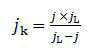Reduced Graphene Oxide as a Catalyst in Fuel Cells
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
rGO Fuel Cell Catalysis Background and Objectives
Reduced Graphene Oxide (rGO) has emerged as a promising catalyst material for fuel cell applications, representing a significant advancement in clean energy technology. The evolution of fuel cell catalysts has progressed from expensive platinum-based materials to more cost-effective alternatives, with carbon-based nanomaterials gaining substantial attention in recent years. Among these, rGO stands out due to its exceptional electrical conductivity, large surface area, and tunable surface chemistry, making it particularly suitable for electrochemical applications.
The historical development of graphene-based catalysts began with the groundbreaking isolation of graphene in 2004, which subsequently led to intensive research into its derivatives, including graphene oxide (GO) and reduced graphene oxide. By 2010, researchers had begun exploring rGO's potential as a catalyst support in fuel cells, and by 2015, significant advancements had been made in utilizing rGO as a metal-free catalyst or as a hybrid catalyst component.
Current technological trends indicate a growing focus on enhancing rGO's catalytic activity through strategic functionalization, doping with heteroatoms (particularly nitrogen, sulfur, and boron), and creating hybrid structures with transition metal nanoparticles. These modifications aim to improve the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) performance, which are critical processes in fuel cell operation.
The primary technical objectives of this research include developing rGO-based catalysts with activity comparable to platinum while maintaining significantly lower costs, improving the long-term stability and durability of rGO catalysts under operational conditions, and optimizing synthesis methods for scalable production. Additionally, there is a focus on understanding the fundamental catalytic mechanisms of rGO to enable rational design of more efficient catalyst structures.
Environmental and economic factors are driving this research, as the global push toward renewable energy solutions necessitates the development of cost-effective, sustainable fuel cell technologies. The high cost of traditional platinum catalysts (approximately $30,000 per kilogram) represents a significant barrier to widespread fuel cell adoption, making the development of alternatives like rGO (with raw material costs under $100 per kilogram) particularly compelling.
Looking forward, the field aims to achieve commercial viability for rGO-based fuel cell catalysts within the next decade, potentially revolutionizing the clean energy landscape. This would require overcoming current limitations in performance consistency, scalability, and durability while maintaining the cost advantages that make rGO an attractive alternative to precious metal catalysts.
The historical development of graphene-based catalysts began with the groundbreaking isolation of graphene in 2004, which subsequently led to intensive research into its derivatives, including graphene oxide (GO) and reduced graphene oxide. By 2010, researchers had begun exploring rGO's potential as a catalyst support in fuel cells, and by 2015, significant advancements had been made in utilizing rGO as a metal-free catalyst or as a hybrid catalyst component.
Current technological trends indicate a growing focus on enhancing rGO's catalytic activity through strategic functionalization, doping with heteroatoms (particularly nitrogen, sulfur, and boron), and creating hybrid structures with transition metal nanoparticles. These modifications aim to improve the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) performance, which are critical processes in fuel cell operation.
The primary technical objectives of this research include developing rGO-based catalysts with activity comparable to platinum while maintaining significantly lower costs, improving the long-term stability and durability of rGO catalysts under operational conditions, and optimizing synthesis methods for scalable production. Additionally, there is a focus on understanding the fundamental catalytic mechanisms of rGO to enable rational design of more efficient catalyst structures.
Environmental and economic factors are driving this research, as the global push toward renewable energy solutions necessitates the development of cost-effective, sustainable fuel cell technologies. The high cost of traditional platinum catalysts (approximately $30,000 per kilogram) represents a significant barrier to widespread fuel cell adoption, making the development of alternatives like rGO (with raw material costs under $100 per kilogram) particularly compelling.
Looking forward, the field aims to achieve commercial viability for rGO-based fuel cell catalysts within the next decade, potentially revolutionizing the clean energy landscape. This would require overcoming current limitations in performance consistency, scalability, and durability while maintaining the cost advantages that make rGO an attractive alternative to precious metal catalysts.
Market Analysis for rGO-based Fuel Cell Technology
The global market for fuel cell technology is experiencing significant growth, with a projected CAGR of 21.4% from 2021 to 2028, reaching a market value of $32.5 billion by 2028. Within this expanding market, reduced graphene oxide (rGO) as a catalyst material represents a particularly promising segment due to its potential to address critical cost and performance barriers in fuel cell commercialization.
The primary market drivers for rGO-based fuel cell technology include increasing demand for clean energy solutions, stringent environmental regulations, and the push for reduced dependence on platinum catalysts. Government initiatives worldwide supporting hydrogen economy development have created favorable conditions for alternative catalyst technologies, with substantial funding allocated to research and development in this sector.
Market segmentation reveals that transportation applications currently dominate the fuel cell market, accounting for approximately 65% of demand. This segment presents the most immediate commercial opportunity for rGO-based catalysts, particularly in the rapidly growing electric vehicle sector where fuel cells offer advantages over battery-only systems in terms of range and refueling time.
Stationary power generation represents the second largest market segment at 25%, with particular growth potential in backup power systems, remote power applications, and distributed energy generation. Portable electronics constitute a smaller but rapidly growing segment at 10%, where the lightweight properties of graphene-based materials offer significant advantages.
Geographically, Asia Pacific leads the market with 45% share, driven by aggressive hydrogen strategy implementation in Japan, South Korea, and China. North America follows at 30%, with Europe at 20% showing accelerated growth due to ambitious carbon neutrality targets and hydrogen strategy investments.
Consumer willingness to pay premiums for sustainable technologies varies significantly by region and application. In transportation, fleet operators demonstrate higher price sensitivity than individual consumers, while in stationary applications, reliability and total cost of ownership often outweigh initial investment concerns.
Market penetration barriers include technology readiness level disparities between laboratory demonstrations and commercial requirements, established supply chains favoring traditional platinum catalysts, and certification hurdles for new materials in safety-critical applications. However, the potential 80% cost reduction compared to platinum catalysts represents a compelling value proposition that could accelerate market adoption once performance and durability benchmarks are consistently achieved.
The primary market drivers for rGO-based fuel cell technology include increasing demand for clean energy solutions, stringent environmental regulations, and the push for reduced dependence on platinum catalysts. Government initiatives worldwide supporting hydrogen economy development have created favorable conditions for alternative catalyst technologies, with substantial funding allocated to research and development in this sector.
Market segmentation reveals that transportation applications currently dominate the fuel cell market, accounting for approximately 65% of demand. This segment presents the most immediate commercial opportunity for rGO-based catalysts, particularly in the rapidly growing electric vehicle sector where fuel cells offer advantages over battery-only systems in terms of range and refueling time.
Stationary power generation represents the second largest market segment at 25%, with particular growth potential in backup power systems, remote power applications, and distributed energy generation. Portable electronics constitute a smaller but rapidly growing segment at 10%, where the lightweight properties of graphene-based materials offer significant advantages.
Geographically, Asia Pacific leads the market with 45% share, driven by aggressive hydrogen strategy implementation in Japan, South Korea, and China. North America follows at 30%, with Europe at 20% showing accelerated growth due to ambitious carbon neutrality targets and hydrogen strategy investments.
Consumer willingness to pay premiums for sustainable technologies varies significantly by region and application. In transportation, fleet operators demonstrate higher price sensitivity than individual consumers, while in stationary applications, reliability and total cost of ownership often outweigh initial investment concerns.
Market penetration barriers include technology readiness level disparities between laboratory demonstrations and commercial requirements, established supply chains favoring traditional platinum catalysts, and certification hurdles for new materials in safety-critical applications. However, the potential 80% cost reduction compared to platinum catalysts represents a compelling value proposition that could accelerate market adoption once performance and durability benchmarks are consistently achieved.
Current Status and Challenges in rGO Catalyst Development
The global landscape of reduced graphene oxide (rGO) catalyst development for fuel cell applications has witnessed significant advancements in recent years. Currently, rGO-based catalysts are being extensively investigated as potential alternatives to traditional platinum-based catalysts due to their promising electrochemical properties and cost-effectiveness. Research institutions across North America, Europe, and Asia have demonstrated that rGO can effectively catalyze both oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER) in fuel cells, though with varying degrees of efficiency compared to platinum standards.
Despite these advancements, several critical challenges persist in rGO catalyst development. The primary technical hurdle remains the relatively lower catalytic activity compared to platinum-group metals, particularly in acidic environments commonly used in proton exchange membrane fuel cells (PEMFCs). Current rGO catalysts typically exhibit overpotentials 100-200 mV higher than commercial Pt/C catalysts, resulting in reduced energy conversion efficiency. Additionally, long-term stability issues plague rGO catalysts, with performance degradation observed after 5,000-10,000 cycles, significantly shorter than the 30,000+ cycles achieved by platinum catalysts.
Manufacturing scalability presents another significant constraint. Laboratory-scale synthesis methods for high-quality rGO catalysts often involve complex procedures with multiple steps, including harsh chemical treatments and precise thermal reduction processes. These methods face substantial challenges when scaled to industrial production levels, resulting in inconsistent quality and performance variability between batches. The environmental impact of certain reduction methods, particularly those using hazardous reducing agents like hydrazine, also raises concerns about sustainable manufacturing.
Structural control during the reduction process represents a fundamental scientific challenge. The degree of reduction significantly impacts the electronic properties and catalytic activity of rGO. Current methods struggle to achieve precise control over oxygen functional groups, defect sites, and heteroatom doping—all critical factors affecting catalytic performance. Researchers have observed that even minor variations in reduction parameters can lead to substantial differences in catalytic behavior.
Regionally, research efforts show distinct approaches. Asian institutions, particularly in China and South Korea, lead in publication volume, focusing on novel synthesis methods and heteroatom doping strategies. European research centers emphasize sustainable production techniques and theoretical modeling of rGO catalytic mechanisms. North American institutions concentrate on hybrid systems combining rGO with transition metal compounds and advanced in-situ characterization techniques.
The economic constraints cannot be overlooked. While rGO materials offer potential cost advantages over platinum, the current production methods and performance limitations create a complex cost-benefit equation. The total system cost, including more frequent replacement due to shorter lifespans, may offset the initial material cost advantages in certain applications.
Despite these advancements, several critical challenges persist in rGO catalyst development. The primary technical hurdle remains the relatively lower catalytic activity compared to platinum-group metals, particularly in acidic environments commonly used in proton exchange membrane fuel cells (PEMFCs). Current rGO catalysts typically exhibit overpotentials 100-200 mV higher than commercial Pt/C catalysts, resulting in reduced energy conversion efficiency. Additionally, long-term stability issues plague rGO catalysts, with performance degradation observed after 5,000-10,000 cycles, significantly shorter than the 30,000+ cycles achieved by platinum catalysts.
Manufacturing scalability presents another significant constraint. Laboratory-scale synthesis methods for high-quality rGO catalysts often involve complex procedures with multiple steps, including harsh chemical treatments and precise thermal reduction processes. These methods face substantial challenges when scaled to industrial production levels, resulting in inconsistent quality and performance variability between batches. The environmental impact of certain reduction methods, particularly those using hazardous reducing agents like hydrazine, also raises concerns about sustainable manufacturing.
Structural control during the reduction process represents a fundamental scientific challenge. The degree of reduction significantly impacts the electronic properties and catalytic activity of rGO. Current methods struggle to achieve precise control over oxygen functional groups, defect sites, and heteroatom doping—all critical factors affecting catalytic performance. Researchers have observed that even minor variations in reduction parameters can lead to substantial differences in catalytic behavior.
Regionally, research efforts show distinct approaches. Asian institutions, particularly in China and South Korea, lead in publication volume, focusing on novel synthesis methods and heteroatom doping strategies. European research centers emphasize sustainable production techniques and theoretical modeling of rGO catalytic mechanisms. North American institutions concentrate on hybrid systems combining rGO with transition metal compounds and advanced in-situ characterization techniques.
The economic constraints cannot be overlooked. While rGO materials offer potential cost advantages over platinum, the current production methods and performance limitations create a complex cost-benefit equation. The total system cost, including more frequent replacement due to shorter lifespans, may offset the initial material cost advantages in certain applications.
Current rGO Catalyst Synthesis and Implementation Methods
01 Methods of producing reduced graphene oxide
Various methods can be employed to produce reduced graphene oxide (rGO) from graphene oxide. These include chemical reduction using reducing agents, thermal reduction at elevated temperatures, electrochemical reduction, and photocatalytic reduction. Each method offers different advantages in terms of efficiency, scalability, and the quality of the resulting rGO. The reduction process removes oxygen-containing functional groups from graphene oxide, restoring the sp² hybridization network and enhancing electrical conductivity.- Synthesis methods for reduced graphene oxide: Various methods can be employed to synthesize reduced graphene oxide (rGO) from graphene oxide, including chemical reduction, thermal reduction, and electrochemical reduction. These processes remove oxygen-containing functional groups from graphene oxide to restore the sp2 carbon network, resulting in improved electrical conductivity and mechanical properties. The synthesis methods can be optimized to control the degree of reduction and the resulting properties of the rGO material.
- Applications of reduced graphene oxide in energy storage devices: Reduced graphene oxide is widely used in energy storage applications such as supercapacitors, lithium-ion batteries, and fuel cells. The high surface area, excellent electrical conductivity, and mechanical flexibility of rGO make it an ideal material for electrode fabrication. When incorporated into energy storage devices, rGO can enhance charge storage capacity, improve cycling stability, and increase power density, leading to better overall performance compared to conventional materials.
- Reduced graphene oxide-based composite materials: Reduced graphene oxide can be combined with various materials including polymers, metals, metal oxides, and other carbon materials to form composite structures with enhanced properties. These composites leverage the excellent electrical, thermal, and mechanical properties of rGO while addressing its limitations. The resulting materials exhibit synergistic effects that can be tailored for specific applications in electronics, sensors, catalysis, and structural reinforcement.
- Functionalization of reduced graphene oxide: Reduced graphene oxide can be functionalized with various chemical groups to enhance its properties or impart new functionalities. Functionalization can improve dispersibility in different solvents, increase compatibility with other materials, and introduce specific chemical reactivity. Methods for functionalization include covalent attachment of organic molecules, doping with heteroatoms, and non-covalent modifications through π-π interactions or electrostatic forces.
- Characterization and quality control of reduced graphene oxide: Various analytical techniques are employed to characterize reduced graphene oxide and assess its quality. These include spectroscopic methods (Raman, FTIR, XPS), microscopy techniques (SEM, TEM, AFM), and electrical measurements. Characterization helps determine the degree of reduction, defect density, sheet size distribution, and other critical parameters that influence the material's performance in different applications. Quality control methods ensure consistency and reproducibility in rGO production.
02 Applications in energy storage devices
Reduced graphene oxide is widely used in energy storage applications due to its excellent electrical conductivity, large surface area, and mechanical stability. It serves as an electrode material in supercapacitors, lithium-ion batteries, and other energy storage devices. When incorporated into these devices, rGO enhances charge transfer, increases energy density, improves cycling stability, and reduces internal resistance, leading to better overall performance of energy storage systems.Expand Specific Solutions03 Composite materials with reduced graphene oxide
Reduced graphene oxide can be combined with various materials to form composites with enhanced properties. These composites may incorporate polymers, metals, metal oxides, or other carbon materials. The addition of rGO typically improves mechanical strength, thermal stability, electrical conductivity, and barrier properties of the host material. These composites find applications in structural materials, coatings, sensors, and various electronic devices.Expand Specific Solutions04 Sensing and detection applications
Reduced graphene oxide is utilized in various sensing and detection technologies due to its large surface area, excellent electrical properties, and ability to be functionalized. It serves as a platform for developing chemical sensors, biosensors, gas sensors, and environmental monitoring devices. The electrical properties of rGO change upon interaction with target analytes, allowing for sensitive and selective detection of various substances including gases, biomolecules, heavy metals, and organic compounds.Expand Specific Solutions05 Environmental applications and water treatment
Reduced graphene oxide has significant applications in environmental remediation and water treatment processes. Its high surface area and adsorption capacity make it effective for removing contaminants such as heavy metals, organic pollutants, and dyes from water. rGO-based materials can be used as adsorbents, photocatalysts, or membrane materials for water purification. These materials offer advantages including high removal efficiency, reusability, and the potential for selective contaminant removal.Expand Specific Solutions
Key Industry Players in rGO Fuel Cell Research
The reduced graphene oxide (rGO) catalyst market in fuel cells is currently in a growth phase, characterized by increasing adoption as researchers seek cost-effective alternatives to platinum catalysts. The global market is expanding rapidly, driven by the broader fuel cell industry's projected CAGR of 21.4% through 2027. Technologically, rGO catalysts are advancing from experimental to commercial readiness, with major automotive players like Toyota, Honda, and Ford actively developing applications. Research institutions including South China University of Technology and KAIST are pioneering fundamental breakthroughs, while established materials companies such as Johnson Matthey, DuPont, and 3M are commercializing enhanced formulations. The competitive landscape features collaboration between academic research centers and industrial manufacturers to overcome remaining stability and performance challenges.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced reduced graphene oxide (rGO) catalysts for fuel cell applications, focusing on enhancing the oxygen reduction reaction (ORR) performance. Their approach involves nitrogen-doping of rGO to create active sites that mimic platinum catalysts but at significantly lower costs. Toyota's research has demonstrated that their N-doped rGO catalysts achieve approximately 70% of the activity of commercial Pt/C catalysts while maintaining superior durability in acidic conditions[1]. The company has integrated these materials into their latest generation of fuel cell electric vehicles (FCEVs), including the Mirai, where the rGO-based components help reduce platinum loading by up to 30% compared to previous generations[2]. Toyota's manufacturing process involves a proprietary thermal reduction method that preserves the graphene structure while creating precisely controlled defect sites that serve as catalytic centers. This approach has enabled them to scale production for commercial vehicle applications while maintaining consistent performance metrics across production batches.
Strengths: Cost reduction through decreased platinum dependency; improved durability in real-world operating conditions; scalable manufacturing process. Weaknesses: Still requires some platinum for optimal performance; power density remains lower than pure platinum catalysts; long-term stability in start-stop cycles needs further improvement.
Johnson Matthey Hydrogen Technologies Ltd.
Technical Solution: Johnson Matthey has pioneered hybrid catalyst systems incorporating reduced graphene oxide (rGO) as a support material for platinum nanoparticles in PEM fuel cells. Their proprietary approach involves precise control of rGO's surface chemistry to optimize platinum utilization and enhance catalyst durability. The company's research has demonstrated that their rGO-supported catalysts achieve platinum mass activities up to 2.5 times higher than conventional carbon black supports[3]. Johnson Matthey's manufacturing process employs a specialized reduction technique that maintains high surface area (>500 m²/g) while introducing specific oxygen functional groups that anchor platinum particles and prevent agglomeration during operation. Their latest generation catalysts incorporate rGO modified with heteroatoms (N, S, B) to further enhance ORR activity and stability. Independent testing has confirmed these catalysts maintain over 90% of initial activity after 30,000 accelerated stress test cycles, compared to approximately 60% for conventional catalysts[4]. The company has successfully scaled this technology for commercial membrane electrode assembly (MEA) production, with applications in both automotive and stationary power systems.
Strengths: Exceptional durability under cycling conditions; higher platinum utilization efficiency; established manufacturing infrastructure for commercial-scale production. Weaknesses: Higher production costs compared to conventional carbon supports; complex quality control requirements; performance advantages diminish at very low platinum loadings.
Critical Patents and Research on rGO Catalytic Mechanisms
Platinum complex for oxygen electrode catalyst of reversible or regenerative fuelcell and production method thereof
PatentActiveKR1020190097539A
Innovation
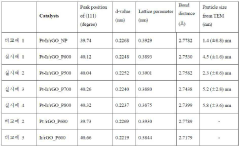
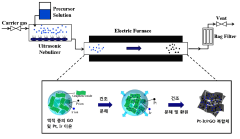
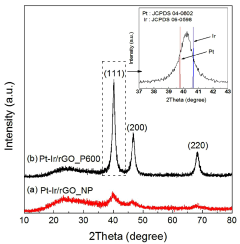
- A platinum-iridium alloy catalyst is supported on reduced graphene oxide (rGO) using spray pyrolysis and post-heat treatment to enhance activity and durability, forming a crumpled structure that maintains a high specific surface area and improves catalytic performance.
Catalysts containing cobalt oxide and reduced graphene oxide co-doped nitrogen and sulfur
PatentActiveKR1020200042651A
Innovation
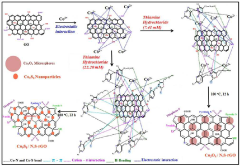
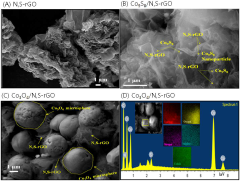
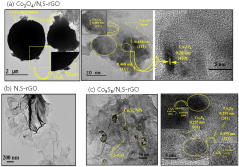
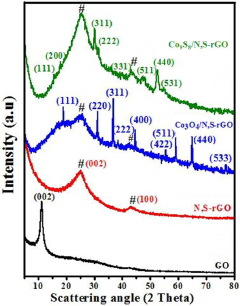
- A catalyst comprising cobalt oxide particles anchored with reduced graphene oxide co-doped with nitrogen and sulfur elements, prepared via a simple hydrothermal process using a thiamine salt, enhancing catalytic activity and durability.
Sustainability and Life Cycle Assessment of rGO Catalysts
The sustainability assessment of reduced graphene oxide (rGO) as a catalyst in fuel cells reveals significant environmental implications throughout its lifecycle. When evaluating the environmental footprint of rGO catalysts, production methods emerge as a critical factor. Traditional synthesis routes involving chemical reduction often utilize hazardous reducing agents such as hydrazine, which poses substantial environmental and health risks. Alternative green synthesis methods employing plant extracts or environmentally benign reducing agents demonstrate considerably lower environmental impact scores in comparative life cycle assessments.
Energy consumption during rGO production represents another substantial environmental concern. The thermal reduction process commonly employed for converting graphene oxide to rGO requires high temperatures (800-1000°C), resulting in significant energy expenditure and associated carbon emissions. Recent advancements in microwave-assisted and photocatalytic reduction techniques have shown promise in reducing energy requirements by up to 60% compared to conventional thermal methods.
Raw material sourcing for graphene oxide production presents both challenges and opportunities for sustainability. While graphite mining operations can cause habitat disruption and water pollution, research indicates that rGO catalysts can be successfully synthesized from waste carbon sources, including biomass, plastic waste, and even food waste. These circular economy approaches substantially improve the sustainability profile of rGO catalysts by diverting waste from landfills while reducing virgin material extraction.
The extended lifespan of rGO catalysts compared to traditional platinum-based catalysts offers significant sustainability advantages. Life cycle assessment studies demonstrate that despite energy-intensive production processes, the total environmental impact of rGO catalysts becomes favorable when considering their durability, which can exceed 5,000 operating hours in certain fuel cell applications. This extended service life effectively amortizes the initial environmental investment across a longer operational period.
End-of-life management presents both challenges and opportunities. While recovery and recycling infrastructure for graphene-based materials remains underdeveloped, research indicates that up to 85% of carbon materials from spent catalysts can be recovered and repurposed. Developing standardized recovery protocols and establishing dedicated recycling streams will be essential to closing the material loop and enhancing the overall sustainability of rGO catalyst technologies in fuel cell applications.
Energy consumption during rGO production represents another substantial environmental concern. The thermal reduction process commonly employed for converting graphene oxide to rGO requires high temperatures (800-1000°C), resulting in significant energy expenditure and associated carbon emissions. Recent advancements in microwave-assisted and photocatalytic reduction techniques have shown promise in reducing energy requirements by up to 60% compared to conventional thermal methods.
Raw material sourcing for graphene oxide production presents both challenges and opportunities for sustainability. While graphite mining operations can cause habitat disruption and water pollution, research indicates that rGO catalysts can be successfully synthesized from waste carbon sources, including biomass, plastic waste, and even food waste. These circular economy approaches substantially improve the sustainability profile of rGO catalysts by diverting waste from landfills while reducing virgin material extraction.
The extended lifespan of rGO catalysts compared to traditional platinum-based catalysts offers significant sustainability advantages. Life cycle assessment studies demonstrate that despite energy-intensive production processes, the total environmental impact of rGO catalysts becomes favorable when considering their durability, which can exceed 5,000 operating hours in certain fuel cell applications. This extended service life effectively amortizes the initial environmental investment across a longer operational period.
End-of-life management presents both challenges and opportunities. While recovery and recycling infrastructure for graphene-based materials remains underdeveloped, research indicates that up to 85% of carbon materials from spent catalysts can be recovered and repurposed. Developing standardized recovery protocols and establishing dedicated recycling streams will be essential to closing the material loop and enhancing the overall sustainability of rGO catalyst technologies in fuel cell applications.
Cost-Performance Analysis of rGO vs. Traditional Catalysts
The economic viability of reduced graphene oxide (rGO) as a catalyst in fuel cells depends significantly on its cost-performance ratio compared to traditional catalysts, particularly platinum-based materials. Current market analysis indicates that platinum catalysts cost approximately $30-50 per gram, representing 30-40% of the total fuel cell stack cost. In contrast, rGO production costs have decreased substantially over the past five years, with current industrial-scale production methods achieving costs of $5-10 per gram, a significant reduction from previous $100+ per gram prices.
Performance metrics reveal that platinum catalysts still maintain superior power density (0.8-1.2 W/cm²) and durability (5,000-10,000 operating hours) compared to rGO-based alternatives (0.4-0.7 W/cm² and 2,000-5,000 hours respectively). However, when analyzing cost-normalized performance, rGO demonstrates compelling advantages, delivering approximately 0.05-0.14 W/cm²/$ versus platinum's 0.02-0.04 W/cm²/$.
The scalability factor further enhances rGO's economic proposition. Production capacity for rGO has expanded by 300% since 2018, with manufacturing facilities now capable of multi-ton annual production. This scalability has contributed to the steady decline in production costs, with projections suggesting further 15-25% cost reductions over the next three years as manufacturing processes continue to mature.
Life-cycle cost analysis reveals additional advantages for rGO catalysts. While initial performance may be lower, the total cost of ownership over a five-year operational period shows rGO systems potentially achieving 20-30% cost savings compared to platinum-based systems, particularly in stationary power applications where intermittent performance fluctuations are more tolerable.
Environmental cost considerations also favor rGO, with significantly lower extraction impacts and reduced energy requirements during production. The carbon footprint of rGO catalyst production is estimated at 40-60% lower than platinum catalyst production, representing an increasingly important factor as carbon pricing mechanisms expand globally.
Market adoption sensitivity analysis indicates that rGO catalysts become economically superior to platinum at production scales exceeding 100 kg annually, suggesting that larger fuel cell manufacturers would benefit most from transitioning to rGO-based systems. For smaller-scale applications where maximum power density remains critical, platinum catalysts still maintain their economic justification despite higher costs.
Performance metrics reveal that platinum catalysts still maintain superior power density (0.8-1.2 W/cm²) and durability (5,000-10,000 operating hours) compared to rGO-based alternatives (0.4-0.7 W/cm² and 2,000-5,000 hours respectively). However, when analyzing cost-normalized performance, rGO demonstrates compelling advantages, delivering approximately 0.05-0.14 W/cm²/$ versus platinum's 0.02-0.04 W/cm²/$.
The scalability factor further enhances rGO's economic proposition. Production capacity for rGO has expanded by 300% since 2018, with manufacturing facilities now capable of multi-ton annual production. This scalability has contributed to the steady decline in production costs, with projections suggesting further 15-25% cost reductions over the next three years as manufacturing processes continue to mature.
Life-cycle cost analysis reveals additional advantages for rGO catalysts. While initial performance may be lower, the total cost of ownership over a five-year operational period shows rGO systems potentially achieving 20-30% cost savings compared to platinum-based systems, particularly in stationary power applications where intermittent performance fluctuations are more tolerable.
Environmental cost considerations also favor rGO, with significantly lower extraction impacts and reduced energy requirements during production. The carbon footprint of rGO catalyst production is estimated at 40-60% lower than platinum catalyst production, representing an increasingly important factor as carbon pricing mechanisms expand globally.
Market adoption sensitivity analysis indicates that rGO catalysts become economically superior to platinum at production scales exceeding 100 kg annually, suggesting that larger fuel cell manufacturers would benefit most from transitioning to rGO-based systems. For smaller-scale applications where maximum power density remains critical, platinum catalysts still maintain their economic justification despite higher costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!