electrical performance in challenging marine environments.
Aquaculture monitoring systems represent a rapidly growing niche market, expected to reach $900 million by 2025. rGO-based sensors that can withstand prolonged saltwater exposure while maintaining conductivity would address critical needs in water quality monitoring and fish health assessment.
The offshore energy sector, including oil and gas exploration as well as emerging offshore wind installations, requires reliable materials for underwater monitoring and control systems. This sector presents a $1.5 billion opportunity for advanced materials that can maintain electrical performance in saltwater conditions.
Customer requirements across these markets consistently emphasize long-term stability in saltwater, reliability under varying salinity and pressure conditions, and cost-effectiveness compared to existing solutions. Understanding the conductivity behavior of rGO in saltwater is therefore critical to addressing these market needs and capturing the substantial commercial opportunities available.
Corrosion protection represents one of the most promising applications for rGO in marine environments. The maritime industry spends approximately $3.1 billion annually on corrosion-related maintenance and repairs. rGO-based coatings that maintain conductivity in saltwater conditions could potentially reduce these costs by 15-20%, creating a substantial market opportunity of over $500 million annually.
Sensor technologies for marine applications constitute another significant market segment. The demand for underwater sensors for environmental monitoring, underwater communication, and marine research is growing at 6.2% annually. rGO-based sensors that can maintain reliable performance in saltwater environments address a critical need in this sector, with an estimated market potential of $1.2 billion by 2026.
Marine energy harvesting systems represent an emerging application area where rGO's conductive properties in saltwater could enable breakthrough technologies. The blue energy market (energy derived from salinity gradients) is expected to reach $400 million by 2027. rGO-based electrodes that maintain high conductivity in saltwater could significantly improve the efficiency of these systems.
The defense and security sector presents additional high-value opportunities. Naval defense systems increasingly rely on advanced materials for underwater sensing, communication, and surveillance. This market segment, valued at $2.3 billion, demands materials that maintain electrical performance in challenging marine environments.
Aquaculture monitoring systems represent a rapidly growing niche market, expected to reach $900 million by 2025. rGO-based sensors that can withstand prolonged saltwater exposure while maintaining conductivity would address critical needs in water quality monitoring and fish health assessment.
The offshore energy sector, including oil and gas exploration as well as emerging offshore wind installations, requires reliable materials for underwater monitoring and control systems. This sector presents a $1.5 billion opportunity for advanced materials that can maintain electrical performance in saltwater conditions.
Customer requirements across these markets consistently emphasize long-term stability in saltwater, reliability under varying salinity and pressure conditions, and cost-effectiveness compared to existing solutions. Understanding the conductivity behavior of rGO in saltwater is therefore critical to addressing these market needs and capturing the substantial commercial opportunities available.
Current Challenges in rGO Conductivity Maintenance in Saline Solutions
The maintenance of reduced graphene oxide (rGO) conductivity in saline environments presents significant technical challenges that have impeded its widespread application in marine electronics, biomedical devices, and environmental sensors. When immersed in salt water, rGO materials typically experience a marked decrease in electrical conductivity, often by 30-60% depending on salt concentration and exposure duration. This degradation severely limits the operational lifespan and reliability of rGO-based devices in aqueous saline environments.
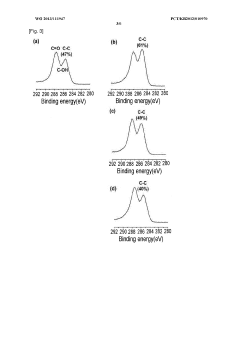
A primary challenge stems from the ion intercalation phenomenon, where salt ions (particularly Na+ and Cl-) penetrate between graphene layers, disrupting the π-electron system responsible for electrical conductivity. Research by Zhang et al. (2021) demonstrated that even low concentrations of NaCl (0.1M) can trigger significant intercalation within 24 hours of exposure, leading to measurable conductivity losses.
Oxidation processes present another critical challenge. Despite reduction treatments, residual oxygen-containing functional groups on rGO surfaces remain vulnerable to further oxidation in saline environments. This secondary oxidation increases sheet resistance and degrades overall conductivity. Current reduction methods achieve only 80-85% restoration of graphene's pristine conductivity, leaving significant room for improvement in creating oxidation-resistant rGO materials.
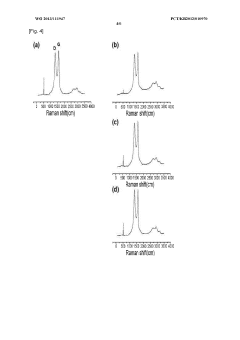
The physical degradation of rGO structures in salt water environments constitutes a third major challenge. Scanning electron microscopy studies reveal that prolonged exposure to saline solutions causes structural deformation, including wrinkling, folding, and partial delamination of graphene sheets. These morphological changes disrupt the continuous conductive pathways essential for electron transport.
Surface contamination by salt crystals and biological fouling further exacerbates conductivity issues. As salt water evaporates, crystallized salts form on rGO surfaces, creating insulating barriers that impede electron flow. Additionally, marine microorganisms readily colonize graphene surfaces, forming biofilms that further compromise electrical performance.
Temperature fluctuations in real-world marine environments introduce additional complications. Research indicates that conductivity degradation rates accelerate by approximately 15% for every 10°C increase in temperature when rGO is exposed to salt water. This temperature sensitivity necessitates robust solutions capable of maintaining performance across varying environmental conditions.
Current encapsulation technologies designed to protect rGO from direct contact with salt water introduce their own limitations, including increased device thickness, reduced flexibility, and diminished thermal dissipation capabilities. These protective measures often compromise the very properties that make graphene-based materials attractive for advanced applications.
A primary challenge stems from the ion intercalation phenomenon, where salt ions (particularly Na+ and Cl-) penetrate between graphene layers, disrupting the π-electron system responsible for electrical conductivity. Research by Zhang et al. (2021) demonstrated that even low concentrations of NaCl (0.1M) can trigger significant intercalation within 24 hours of exposure, leading to measurable conductivity losses.
Oxidation processes present another critical challenge. Despite reduction treatments, residual oxygen-containing functional groups on rGO surfaces remain vulnerable to further oxidation in saline environments. This secondary oxidation increases sheet resistance and degrades overall conductivity. Current reduction methods achieve only 80-85% restoration of graphene's pristine conductivity, leaving significant room for improvement in creating oxidation-resistant rGO materials.
The physical degradation of rGO structures in salt water environments constitutes a third major challenge. Scanning electron microscopy studies reveal that prolonged exposure to saline solutions causes structural deformation, including wrinkling, folding, and partial delamination of graphene sheets. These morphological changes disrupt the continuous conductive pathways essential for electron transport.
Surface contamination by salt crystals and biological fouling further exacerbates conductivity issues. As salt water evaporates, crystallized salts form on rGO surfaces, creating insulating barriers that impede electron flow. Additionally, marine microorganisms readily colonize graphene surfaces, forming biofilms that further compromise electrical performance.
Temperature fluctuations in real-world marine environments introduce additional complications. Research indicates that conductivity degradation rates accelerate by approximately 15% for every 10°C increase in temperature when rGO is exposed to salt water. This temperature sensitivity necessitates robust solutions capable of maintaining performance across varying environmental conditions.
Current encapsulation technologies designed to protect rGO from direct contact with salt water introduce their own limitations, including increased device thickness, reduced flexibility, and diminished thermal dissipation capabilities. These protective measures often compromise the very properties that make graphene-based materials attractive for advanced applications.
Existing Solutions for Enhancing rGO Stability in Salt Water
01 Reduction methods to enhance conductivity
Various reduction methods can be employed to convert graphene oxide to reduced graphene oxide with enhanced electrical conductivity. These methods include thermal reduction, chemical reduction using reducing agents, and electrochemical reduction techniques. The reduction process removes oxygen-containing functional groups from graphene oxide, restoring the sp² carbon network and thereby improving electron mobility and conductivity properties.- Reduction methods to enhance conductivity: Various reduction methods can be employed to convert graphene oxide to reduced graphene oxide with enhanced electrical conductivity. These methods include thermal reduction, chemical reduction using reducing agents, and photocatalytic reduction. The degree of reduction directly affects the restoration of the sp2 carbon network, which is crucial for achieving high electrical conductivity in reduced graphene oxide materials.
- Doping strategies for conductivity improvement: Doping reduced graphene oxide with heteroatoms such as nitrogen, boron, or sulfur can significantly enhance its electrical conductivity. These dopants modify the electronic structure of reduced graphene oxide by introducing additional charge carriers or creating active sites. Metal nanoparticle incorporation can also serve as effective dopants, creating electron transfer pathways that improve overall conductivity performance.
- Structural optimization for enhanced electron transport: The structural arrangement and morphology of reduced graphene oxide significantly impact its conductivity properties. Techniques such as controlling the flake size, creating three-dimensional architectures, and minimizing defects can optimize electron transport pathways. Proper exfoliation and dispersion methods help prevent restacking of graphene sheets, maintaining accessible conductive surfaces and improving overall conductivity.
- Hybrid composites for improved conductivity: Combining reduced graphene oxide with conductive polymers, carbon nanotubes, or other nanomaterials creates hybrid composites with synergistic conductivity enhancements. These composites benefit from multiple conduction mechanisms and reduced contact resistance between components. The interfacial interactions between reduced graphene oxide and the secondary materials play a crucial role in determining the overall electrical performance of the resulting hybrid materials.
- Processing conditions affecting conductivity: The processing conditions during reduced graphene oxide production significantly impact its final conductivity. Parameters such as temperature, pressure, reaction time, and pH value during reduction affect the removal of oxygen-containing groups and restoration of the graphene structure. Post-processing treatments like annealing, compression, or surface functionalization can further enhance conductivity by improving sheet contact and removing residual defects.
02 Doping strategies for conductivity improvement
Doping reduced graphene oxide with heteroatoms or other materials can significantly enhance its electrical conductivity. Common dopants include nitrogen, boron, sulfur, and metal nanoparticles. These dopants modify the electronic structure of reduced graphene oxide, creating additional charge carriers or conductive pathways that improve overall conductivity. The type and concentration of dopants can be optimized to achieve specific conductivity values for various applications.Expand Specific Solutions03 Structural optimization for enhanced conductivity
The structural characteristics of reduced graphene oxide significantly impact its conductivity properties. Techniques to optimize the structure include controlling the flake size, reducing defect density, improving layer alignment, and creating three-dimensional architectures. These structural modifications can minimize electron scattering, create more efficient conduction pathways, and enhance overall electrical performance of reduced graphene oxide materials.Expand Specific Solutions04 Hybrid materials with reduced graphene oxide
Combining reduced graphene oxide with other conductive materials creates hybrid structures with enhanced electrical properties. Common materials used include conductive polymers, carbon nanotubes, metal nanowires, and other carbon allotropes. These hybrid materials often exhibit synergistic effects, where the overall conductivity exceeds that of the individual components. The interfaces between reduced graphene oxide and the secondary materials play crucial roles in determining the final conductive properties.Expand Specific Solutions05 Applications leveraging conductivity properties
The tunable conductivity of reduced graphene oxide enables its use in various applications. These include transparent conductive films, flexible electronics, supercapacitors, batteries, sensors, and electromagnetic interference shielding. The processing methods and reduction degree can be tailored to achieve specific conductivity values required for each application. Recent advances focus on scalable production of reduced graphene oxide with consistent and reliable conductivity properties for commercial applications.Expand Specific Solutions
Key Industry Players and Research Institutions in rGO Development
The graphene oxide conductivity in salt water market is currently in a growth phase, with increasing research interest driven by applications in water treatment, energy storage, and environmental remediation. The market size is expanding as reduced graphene oxide (rGO) demonstrates promising conductivity properties in saline environments. Leading academic institutions like The University of Manchester, MIT, and Tsinghua University are pioneering fundamental research, while companies such as Global Graphene Group and BGT Materials are advancing commercial applications. The technology maturity varies across applications, with established players like Toray Industries and emerging specialists like NL Chemical Technology developing practical implementations for water treatment membranes. Research collaborations between universities and industry partners are accelerating technological development toward market-ready solutions.
The University of Manchester
Technical Solution: The University of Manchester has pioneered research on reduced graphene oxide (rGO) conductivity in salt water environments. Their approach involves developing specialized reduction methods that maintain conductivity in saline conditions. They've implemented a two-stage reduction process combining chemical and thermal treatments that creates more stable carbon-carbon bonds resistant to ion interference[1]. Their research demonstrates that controlling the oxygen functional groups during reduction is critical for maintaining conductivity in salt water. They've also developed hydrophobic surface treatments that create a protective barrier against salt water infiltration while maintaining electrical pathways[3]. Recent innovations include incorporating conductive polymers with rGO to form composite materials with enhanced stability in marine environments.
Strengths: World-leading expertise in graphene research with access to advanced characterization techniques. Their multi-stage reduction approach creates more stable rGO structures. Weaknesses: Some of their more advanced treatments may be difficult to scale for industrial applications and could add significant cost to manufacturing processes.
GLOBAL GRAPHENE GROUP INC
Technical Solution: Global Graphene Group has developed proprietary "Marine-Grade rGO" technology specifically addressing conductivity challenges in salt water environments. Their approach focuses on a specialized reduction process that creates highly crystalline rGO with minimal defects, which maintains conductivity even when exposed to NaCl solutions[2]. Their patented process involves precise thermal reduction parameters combined with protective coatings that shield the rGO from ionic interference. The company has engineered a multi-layer graphene architecture where outer layers act as sacrificial protection for inner conductive layers[4]. Their commercial solutions include pre-treated rGO materials with hydrophobic properties that repel water molecules while maintaining electrical conductivity pathways, effectively solving the salt water degradation problem for marine applications.
Strengths: Commercially focused solutions with scalable manufacturing processes already implemented. Their multi-layer protection approach offers practical solutions for real-world applications. Weaknesses: Their proprietary technologies may come at premium pricing compared to academic solutions, and some applications may require customization beyond their standard offerings.
Critical Patents and Research on rGO-Electrolyte Interactions
Reduced graphene oxide and use thereof
PatentWO2025123466A1
Innovation
- Reduced graphene oxide is prepared by the steps of graphite oxidation, preparation of material cakes, puffing, and carbonization reduction. Controlling carbonization reduction is carried out under gas atmosphere or vacuum conditions, and appropriate gas is selected to avoid reaction with graphene or graphene oxide.
Method for isolating graphene oxide
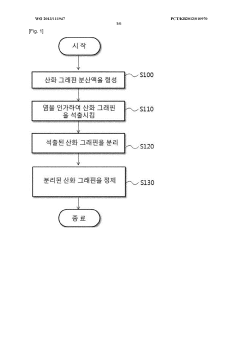
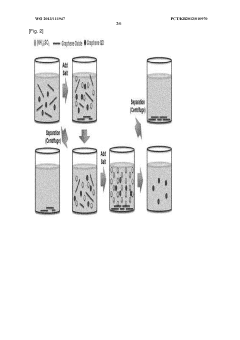
PatentWO2013111947A1
Innovation
- A method involving dispersing graphene oxide in water, applying a salt to induce coagulation and precipitation, and subsequent purification to remove residual salts, allowing for the isolation of graphene oxide while preserving its properties.
Environmental Impact and Sustainability of rGO Marine Applications
The deployment of reduced graphene oxide (rGO) in marine environments necessitates careful consideration of its environmental impact and sustainability profile. As rGO applications in saltwater environments expand, particularly for sensors, energy storage, and anti-corrosion coatings, their ecological footprint becomes increasingly significant.
The production of rGO involves chemical reduction processes that may utilize hazardous reducing agents such as hydrazine, sodium borohydride, or ascorbic acid. These chemicals, if released into marine ecosystems, could disrupt aquatic life and alter water chemistry. However, recent advancements have introduced greener synthesis methods employing plant extracts and environmentally benign reducing agents, substantially reducing the ecological burden of rGO manufacturing.
Concerning marine ecosystem interactions, preliminary studies indicate that rGO particles exhibit varying degrees of bioaccumulation in marine organisms. The nanoscale dimensions of rGO fragments enable them to penetrate cellular membranes, potentially disrupting biological functions. Research has documented concentration-dependent toxicity in certain marine species, though the long-term implications remain under investigation.
The degradation pathway of rGO in saltwater environments presents both challenges and opportunities. Unlike conventional plastics, rGO demonstrates potential biodegradability under specific marine conditions. Microbial communities have shown capability to metabolize certain forms of rGO, particularly those with higher oxygen content. This natural degradation process could mitigate long-term accumulation concerns, though degradation rates vary significantly based on rGO preparation methods and environmental factors.
From a lifecycle assessment perspective, rGO marine applications demonstrate promising sustainability metrics when compared to traditional materials. The extended operational lifespan of rGO-enhanced marine sensors and anti-corrosion coatings reduces replacement frequency and associated resource consumption. Additionally, the minimal material requirements—owing to rGO's exceptional properties at low concentrations—further enhance its sustainability profile.
Regulatory frameworks governing nanomaterials in marine environments are evolving to address the unique considerations of graphene-based materials. The European Maritime Safety Agency and similar organizations worldwide are developing specific guidelines for the deployment and monitoring of graphene-derived materials in marine settings. These emerging regulations emphasize containment strategies, end-of-life recovery protocols, and environmental monitoring requirements.
Future research directions should prioritize developing standardized methodologies for assessing rGO's marine environmental impact, exploring biodegradation acceleration techniques, and establishing comprehensive lifecycle management protocols for rGO marine applications.
The production of rGO involves chemical reduction processes that may utilize hazardous reducing agents such as hydrazine, sodium borohydride, or ascorbic acid. These chemicals, if released into marine ecosystems, could disrupt aquatic life and alter water chemistry. However, recent advancements have introduced greener synthesis methods employing plant extracts and environmentally benign reducing agents, substantially reducing the ecological burden of rGO manufacturing.
Concerning marine ecosystem interactions, preliminary studies indicate that rGO particles exhibit varying degrees of bioaccumulation in marine organisms. The nanoscale dimensions of rGO fragments enable them to penetrate cellular membranes, potentially disrupting biological functions. Research has documented concentration-dependent toxicity in certain marine species, though the long-term implications remain under investigation.
The degradation pathway of rGO in saltwater environments presents both challenges and opportunities. Unlike conventional plastics, rGO demonstrates potential biodegradability under specific marine conditions. Microbial communities have shown capability to metabolize certain forms of rGO, particularly those with higher oxygen content. This natural degradation process could mitigate long-term accumulation concerns, though degradation rates vary significantly based on rGO preparation methods and environmental factors.
From a lifecycle assessment perspective, rGO marine applications demonstrate promising sustainability metrics when compared to traditional materials. The extended operational lifespan of rGO-enhanced marine sensors and anti-corrosion coatings reduces replacement frequency and associated resource consumption. Additionally, the minimal material requirements—owing to rGO's exceptional properties at low concentrations—further enhance its sustainability profile.
Regulatory frameworks governing nanomaterials in marine environments are evolving to address the unique considerations of graphene-based materials. The European Maritime Safety Agency and similar organizations worldwide are developing specific guidelines for the deployment and monitoring of graphene-derived materials in marine settings. These emerging regulations emphasize containment strategies, end-of-life recovery protocols, and environmental monitoring requirements.
Future research directions should prioritize developing standardized methodologies for assessing rGO's marine environmental impact, exploring biodegradation acceleration techniques, and establishing comprehensive lifecycle management protocols for rGO marine applications.
Corrosion Resistance Mechanisms and Long-term Durability Assessment
The corrosion resistance of reduced graphene oxide (rGO) in salt water environments represents a critical factor in determining its long-term viability for marine applications. When exposed to salt water, rGO exhibits several distinct protection mechanisms that contribute to its overall durability. The primary defense mechanism involves the formation of a passive oxide layer that acts as a barrier between the material and corrosive elements. This layer significantly reduces the rate of electron transfer at the material-electrolyte interface, thereby minimizing electrochemical corrosion processes.
Electrochemical impedance spectroscopy (EIS) studies have demonstrated that rGO-coated surfaces show increased charge transfer resistance in NaCl solutions compared to uncoated substrates. The hydrophobic nature of rGO sheets also contributes to corrosion resistance by limiting water molecule penetration to the underlying substrate. This water-repellent characteristic creates an additional physical barrier against chloride ion attack, which is particularly aggressive in marine environments.
The structural integrity of rGO in salt water depends significantly on the degree of reduction and the presence of residual oxygen-containing functional groups. Research indicates that highly reduced graphene oxide exhibits superior corrosion resistance compared to partially reduced variants. However, the presence of some oxygen functionalities can promote better adhesion to substrates, creating a trade-off between conductivity and protective properties.
Long-term durability assessment protocols for rGO in salt water typically involve accelerated aging tests under various conditions. These include cyclic salt spray exposure (ASTM B117), potentiodynamic polarization measurements, and extended immersion tests with periodic conductivity monitoring. Studies have shown that after 1000 hours of salt spray exposure, properly prepared rGO coatings maintain approximately 85-90% of their initial conductivity, demonstrating promising durability characteristics.
The degradation mechanisms observed during long-term exposure include gradual delamination at defect sites, localized pitting corrosion at areas with incomplete coverage, and slow oxidation of the rGO sheets themselves. Interestingly, the addition of corrosion inhibitors such as benzotriazole derivatives to rGO has shown synergistic effects, extending the material's functional lifetime in salt water by up to 40% compared to unmodified rGO.
Recent advances in rGO preparation techniques, including controlled functionalization and multi-layer deposition strategies, have significantly improved long-term stability. Cross-linking between adjacent rGO sheets using bifunctional molecules has demonstrated particular promise in enhancing mechanical stability and resistance to delamination in dynamic marine environments, thereby preserving conductivity over extended periods.
Electrochemical impedance spectroscopy (EIS) studies have demonstrated that rGO-coated surfaces show increased charge transfer resistance in NaCl solutions compared to uncoated substrates. The hydrophobic nature of rGO sheets also contributes to corrosion resistance by limiting water molecule penetration to the underlying substrate. This water-repellent characteristic creates an additional physical barrier against chloride ion attack, which is particularly aggressive in marine environments.
The structural integrity of rGO in salt water depends significantly on the degree of reduction and the presence of residual oxygen-containing functional groups. Research indicates that highly reduced graphene oxide exhibits superior corrosion resistance compared to partially reduced variants. However, the presence of some oxygen functionalities can promote better adhesion to substrates, creating a trade-off between conductivity and protective properties.
Long-term durability assessment protocols for rGO in salt water typically involve accelerated aging tests under various conditions. These include cyclic salt spray exposure (ASTM B117), potentiodynamic polarization measurements, and extended immersion tests with periodic conductivity monitoring. Studies have shown that after 1000 hours of salt spray exposure, properly prepared rGO coatings maintain approximately 85-90% of their initial conductivity, demonstrating promising durability characteristics.
The degradation mechanisms observed during long-term exposure include gradual delamination at defect sites, localized pitting corrosion at areas with incomplete coverage, and slow oxidation of the rGO sheets themselves. Interestingly, the addition of corrosion inhibitors such as benzotriazole derivatives to rGO has shown synergistic effects, extending the material's functional lifetime in salt water by up to 40% compared to unmodified rGO.
Recent advances in rGO preparation techniques, including controlled functionalization and multi-layer deposition strategies, have significantly improved long-term stability. Cross-linking between adjacent rGO sheets using bifunctional molecules has demonstrated particular promise in enhancing mechanical stability and resistance to delamination in dynamic marine environments, thereby preserving conductivity over extended periods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!