Safety Standards for Reduced Graphene Oxide Handling and Processing
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
rGO Safety Background and Objectives
Reduced graphene oxide (rGO) has emerged as a revolutionary material in the advanced materials landscape over the past decade. Derived from graphene oxide through various reduction processes, rGO combines exceptional electrical conductivity, mechanical strength, and thermal properties with relatively economical production methods. The evolution of rGO technology has progressed from laboratory curiosity to industrial application, with significant advancements in production techniques, quality control, and application development since its initial discovery.
The safety considerations surrounding rGO handling and processing have become increasingly important as production scales expand from laboratory to industrial levels. Early research focused primarily on material properties rather than safety implications, creating a knowledge gap that now requires urgent attention. Recent studies have indicated potential respiratory, dermal, and environmental concerns associated with rGO particles, particularly at the nanoscale where unique physical properties may translate to distinct biological interactions.
Current safety protocols for rGO handling vary significantly across regions and organizations, with inconsistent standards creating challenges for global research collaboration and commercial development. The fragmented regulatory landscape has resulted in varying approaches to risk assessment and mitigation strategies, highlighting the need for harmonized safety standards that can be universally applied while accommodating the diverse forms and applications of rGO materials.
The primary objective of this technical research report is to establish a comprehensive framework for safety standards in rGO handling and processing that balances innovation enablement with appropriate risk management. This includes identifying current best practices, evaluating their effectiveness, and proposing standardized protocols that can be implemented across research, development, and manufacturing environments.
Secondary objectives include mapping the relationship between specific rGO production methods and their associated safety profiles, developing scalable safety protocols that remain effective from laboratory to industrial settings, and creating clear guidelines for personal protective equipment, engineering controls, and administrative measures specific to different rGO handling scenarios.
The technological trajectory suggests increasing industrial adoption of rGO in the coming years, particularly in energy storage, electronics, composites, and biomedical applications. This anticipated growth underscores the urgency of establishing robust safety standards that can evolve alongside technological advancements while maintaining appropriate protection for workers, consumers, and the environment throughout the rGO lifecycle.
The safety considerations surrounding rGO handling and processing have become increasingly important as production scales expand from laboratory to industrial levels. Early research focused primarily on material properties rather than safety implications, creating a knowledge gap that now requires urgent attention. Recent studies have indicated potential respiratory, dermal, and environmental concerns associated with rGO particles, particularly at the nanoscale where unique physical properties may translate to distinct biological interactions.
Current safety protocols for rGO handling vary significantly across regions and organizations, with inconsistent standards creating challenges for global research collaboration and commercial development. The fragmented regulatory landscape has resulted in varying approaches to risk assessment and mitigation strategies, highlighting the need for harmonized safety standards that can be universally applied while accommodating the diverse forms and applications of rGO materials.
The primary objective of this technical research report is to establish a comprehensive framework for safety standards in rGO handling and processing that balances innovation enablement with appropriate risk management. This includes identifying current best practices, evaluating their effectiveness, and proposing standardized protocols that can be implemented across research, development, and manufacturing environments.
Secondary objectives include mapping the relationship between specific rGO production methods and their associated safety profiles, developing scalable safety protocols that remain effective from laboratory to industrial settings, and creating clear guidelines for personal protective equipment, engineering controls, and administrative measures specific to different rGO handling scenarios.
The technological trajectory suggests increasing industrial adoption of rGO in the coming years, particularly in energy storage, electronics, composites, and biomedical applications. This anticipated growth underscores the urgency of establishing robust safety standards that can evolve alongside technological advancements while maintaining appropriate protection for workers, consumers, and the environment throughout the rGO lifecycle.
Market Analysis for rGO Applications
The reduced graphene oxide (rGO) market has been experiencing significant growth, driven by the material's exceptional properties and versatility across multiple industries. The global rGO market was valued at approximately 23.9 million USD in 2022 and is projected to reach 47.6 million USD by 2028, growing at a CAGR of 12.1% during the forecast period. This growth trajectory reflects the increasing adoption of rGO in various high-value applications.
Electronics represents the largest application segment for rGO, accounting for roughly 35% of the market share. The material's excellent electrical conductivity, flexibility, and thermal properties make it ideal for next-generation electronic devices, including flexible displays, wearable technology, and high-performance transistors. Major electronics manufacturers are increasingly incorporating rGO into their product development roadmaps.
The energy storage sector constitutes another substantial market for rGO applications, particularly in supercapacitors and lithium-ion batteries. The material's high surface area and conductivity properties enable faster charging times and improved energy density. This segment is expected to grow at the fastest rate among all application areas, with a projected CAGR of 15.3% through 2028, driven by the expanding electric vehicle market and renewable energy storage requirements.
Biomedical applications represent an emerging but rapidly growing segment for rGO. The material's biocompatibility, large surface area, and functionalization capabilities make it suitable for drug delivery systems, biosensors, and tissue engineering. Though currently accounting for only about 8% of the market, this segment is expected to double its share within the next five years as safety standards and regulatory frameworks mature.
Regionally, Asia-Pacific dominates the rGO market with approximately 45% share, led by China, Japan, and South Korea. These countries have established robust manufacturing capabilities and research infrastructure dedicated to graphene-based materials. North America follows with 30% market share, with significant investments in rGO research and commercialization efforts, particularly in the United States.
The competitive landscape features both specialized graphene producers and large chemical companies. Key market players include Sixth Element Materials, Garmor, Graphenea, and XG Sciences, alongside chemical giants like BASF and Dow Chemical that have entered the space. Market consolidation is expected as safety standards become more established and production scales up to meet growing demand.
Customer adoption barriers primarily revolve around cost considerations, safety concerns, and standardization issues. As safety protocols for handling and processing rGO become more established, market penetration is expected to accelerate across all application segments, particularly in consumer electronics and biomedical fields where safety considerations are paramount.
Electronics represents the largest application segment for rGO, accounting for roughly 35% of the market share. The material's excellent electrical conductivity, flexibility, and thermal properties make it ideal for next-generation electronic devices, including flexible displays, wearable technology, and high-performance transistors. Major electronics manufacturers are increasingly incorporating rGO into their product development roadmaps.
The energy storage sector constitutes another substantial market for rGO applications, particularly in supercapacitors and lithium-ion batteries. The material's high surface area and conductivity properties enable faster charging times and improved energy density. This segment is expected to grow at the fastest rate among all application areas, with a projected CAGR of 15.3% through 2028, driven by the expanding electric vehicle market and renewable energy storage requirements.
Biomedical applications represent an emerging but rapidly growing segment for rGO. The material's biocompatibility, large surface area, and functionalization capabilities make it suitable for drug delivery systems, biosensors, and tissue engineering. Though currently accounting for only about 8% of the market, this segment is expected to double its share within the next five years as safety standards and regulatory frameworks mature.
Regionally, Asia-Pacific dominates the rGO market with approximately 45% share, led by China, Japan, and South Korea. These countries have established robust manufacturing capabilities and research infrastructure dedicated to graphene-based materials. North America follows with 30% market share, with significant investments in rGO research and commercialization efforts, particularly in the United States.
The competitive landscape features both specialized graphene producers and large chemical companies. Key market players include Sixth Element Materials, Garmor, Graphenea, and XG Sciences, alongside chemical giants like BASF and Dow Chemical that have entered the space. Market consolidation is expected as safety standards become more established and production scales up to meet growing demand.
Customer adoption barriers primarily revolve around cost considerations, safety concerns, and standardization issues. As safety protocols for handling and processing rGO become more established, market penetration is expected to accelerate across all application segments, particularly in consumer electronics and biomedical fields where safety considerations are paramount.
Current Safety Challenges in rGO Handling
The handling and processing of reduced graphene oxide (rGO) present significant safety challenges that require immediate attention from industry stakeholders. Current safety protocols remain inadequate due to the unique physicochemical properties of rGO, which differ substantially from both graphene oxide and pristine graphene. The nanoscale dimensions and high surface area of rGO particles create substantial inhalation risks in laboratory and manufacturing environments, with airborne particles capable of deep lung penetration and potential translocation to other organs.
Exposure assessment methods for rGO remain underdeveloped, with limited standardized protocols for measuring workplace concentrations. Conventional particulate monitoring equipment often fails to accurately detect and characterize rGO nanoparticles, creating significant blind spots in occupational exposure monitoring. This technical gap has resulted in inconsistent safety practices across research institutions and manufacturing facilities.
The chemical reduction processes used to convert graphene oxide to rGO introduce additional hazards. Reducing agents such as hydrazine, sodium borohydride, and ascorbic acid present their own toxicity concerns, while thermal reduction methods generate potentially harmful gaseous byproducts. Current ventilation standards and personal protective equipment recommendations have not been specifically validated for these complex exposure scenarios.
Dermal exposure represents another significant challenge, as rGO particles can potentially penetrate skin barriers, especially when suspended in various solvents used during processing. The current understanding of dermal absorption kinetics for rGO remains limited, hampering the development of appropriate protective measures and exposure limits.
Fire and explosion risks constitute a critical safety concern, particularly during the production and handling of dry rGO powders. The high surface area and potential for static electricity accumulation create conditions conducive to dust explosions, yet specific prevention protocols tailored to rGO's unique properties remain underdeveloped. Standard dust explosion prevention measures may require significant modification to address rGO's distinctive characteristics.
Waste management presents ongoing challenges, with unclear guidelines for the disposal of rGO-containing materials and process byproducts. The potential for environmental persistence and mobility of rGO particles raises concerns about appropriate containment and treatment methods. Current hazardous waste regulations may not adequately address the unique properties and potential environmental impacts of rGO materials.
Cross-contamination control within laboratory and manufacturing settings remains problematic, with limited validated decontamination procedures for equipment and surfaces. The potential for rGO particles to adhere to various surfaces and subsequently become resuspended creates persistent exposure risks that current cleaning protocols may not effectively address.
Exposure assessment methods for rGO remain underdeveloped, with limited standardized protocols for measuring workplace concentrations. Conventional particulate monitoring equipment often fails to accurately detect and characterize rGO nanoparticles, creating significant blind spots in occupational exposure monitoring. This technical gap has resulted in inconsistent safety practices across research institutions and manufacturing facilities.
The chemical reduction processes used to convert graphene oxide to rGO introduce additional hazards. Reducing agents such as hydrazine, sodium borohydride, and ascorbic acid present their own toxicity concerns, while thermal reduction methods generate potentially harmful gaseous byproducts. Current ventilation standards and personal protective equipment recommendations have not been specifically validated for these complex exposure scenarios.
Dermal exposure represents another significant challenge, as rGO particles can potentially penetrate skin barriers, especially when suspended in various solvents used during processing. The current understanding of dermal absorption kinetics for rGO remains limited, hampering the development of appropriate protective measures and exposure limits.
Fire and explosion risks constitute a critical safety concern, particularly during the production and handling of dry rGO powders. The high surface area and potential for static electricity accumulation create conditions conducive to dust explosions, yet specific prevention protocols tailored to rGO's unique properties remain underdeveloped. Standard dust explosion prevention measures may require significant modification to address rGO's distinctive characteristics.
Waste management presents ongoing challenges, with unclear guidelines for the disposal of rGO-containing materials and process byproducts. The potential for environmental persistence and mobility of rGO particles raises concerns about appropriate containment and treatment methods. Current hazardous waste regulations may not adequately address the unique properties and potential environmental impacts of rGO materials.
Cross-contamination control within laboratory and manufacturing settings remains problematic, with limited validated decontamination procedures for equipment and surfaces. The potential for rGO particles to adhere to various surfaces and subsequently become resuspended creates persistent exposure risks that current cleaning protocols may not effectively address.
Existing rGO Handling Safety Solutions
01 Handling and exposure safety protocols for reduced graphene oxide
Safety standards for handling reduced graphene oxide (rGO) include protocols for minimizing exposure risks during manufacturing, processing, and application. These protocols address proper ventilation systems, personal protective equipment requirements, and containment measures to prevent inhalation or dermal contact with rGO particles. Guidelines also cover safe disposal methods and emergency procedures for accidental exposure to ensure worker safety when handling this nanomaterial.- Handling and exposure safety protocols for reduced graphene oxide: Safety standards for handling reduced graphene oxide (rGO) include protocols for minimizing exposure risks during manufacturing, processing, and application. These protocols address proper ventilation systems, personal protective equipment requirements, and containment measures to prevent inhalation or skin contact with rGO particles. Guidelines also cover emergency procedures for accidental exposure and spill management to protect workers and the environment.
- Environmental impact assessment and disposal regulations: Standards for evaluating the environmental impact of reduced graphene oxide include testing protocols for assessing biodegradability, persistence in ecosystems, and potential bioaccumulation. Regulations specify proper disposal methods to prevent environmental contamination, including waste classification, treatment requirements before disposal, and monitoring procedures. These standards aim to minimize the ecological footprint of rGO throughout its lifecycle.
- Toxicological evaluation and health risk assessment: Safety standards for reduced graphene oxide include comprehensive toxicological evaluation frameworks to assess potential health hazards. These frameworks specify testing methodologies for acute and chronic toxicity, genotoxicity, respiratory effects, and dermal sensitization. Risk assessment protocols consider exposure scenarios, dose-response relationships, and identification of vulnerable populations to establish occupational exposure limits and consumer safety thresholds.
- Quality control and characterization requirements: Standards for reduced graphene oxide quality control include specifications for purity levels, oxygen content, sheet size distribution, and defect density. Standardized characterization methods such as Raman spectroscopy, X-ray photoelectron spectroscopy, and electron microscopy are prescribed to ensure consistent material properties. These requirements help ensure safety by preventing variability in rGO materials that could lead to unpredictable hazards during handling or application.
- Application-specific safety guidelines and certifications: Safety standards for reduced graphene oxide vary by application field, with specific guidelines for electronics, biomedical, energy storage, and consumer products. These include biocompatibility testing for medical applications, electrical safety standards for electronic devices, and migration limits for food contact materials. Certification processes verify compliance with these application-specific requirements, often involving third-party testing and regular audits to maintain safety certifications throughout the product lifecycle.
02 Environmental impact assessment and disposal standards
Standards for evaluating the environmental impact of reduced graphene oxide include protocols for assessing potential ecological risks, biodegradation pathways, and aquatic toxicity. These standards establish proper disposal methods to prevent environmental contamination, including waste treatment processes that neutralize potential hazards before release. Guidelines also cover monitoring requirements for manufacturing facilities to ensure compliance with environmental protection regulations.Expand Specific Solutions03 Toxicological evaluation and biocompatibility standards
Safety standards for reduced graphene oxide include comprehensive toxicological evaluation protocols to assess potential health hazards. These standards specify methods for testing cytotoxicity, genotoxicity, and inflammatory responses in various biological systems. Biocompatibility assessments are particularly important for applications in biomedical fields, with standards covering dose-dependent effects, long-term exposure impacts, and potential accumulation in tissues to ensure safe use in products that may contact human tissues.Expand Specific Solutions04 Quality control and characterization requirements
Safety standards for reduced graphene oxide establish quality control parameters and characterization requirements to ensure consistent safety profiles. These standards specify acceptable levels of residual chemicals from the reduction process, particle size distributions, and surface chemistry characteristics. Testing protocols include methods for detecting impurities, verifying reduction status, and confirming structural integrity. Standardized characterization techniques ensure that rGO materials meet safety specifications before use in commercial applications.Expand Specific Solutions05 Application-specific safety guidelines and regulatory frameworks
Safety standards for reduced graphene oxide vary based on intended applications, with specific guidelines for electronics, energy storage, biomedical, and consumer products. These application-specific standards address unique risks associated with each use case and establish appropriate safety margins. Regulatory frameworks include certification requirements, labeling standards, and compliance testing protocols. International harmonization efforts aim to standardize safety requirements across different jurisdictions while accommodating technological advancements in rGO production and application.Expand Specific Solutions
Key Organizations in rGO Safety Standards
The safety standards for reduced graphene oxide (rGO) handling and processing are evolving within a rapidly growing market that is still in its early maturity phase. The global graphene market, estimated at approximately $200 million, is expected to expand significantly as applications diversify across industries. Technical maturity varies considerably among key players, with research institutions like King Abdullah University of Science & Technology and Indian Institutes of Technology leading fundamental safety research, while commercial entities such as Global Graphene Group, Graphenea, and Cabot Corporation are developing standardized handling protocols. Major industrial players including LG Electronics, ArcelorMittal, and Tata Steel are integrating these safety standards into manufacturing processes, though comprehensive regulatory frameworks remain under development as the industry continues to address occupational exposure limits and waste management concerns.
Cabot Corp.
Technical Solution: Cabot Corporation has developed an integrated safety management system for rGO handling called "SafeGraph" that emphasizes exposure minimization through process design. Their approach begins with material characterization to understand specific hazard profiles of different rGO formulations, allowing tailored safety measures. Cabot has engineered specialized containment systems for different processing stages, including custom glove box setups for laboratory work and automated handling systems for production environments. Their safety protocols incorporate regular workplace monitoring using advanced particulate detection technologies capable of identifying nanoscale materials. Cabot has established comprehensive waste management procedures specifically for rGO materials, including specialized filtration systems for liquid waste and secure containment for solid waste. The company maintains detailed exposure registries for all workers handling rGO materials and conducts regular health monitoring. Additionally, Cabot has developed specialized decontamination procedures for equipment and facilities that have been exposed to rGO.
Strengths: Material-specific approach allows tailored safety measures; comprehensive monitoring program provides early detection of potential exposures; specialized containment systems for different processing environments. Weaknesses: Extensive material characterization requirements add time and cost to processes; specialized equipment needs create vendor dependencies; system complexity requires significant technical expertise.
Nanjing Tech University
Technical Solution: Nanjing Tech University has developed the "Hierarchical Control Framework for rGO Safety" that establishes a systematic approach to exposure prevention in research and development settings. Their system prioritizes process modification and engineering controls over personal protective equipment, with specific protocols for different scales of rGO handling from laboratory to pilot production. The university has pioneered specialized fume hood designs optimized for nanomaterials handling, incorporating advanced airflow patterns and filtration systems specific to graphene materials. Their approach includes detailed standard operating procedures for common rGO processes with step-by-step safety measures integrated directly into research protocols. Nanjing Tech has developed specialized cleaning and decontamination procedures for laboratory equipment and surfaces that have been exposed to rGO, including validation methods to confirm decontamination effectiveness. Additionally, they've established comprehensive waste segregation and treatment protocols specifically for rGO-contaminated materials, addressing both solid and liquid waste streams. The university maintains a centralized incident reporting system for any rGO exposure events, allowing continuous improvement of safety protocols.
Strengths: Research-focused approach provides practical guidance for laboratory environments; integrated safety procedures within research protocols improve compliance; specialized fume hood designs address specific challenges of nanomaterials. Weaknesses: May have limited applicability to large-scale industrial processes; academic setting may not fully address commercial production challenges; potential resource constraints for implementing all recommended engineering controls.
Critical Safety Research for rGO Processing
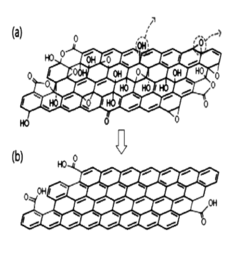
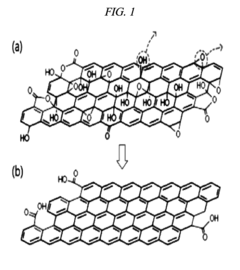
Graphene oxide reducing agent comprising a reducing agent containing a halogen element, method for manufacturing a reduced graphene oxide using same, and use of the reduced graphene oxide manufactured by the method
PatentActiveUS9090805B2
Innovation
- A graphene oxide reducer containing a halogen element, preferably hydroiodic acid (HI), is used to react with graphene oxide at a temperature of 10°C or more, optionally with a weaker acid like acetic acid, to produce high-purity reduced graphene oxide with improved electrical conductivity.
A method of preparation of reduced graphene oxide nanoparticles
PatentActiveIN201911000878A
Innovation
- Single-step cost-effective method for preparing reduced graphene oxide (rGO) nanoparticles through combustion of carbon source under high pressure in the presence of oxygen.
- Time-efficient process compared to conventional methods that require 10-17 hours, reducing energy consumption by eliminating the need for high temperatures (300°C) used in traditional methods.
- Direct production of rGO nanoparticles without requiring intermediate steps like hydrazine reduction or thermal treatment, making the process more economical and environmentally friendly.
Regulatory Framework for Nanomaterials
The regulatory landscape for nanomaterials, including reduced graphene oxide (rGO), is complex and evolving globally. Currently, nanomaterials are regulated under various frameworks that were not specifically designed for these novel materials. In the United States, the Environmental Protection Agency (EPA) regulates nanomaterials primarily under the Toxic Substances Control Act (TSCA), requiring manufacturers to submit premanufacture notices for new chemical substances, including nanomaterials. The EPA has issued significant new use rules (SNURs) for certain carbon nanomaterials, though specific regulations for rGO are still developing.
The European Union has implemented a more comprehensive approach through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which requires registration of substances manufactured or imported in quantities over one ton per year. The EU has also established the European Chemicals Agency (ECHA) to manage technical aspects of REACH implementation. Notably, the EU has adopted a nanomaterial definition that includes materials with 50% or more particles in the size range of 1-100 nm, which encompasses rGO.
In Asia, regulatory frameworks vary significantly. Japan has implemented a voluntary reporting system for nanomaterials under its Chemical Substances Control Law. China has included nanomaterials in its new chemical substance notification system, while South Korea has established specific registration requirements for nanomaterials under K-REACH.
International organizations have also contributed to the regulatory framework. The Organization for Economic Cooperation and Development (OECD) has established the Working Party on Manufactured Nanomaterials (WPMN) to coordinate international efforts on risk assessment and regulatory approaches. The International Organization for Standardization (ISO) has developed standards for terminology, measurement, and characterization of nanomaterials through its Technical Committee 229.
Industry-specific regulations add another layer of complexity. For medical applications, the FDA in the US and the European Medicines Agency have specific guidelines for nanomaterials in medical devices and pharmaceuticals. For food contact materials, both the FDA and European Food Safety Authority have established evaluation procedures for nanomaterials.
Occupational safety regulations for nanomaterial handling are primarily governed by agencies like OSHA in the US and similar bodies in other countries. These regulations typically require risk assessments, exposure monitoring, and appropriate control measures. However, specific exposure limits for rGO are largely absent, with most jurisdictions applying general dust or particulate matter standards as interim measures.
The European Union has implemented a more comprehensive approach through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which requires registration of substances manufactured or imported in quantities over one ton per year. The EU has also established the European Chemicals Agency (ECHA) to manage technical aspects of REACH implementation. Notably, the EU has adopted a nanomaterial definition that includes materials with 50% or more particles in the size range of 1-100 nm, which encompasses rGO.
In Asia, regulatory frameworks vary significantly. Japan has implemented a voluntary reporting system for nanomaterials under its Chemical Substances Control Law. China has included nanomaterials in its new chemical substance notification system, while South Korea has established specific registration requirements for nanomaterials under K-REACH.
International organizations have also contributed to the regulatory framework. The Organization for Economic Cooperation and Development (OECD) has established the Working Party on Manufactured Nanomaterials (WPMN) to coordinate international efforts on risk assessment and regulatory approaches. The International Organization for Standardization (ISO) has developed standards for terminology, measurement, and characterization of nanomaterials through its Technical Committee 229.
Industry-specific regulations add another layer of complexity. For medical applications, the FDA in the US and the European Medicines Agency have specific guidelines for nanomaterials in medical devices and pharmaceuticals. For food contact materials, both the FDA and European Food Safety Authority have established evaluation procedures for nanomaterials.
Occupational safety regulations for nanomaterial handling are primarily governed by agencies like OSHA in the US and similar bodies in other countries. These regulations typically require risk assessments, exposure monitoring, and appropriate control measures. However, specific exposure limits for rGO are largely absent, with most jurisdictions applying general dust or particulate matter standards as interim measures.
Environmental Impact Assessment of rGO
The environmental impact of reduced graphene oxide (rGO) extends across its entire lifecycle, from production to disposal. During manufacturing processes, chemical reduction methods often employ hazardous reducing agents such as hydrazine, sodium borohydride, and hydroiodic acid, which can release toxic byproducts into air and water systems if not properly contained. Studies indicate that production facilities without adequate filtration systems may release particulate matter containing rGO into the surrounding environment, potentially affecting local ecosystems.
Water contamination represents a significant concern, as rGO particles exhibit high mobility in aquatic environments due to their nanoscale dimensions and surface properties. Research has demonstrated that these particles can persist in water bodies for extended periods, with potential for bioaccumulation in aquatic organisms. Laboratory studies have shown that concentrations as low as 10 mg/L can impair gill function in certain fish species and disrupt growth patterns in aquatic plants.
Atmospheric dispersion of rGO particulates presents another environmental challenge. Dry processing methods can generate airborne particles that may travel considerable distances from their source. These particles can eventually settle on soil and vegetation, potentially altering soil chemistry and microbial communities. Preliminary studies suggest that rGO may influence soil pH and nutrient availability, though long-term effects remain under investigation.
Biodegradability assessments of rGO reveal concerning patterns of environmental persistence. Unlike conventional organic materials, rGO demonstrates remarkable stability against microbial degradation, with estimated environmental half-lives potentially extending to several years or decades. This persistence increases the likelihood of long-term ecosystem exposure and potential bioaccumulation through food chains.
Waste management practices for rGO-containing materials present unique challenges. Current recycling infrastructure is largely unprepared to handle nanomaterial-enhanced products, potentially leading to improper disposal. Incineration of rGO waste may release particulates into the atmosphere, while landfill disposal raises concerns about leachate contamination of groundwater systems.
Emerging research suggests potential for ecological adaptation strategies. Some studies have identified specific bacterial strains capable of partially metabolizing graphene-based materials, offering promising avenues for bioremediation approaches. Additionally, advanced filtration technologies incorporating activated carbon and membrane systems have demonstrated effectiveness in removing rGO from wastewater streams, potentially mitigating environmental release.
Water contamination represents a significant concern, as rGO particles exhibit high mobility in aquatic environments due to their nanoscale dimensions and surface properties. Research has demonstrated that these particles can persist in water bodies for extended periods, with potential for bioaccumulation in aquatic organisms. Laboratory studies have shown that concentrations as low as 10 mg/L can impair gill function in certain fish species and disrupt growth patterns in aquatic plants.
Atmospheric dispersion of rGO particulates presents another environmental challenge. Dry processing methods can generate airborne particles that may travel considerable distances from their source. These particles can eventually settle on soil and vegetation, potentially altering soil chemistry and microbial communities. Preliminary studies suggest that rGO may influence soil pH and nutrient availability, though long-term effects remain under investigation.
Biodegradability assessments of rGO reveal concerning patterns of environmental persistence. Unlike conventional organic materials, rGO demonstrates remarkable stability against microbial degradation, with estimated environmental half-lives potentially extending to several years or decades. This persistence increases the likelihood of long-term ecosystem exposure and potential bioaccumulation through food chains.
Waste management practices for rGO-containing materials present unique challenges. Current recycling infrastructure is largely unprepared to handle nanomaterial-enhanced products, potentially leading to improper disposal. Incineration of rGO waste may release particulates into the atmosphere, while landfill disposal raises concerns about leachate contamination of groundwater systems.
Emerging research suggests potential for ecological adaptation strategies. Some studies have identified specific bacterial strains capable of partially metabolizing graphene-based materials, offering promising avenues for bioremediation approaches. Additionally, advanced filtration technologies incorporating activated carbon and membrane systems have demonstrated effectiveness in removing rGO from wastewater streams, potentially mitigating environmental release.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!