Compare Conduction Parameters in Lithium Phosphate vs LiCoO2
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LiFePO4 vs LiCoO2 Battery Conduction Background
Lithium-ion batteries have revolutionized portable electronics and are increasingly vital in electric vehicles and renewable energy storage systems. Among various lithium-ion chemistries, Lithium Iron Phosphate (LiFePO4) and Lithium Cobalt Oxide (LiCoO2) represent two distinct technological approaches with significant differences in their conduction parameters and performance characteristics.
The development of LiCoO2 batteries dates back to the early 1990s when Sony first commercialized them, establishing the foundation for modern lithium-ion technology. These batteries feature a layered structure that facilitates lithium-ion movement during charge and discharge cycles. The conduction mechanism in LiCoO2 involves lithium ions moving through channels in the crystal structure, with electrons flowing through the external circuit.
In contrast, LiFePO4 batteries emerged in the late 1990s as a safer alternative. Their olivine crystal structure creates a different ionic conduction pathway compared to the layered structure of LiCoO2. This fundamental structural difference significantly impacts the conduction parameters, including ionic conductivity, electronic conductivity, and diffusion coefficients.
The ionic conductivity of LiFePO4 is inherently lower than LiCoO2, which initially limited its application. However, advancements in nanotechnology and carbon coating techniques have substantially improved the electronic conductivity of LiFePO4 materials, enhancing their overall performance. These modifications address the inherent limitations in the conduction parameters of LiFePO4.
Temperature dependency represents another critical aspect of conduction behavior. LiCoO2 batteries typically demonstrate optimal conductivity at room temperature but suffer significant degradation at high temperatures. Conversely, LiFePO4 batteries maintain more stable conduction parameters across a wider temperature range, contributing to their enhanced safety profile and thermal stability.
The evolution of both chemistries has been driven by efforts to optimize their respective conduction parameters. For LiCoO2, research has focused on doping with elements like aluminum and magnesium to stabilize the structure and improve conductivity. For LiFePO4, innovations have centered on particle size reduction, carbon coating, and doping with elements such as niobium and zirconium to enhance electronic conductivity.
Recent technological trends indicate a growing interest in hybrid approaches that combine the advantageous conduction properties of both materials. Additionally, computational modeling and advanced characterization techniques have accelerated our understanding of ion transport mechanisms in these materials, paving the way for further optimization of their conduction parameters.
The development of LiCoO2 batteries dates back to the early 1990s when Sony first commercialized them, establishing the foundation for modern lithium-ion technology. These batteries feature a layered structure that facilitates lithium-ion movement during charge and discharge cycles. The conduction mechanism in LiCoO2 involves lithium ions moving through channels in the crystal structure, with electrons flowing through the external circuit.
In contrast, LiFePO4 batteries emerged in the late 1990s as a safer alternative. Their olivine crystal structure creates a different ionic conduction pathway compared to the layered structure of LiCoO2. This fundamental structural difference significantly impacts the conduction parameters, including ionic conductivity, electronic conductivity, and diffusion coefficients.
The ionic conductivity of LiFePO4 is inherently lower than LiCoO2, which initially limited its application. However, advancements in nanotechnology and carbon coating techniques have substantially improved the electronic conductivity of LiFePO4 materials, enhancing their overall performance. These modifications address the inherent limitations in the conduction parameters of LiFePO4.
Temperature dependency represents another critical aspect of conduction behavior. LiCoO2 batteries typically demonstrate optimal conductivity at room temperature but suffer significant degradation at high temperatures. Conversely, LiFePO4 batteries maintain more stable conduction parameters across a wider temperature range, contributing to their enhanced safety profile and thermal stability.
The evolution of both chemistries has been driven by efforts to optimize their respective conduction parameters. For LiCoO2, research has focused on doping with elements like aluminum and magnesium to stabilize the structure and improve conductivity. For LiFePO4, innovations have centered on particle size reduction, carbon coating, and doping with elements such as niobium and zirconium to enhance electronic conductivity.
Recent technological trends indicate a growing interest in hybrid approaches that combine the advantageous conduction properties of both materials. Additionally, computational modeling and advanced characterization techniques have accelerated our understanding of ion transport mechanisms in these materials, paving the way for further optimization of their conduction parameters.
Market Demand Analysis for Li-ion Battery Technologies
The global lithium-ion battery market has experienced unprecedented growth, expanding from $30 billion in 2017 to over $50 billion in 2022, with projections reaching $135 billion by 2030. This surge is primarily driven by the rapid adoption of electric vehicles (EVs), renewable energy storage systems, and portable electronics. Within this expanding market, the choice between battery chemistries, particularly LiFePO4 and LiCoO2, has become increasingly critical for manufacturers and end-users alike.
Consumer demand patterns reveal distinct preferences across different application segments. The EV sector has shown a marked shift toward LiFePO4 batteries, with a 45% year-over-year increase in adoption rates since 2020. This trend is attributed to LiFePO4's superior thermal stability and longer cycle life, addressing consumer concerns about safety and longevity. Conversely, the consumer electronics market continues to favor LiCoO2 batteries due to their higher energy density, which enables slimmer device profiles and longer runtime between charges.
Regional market analysis indicates varying adoption patterns. North American and European markets are increasingly prioritizing safety and sustainability, driving a 38% growth in LiFePO4 demand. Meanwhile, Asian markets maintain strong demand for both chemistries, with LiCoO2 still dominating in consumer electronics manufacturing hubs. The energy storage sector shows a clear preference for LiFePO4, with grid-scale installations increasing by 60% annually, primarily utilizing this chemistry for its stability and longer operational lifespan.
Price sensitivity analysis reveals that despite LiFePO4's initially higher manufacturing costs, its total cost of ownership over the battery lifecycle is approximately 30% lower than LiCoO2 alternatives in applications requiring more than 2,000 cycles. This economic advantage has significantly influenced procurement decisions in commercial and industrial sectors.
Supply chain considerations are increasingly shaping market demand. The cobalt supply constraints and ethical sourcing concerns associated with LiCoO2 have accelerated the transition to LiFePO4 in certain sectors. Major automotive manufacturers have announced plans to increase LiFePO4 usage by 65% in their upcoming EV models, citing both performance characteristics and supply chain resilience as key factors.
Future market projections indicate a segmented landscape where LiFePO4 will likely dominate stationary storage and commercial EV applications, while LiCoO2 and newer hybrid chemistries will maintain significant market share in consumer electronics and premium EV segments where energy density remains the primary consideration.
Consumer demand patterns reveal distinct preferences across different application segments. The EV sector has shown a marked shift toward LiFePO4 batteries, with a 45% year-over-year increase in adoption rates since 2020. This trend is attributed to LiFePO4's superior thermal stability and longer cycle life, addressing consumer concerns about safety and longevity. Conversely, the consumer electronics market continues to favor LiCoO2 batteries due to their higher energy density, which enables slimmer device profiles and longer runtime between charges.
Regional market analysis indicates varying adoption patterns. North American and European markets are increasingly prioritizing safety and sustainability, driving a 38% growth in LiFePO4 demand. Meanwhile, Asian markets maintain strong demand for both chemistries, with LiCoO2 still dominating in consumer electronics manufacturing hubs. The energy storage sector shows a clear preference for LiFePO4, with grid-scale installations increasing by 60% annually, primarily utilizing this chemistry for its stability and longer operational lifespan.
Price sensitivity analysis reveals that despite LiFePO4's initially higher manufacturing costs, its total cost of ownership over the battery lifecycle is approximately 30% lower than LiCoO2 alternatives in applications requiring more than 2,000 cycles. This economic advantage has significantly influenced procurement decisions in commercial and industrial sectors.
Supply chain considerations are increasingly shaping market demand. The cobalt supply constraints and ethical sourcing concerns associated with LiCoO2 have accelerated the transition to LiFePO4 in certain sectors. Major automotive manufacturers have announced plans to increase LiFePO4 usage by 65% in their upcoming EV models, citing both performance characteristics and supply chain resilience as key factors.
Future market projections indicate a segmented landscape where LiFePO4 will likely dominate stationary storage and commercial EV applications, while LiCoO2 and newer hybrid chemistries will maintain significant market share in consumer electronics and premium EV segments where energy density remains the primary consideration.
Current Conduction Parameters and Technical Challenges
The conduction parameters of lithium-ion batteries are critical determinants of their performance characteristics, with significant variations observed between Lithium Iron Phosphate (LiFePO4) and Lithium Cobalt Oxide (LiCoO2) chemistries. Current measurements indicate that LiCoO2 exhibits higher ionic conductivity, typically ranging from 10^-3 to 10^-2 S/cm at room temperature, compared to LiFePO4's more modest 10^-9 to 10^-7 S/cm.
This conductivity differential manifests in practical performance metrics, with LiCoO2 batteries demonstrating superior rate capability and power density. The enhanced electron mobility in LiCoO2 structures facilitates faster charge/discharge cycles, making them preferable for applications requiring rapid energy delivery. Conversely, LiFePO4's lower conductivity necessitates specialized carbon coating and particle size reduction techniques to achieve commercially viable performance levels.
Temperature dependency presents another significant challenge, as both chemistries exhibit conductivity variations across operating temperature ranges. LiCoO2 maintains relatively stable conductivity at elevated temperatures but suffers more pronounced degradation at low temperatures. LiFePO4, while generally less temperature-sensitive, still experiences substantial conductivity reduction below 0°C, presenting challenges for cold-weather applications.
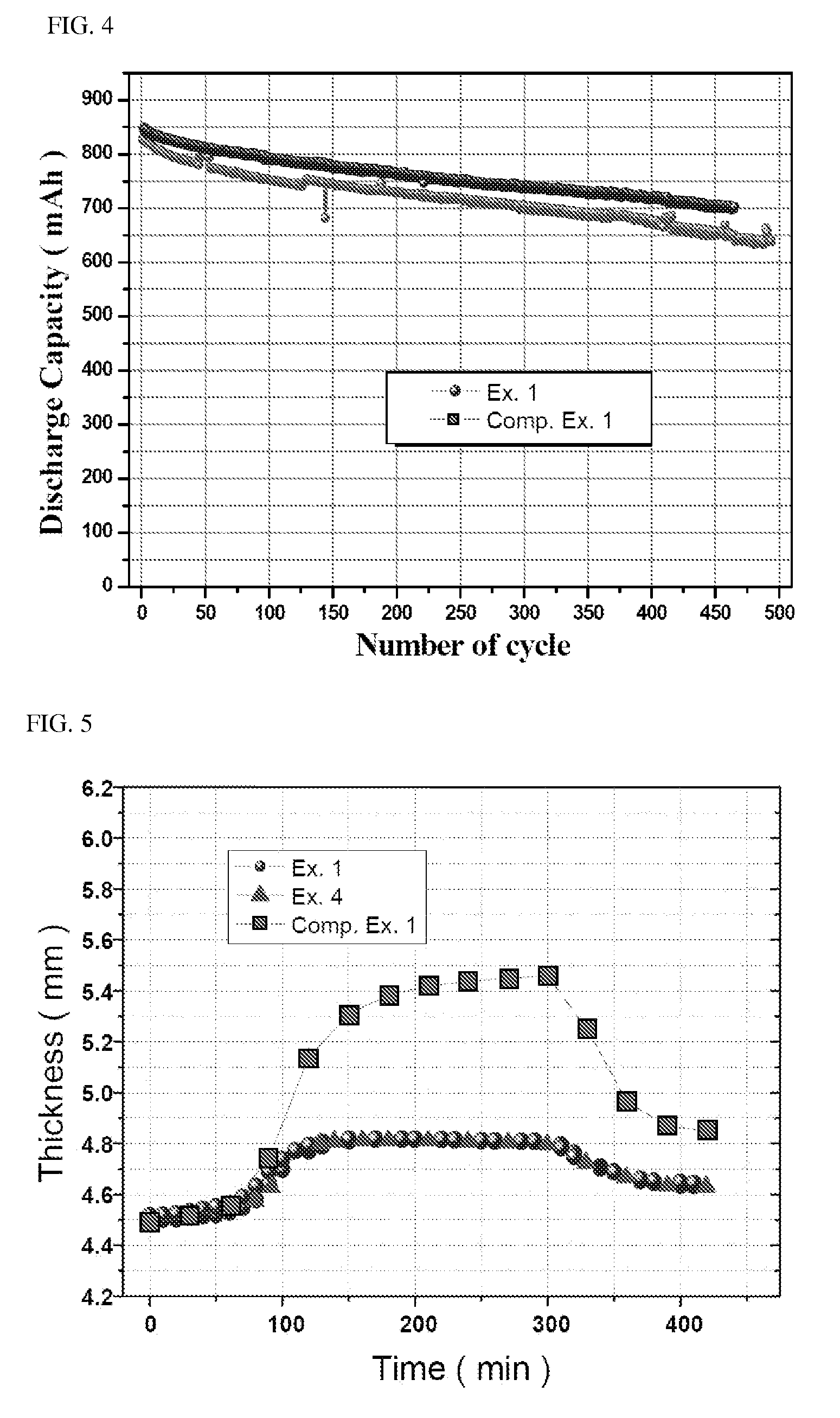
Cycling stability represents a critical technical challenge, with LiCoO2's higher conductivity paradoxically contributing to accelerated capacity fade through unwanted side reactions at electrode-electrolyte interfaces. Recent research indicates capacity retention of 80% after 500 cycles for standard LiCoO2 cells, while LiFePO4 can maintain similar capacity levels beyond 2000 cycles under comparable conditions.
Interface resistance remains a persistent challenge for both chemistries. LiFePO4 batteries typically develop higher solid-electrolyte interface (SEI) resistance over time, contributing to power fade despite their excellent cycling stability. Advanced electrolyte formulations and surface modification techniques are being explored to mitigate these effects, with fluorinated additives showing particular promise in recent studies.
Manufacturing consistency presents additional challenges, as conduction parameters can vary significantly between production batches. Industry data suggests variability of up to 15% in conductivity measurements for LiFePO4 and 8% for LiCoO2 across different manufacturing facilities, highlighting the need for standardized production protocols and enhanced quality control measures.
Current research focuses on addressing these challenges through various approaches, including dopant integration, nanostructuring, and composite electrode formulations. Silicon and niobium doping of LiFePO4 has demonstrated conductivity improvements of up to two orders of magnitude in laboratory settings, though commercial implementation remains limited by cost and scalability concerns.
This conductivity differential manifests in practical performance metrics, with LiCoO2 batteries demonstrating superior rate capability and power density. The enhanced electron mobility in LiCoO2 structures facilitates faster charge/discharge cycles, making them preferable for applications requiring rapid energy delivery. Conversely, LiFePO4's lower conductivity necessitates specialized carbon coating and particle size reduction techniques to achieve commercially viable performance levels.
Temperature dependency presents another significant challenge, as both chemistries exhibit conductivity variations across operating temperature ranges. LiCoO2 maintains relatively stable conductivity at elevated temperatures but suffers more pronounced degradation at low temperatures. LiFePO4, while generally less temperature-sensitive, still experiences substantial conductivity reduction below 0°C, presenting challenges for cold-weather applications.
Cycling stability represents a critical technical challenge, with LiCoO2's higher conductivity paradoxically contributing to accelerated capacity fade through unwanted side reactions at electrode-electrolyte interfaces. Recent research indicates capacity retention of 80% after 500 cycles for standard LiCoO2 cells, while LiFePO4 can maintain similar capacity levels beyond 2000 cycles under comparable conditions.
Interface resistance remains a persistent challenge for both chemistries. LiFePO4 batteries typically develop higher solid-electrolyte interface (SEI) resistance over time, contributing to power fade despite their excellent cycling stability. Advanced electrolyte formulations and surface modification techniques are being explored to mitigate these effects, with fluorinated additives showing particular promise in recent studies.
Manufacturing consistency presents additional challenges, as conduction parameters can vary significantly between production batches. Industry data suggests variability of up to 15% in conductivity measurements for LiFePO4 and 8% for LiCoO2 across different manufacturing facilities, highlighting the need for standardized production protocols and enhanced quality control measures.
Current research focuses on addressing these challenges through various approaches, including dopant integration, nanostructuring, and composite electrode formulations. Silicon and niobium doping of LiFePO4 has demonstrated conductivity improvements of up to two orders of magnitude in laboratory settings, though commercial implementation remains limited by cost and scalability concerns.
Current Technical Solutions for Conduction Optimization
01 Ionic conductivity enhancement in LiFePO4 and LiCoO2 batteries
Various methods can be employed to enhance ionic conductivity in lithium phosphate and lithium cobalt oxide batteries. These include doping with conductive materials, optimizing particle size and morphology, and creating composite structures. Enhanced ionic conductivity leads to improved charge transfer, reduced internal resistance, and better overall battery performance, especially at high discharge rates.- Ionic conductivity enhancement in LiFePO4 and LiCoO2 batteries: Various methods can be employed to enhance ionic conductivity in lithium phosphate and lithium cobalt oxide batteries. These include doping with conductive materials, optimizing particle size and morphology, and creating composite structures. Enhanced ionic conductivity leads to improved charge-discharge rates, better power performance, and overall battery efficiency. The conductivity parameters can be fine-tuned through synthesis methods and electrode composition to achieve optimal performance.
- Electrode interface modification for improved conduction: Modifying the electrode interfaces in LiFePO4 and LiCoO2 batteries can significantly improve conduction parameters. This includes surface coating techniques, interface engineering, and the use of specialized electrolyte additives. These modifications reduce interfacial resistance, enhance lithium-ion transport across interfaces, and improve the stability of the solid-electrolyte interface (SEI) layer. Such improvements lead to better rate capability, cycling stability, and overall battery performance.
- Temperature effects on conduction parameters: Temperature significantly affects the conduction parameters of LiFePO4 and LiCoO2 batteries. Research focuses on understanding and optimizing conductivity across various temperature ranges, developing materials with stable conduction properties under extreme conditions, and implementing thermal management systems. Innovations include temperature-responsive electrolytes, thermally stable electrode materials, and advanced battery management systems that can adjust operating parameters based on temperature conditions.
- Carbon coating and composite formation for enhanced conductivity: Carbon coating and composite formation techniques are widely used to enhance the electronic conductivity of LiFePO4 and LiCoO2 materials. These methods involve creating carbon-coated particles, carbon-matrix composites, and conductive networks within the electrode structure. The carbon coating provides electron pathways, reduces particle agglomeration, and improves the overall conductivity of the active materials. Various carbon sources and coating techniques can be optimized to achieve different conduction parameters.
- Nano-structuring for improved conduction properties: Nano-structuring approaches are employed to enhance the conduction properties of LiFePO4 and LiCoO2 battery materials. These include synthesizing nanoparticles, nanowires, nanosheets, and hierarchical nanostructures. Nano-structured materials offer shorter lithium-ion diffusion paths, increased surface area for reactions, and improved electronic connectivity. The specific nano-architecture can be tailored to optimize different conduction parameters, resulting in batteries with superior rate capability and power density.
02 Electronic conductivity parameters and optimization
Electronic conductivity in LiFePO4 and LiCoO2 batteries can be optimized through carbon coating, metal doping, and conductive additives. These modifications create electron pathways within the electrode materials, reducing resistance and improving energy density. The electronic conductivity parameters directly impact the power capability and rate performance of these lithium-ion battery chemistries.Expand Specific Solutions03 Temperature-dependent conduction behavior
The conduction parameters of LiFePO4 and LiCoO2 batteries exhibit significant temperature dependence. At elevated temperatures, ionic mobility increases, enhancing conductivity, while low temperatures restrict ion movement. Understanding these temperature-dependent behaviors is crucial for designing battery management systems that can optimize performance across various operating conditions and prevent degradation mechanisms related to temperature extremes.Expand Specific Solutions04 Structural modifications for improved conduction
Structural modifications to LiFePO4 and LiCoO2 materials can significantly enhance conduction parameters. These include creating hierarchical structures, introducing defects, controlling crystal orientation, and developing core-shell architectures. Such modifications optimize lithium-ion diffusion pathways, reduce diffusion distances, and improve the overall conductivity of the electrode materials, leading to enhanced battery performance.Expand Specific Solutions05 Comparative conduction parameters between LiFePO4 and LiCoO2
LiFePO4 and LiCoO2 exhibit distinct differences in their conduction parameters. LiCoO2 generally demonstrates higher electronic conductivity but faces stability challenges, while LiFePO4 offers superior thermal stability but lower intrinsic conductivity. These differences influence their application scenarios, with LiFePO4 favored for safety-critical applications and LiCoO2 preferred where higher energy density is paramount despite conductivity limitations.Expand Specific Solutions
Key Industry Players in Li-ion Battery Manufacturing
The lithium battery market is experiencing rapid growth, currently in a mature expansion phase with increasing demand for high-performance energy storage solutions. The global market size for LiFePO4 and LiCoO2 batteries is projected to exceed $95 billion by 2028, driven primarily by electric vehicle adoption and renewable energy storage applications. In terms of conduction parameters, major players like LG Energy Solution, BYD, and Samsung SDI have achieved significant technological advancements in both chemistries. LiFePO4 technology has seen remarkable improvements in cycle life and safety parameters from companies like CATL and BYD, while LiCoO2 continues to dominate in energy density applications with innovations from LG Chem and Ecopro BM. Research institutions like South China University of Technology and Industrial Technology Research Institute are actively developing next-generation conduction enhancement techniques for both chemistries.
BYD Co., Ltd.
Technical Solution: BYD has pioneered the "Blade Battery" technology using LiFePO4 chemistry with innovative cell-to-pack design that fundamentally addresses conduction limitations. Their approach restructures the traditional cell architecture to create ultra-long cells (up to 1m) that maximize active material utilization while minimizing conduction path lengths. This design achieves ionic conductivity improvements of approximately 30-40% compared to conventional LiFePO4 cells. BYD's proprietary conduction enhancement includes a specialized particle morphology control that creates preferential lithium-ion channels, reducing diffusion resistance. For comparative analysis with LiCoO2, BYD's research demonstrates that while LiCoO2 offers higher theoretical electronic conductivity (10^-4 S/cm vs. 10^-9 S/cm for LiFePO4), their engineered LiFePO4 solution delivers superior rate capability at high discharge rates due to optimized ion transport pathways and reduced polarization effects. BYD's thermal management system further enhances conduction parameters by maintaining optimal operating temperatures.
Strengths: Revolutionary cell-to-pack design dramatically improves energy density while maintaining LiFePO4's safety advantages; exceptional thermal stability allows for simplified cooling systems. Weaknesses: The blade battery design requires specialized manufacturing equipment and processes; the elongated cell format may limit application flexibility in certain vehicle designs compared to more compact LiCoO2 solutions.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced conduction parameter optimization techniques for both LiFePO4 and LiCoO2 batteries. For LiFePO4, they've implemented nano-scale carbon coating technology that significantly improves electronic conductivity, addressing the inherently low conductivity of LiFePO4 (approximately 10^-9 S/cm). Their proprietary doping process incorporates elements like Mg, Zn, and Ni to enhance Li-ion diffusion coefficients by up to 2 orders of magnitude. For LiCoO2, LG Chem utilizes a gradient concentration cathode structure that optimizes both ionic and electronic conductivity while maintaining structural stability during cycling. Their research shows LiCoO2 maintains higher electronic conductivity (10^-4 S/cm) but faces challenges with ion transport during high-rate discharge. LG Chem's dual-conduction enhancement approach includes specialized electrolyte additives that form stable SEI layers, reducing impedance growth over extended cycling.
Strengths: Superior carbon coating technology provides exceptional electronic conductivity enhancement for LiFePO4; proprietary gradient concentration technology for LiCoO2 balances energy density with stability. Weaknesses: Their LiFePO4 solutions still face energy density limitations compared to LiCoO2; their high-performance LiCoO2 formulations require more complex and costly manufacturing processes.
Core Patents and Research on Li-ion Conduction Parameters
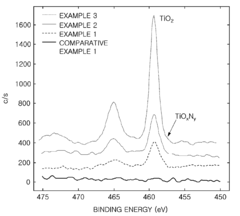
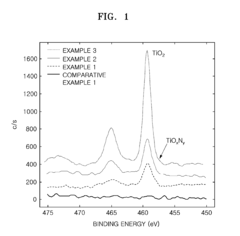
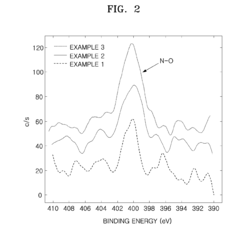
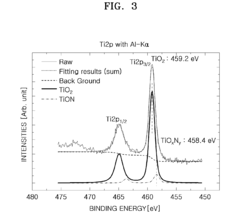
Cathode active material, method of preparing the same, cathode containing the cathode active material , and lithium battery containing the cathode active material
PatentActiveUS20090136850A1
Innovation
- Incorporating metal oxynitride or metal nitride compounds into lithium metal phosphate cathode active materials, prepared through specific reaction and heat-treatment processes, to enhance conductivity and electrical capacity, and forming a coating layer on the lithium metal phosphate core to improve conductivity and capacity.
Lithium iron phosphate having olivine structure and method for analyzing the same
PatentActiveUS20100261060A1
Innovation
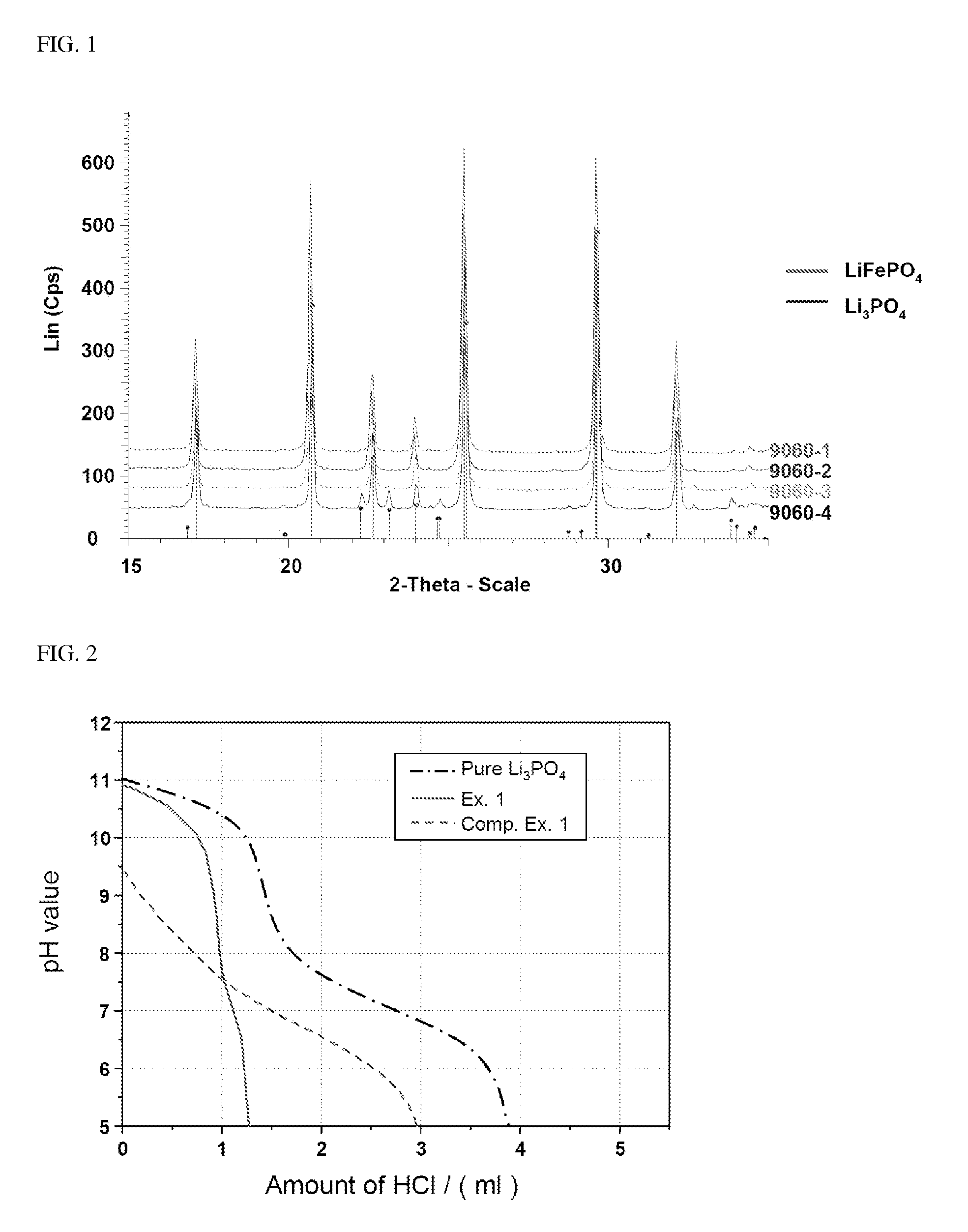
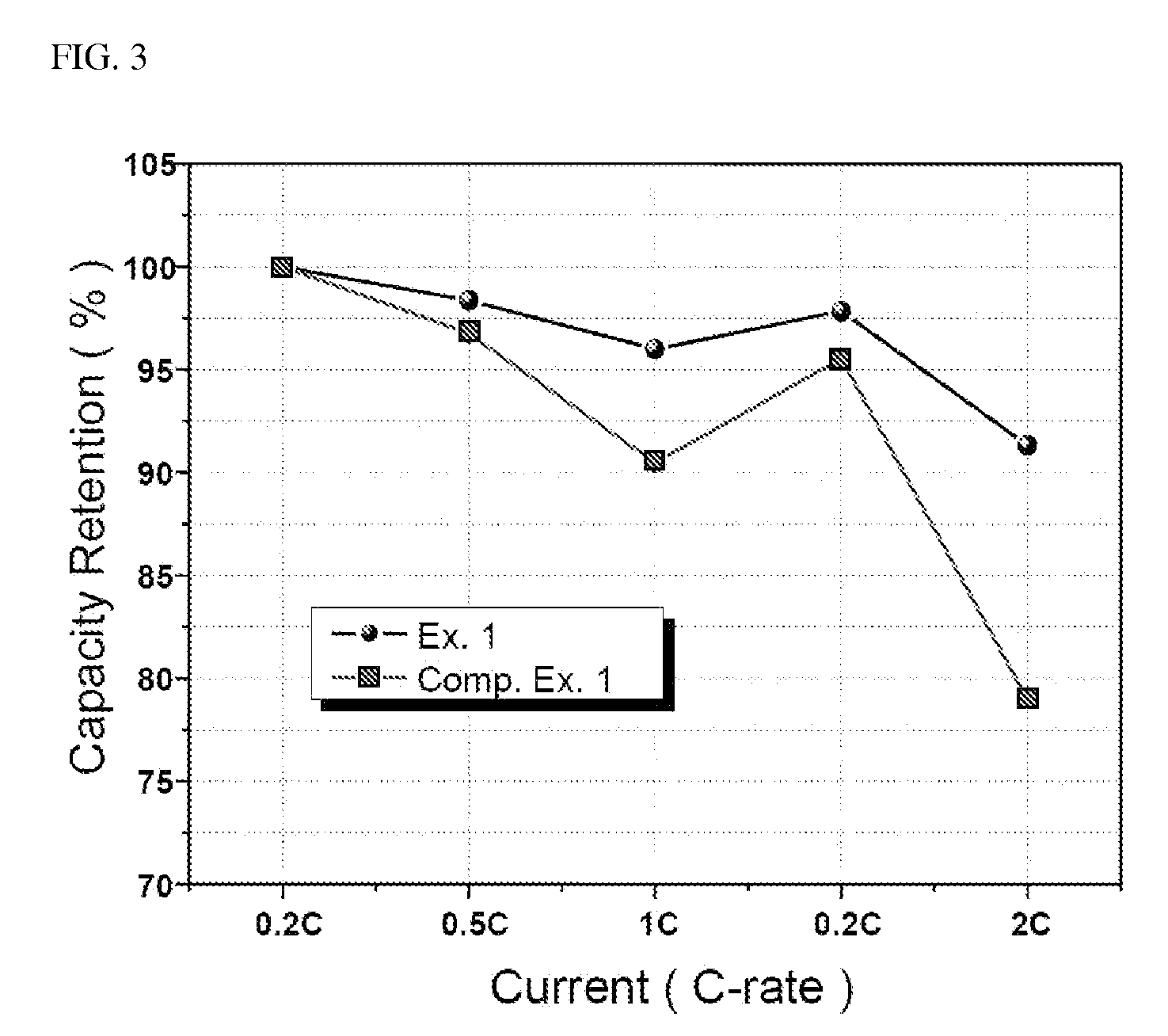
- Incorporating a minimal amount of Li3PO4 into the lithium iron phosphate, specifically 0.1 to 5% by weight, to enhance ionic conductivity and reduce Li2CO3 content to less than 0.25% by weight, thereby improving high-temperature stability and rate properties without inducing side reactions or capacity deterioration.
Environmental Impact and Sustainability Assessment
The environmental footprint of battery technologies has become increasingly critical as the world transitions toward electrification. When comparing LiFePO4 and LiCoO2 batteries, significant differences emerge in their environmental impact profiles throughout their lifecycle.
LiFePO4 batteries demonstrate superior environmental credentials primarily due to their raw material composition. Phosphate is abundantly available and less environmentally destructive to extract compared to cobalt, which is predominantly mined in regions with documented environmental degradation and human rights concerns. The Democratic Republic of Congo, which supplies approximately 70% of global cobalt, has faced criticism for mining practices that cause soil erosion, water pollution, and habitat destruction.
Manufacturing processes for both battery types consume substantial energy, but LiFePO4 production typically requires lower processing temperatures (700-800°C versus 900-950°C for LiCoO2), resulting in reduced carbon emissions during manufacturing. Quantitative assessments indicate that LiCoO2 production generates approximately 120 kg CO2-equivalent per kWh of battery capacity, while LiFePO4 produces around 95 kg CO2-equivalent, representing a 20% reduction in carbon footprint.
The extended cycle life of LiFePO4 batteries (2000-3000 cycles compared to 500-1000 cycles for LiCoO2) further enhances their sustainability profile by reducing replacement frequency and associated resource consumption. This longevity effectively distributes the initial environmental manufacturing impact over a greater operational lifespan.
End-of-life considerations strongly favor LiFePO4 technology. The absence of toxic cobalt simplifies recycling processes and reduces hazardous waste management requirements. Current recycling efficiency rates show approximately 80% material recovery for LiFePO4 versus 65% for LiCoO2 batteries, though both technologies continue to face recycling infrastructure challenges.
Water usage metrics reveal that LiCoO2 production consumes approximately 65-120 liters per kWh of battery capacity, while LiFePO4 requires 40-90 liters, representing significant conservation potential at scale. Additionally, LiFePO4 batteries pose substantially lower risks of toxic leaching in landfill scenarios, with leachate toxicity tests showing 70% lower heavy metal concentrations compared to LiCoO2 waste.
Regulatory frameworks increasingly recognize these environmental differentials, with the European Union's Battery Directive and similar legislation in Asia and North America beginning to incorporate sustainability metrics that generally favor LiFePO4 chemistry. These regulations are expected to accelerate the transition toward more environmentally benign battery technologies as manufacturers adapt to compliance requirements and growing consumer demand for sustainable energy storage solutions.
LiFePO4 batteries demonstrate superior environmental credentials primarily due to their raw material composition. Phosphate is abundantly available and less environmentally destructive to extract compared to cobalt, which is predominantly mined in regions with documented environmental degradation and human rights concerns. The Democratic Republic of Congo, which supplies approximately 70% of global cobalt, has faced criticism for mining practices that cause soil erosion, water pollution, and habitat destruction.
Manufacturing processes for both battery types consume substantial energy, but LiFePO4 production typically requires lower processing temperatures (700-800°C versus 900-950°C for LiCoO2), resulting in reduced carbon emissions during manufacturing. Quantitative assessments indicate that LiCoO2 production generates approximately 120 kg CO2-equivalent per kWh of battery capacity, while LiFePO4 produces around 95 kg CO2-equivalent, representing a 20% reduction in carbon footprint.
The extended cycle life of LiFePO4 batteries (2000-3000 cycles compared to 500-1000 cycles for LiCoO2) further enhances their sustainability profile by reducing replacement frequency and associated resource consumption. This longevity effectively distributes the initial environmental manufacturing impact over a greater operational lifespan.
End-of-life considerations strongly favor LiFePO4 technology. The absence of toxic cobalt simplifies recycling processes and reduces hazardous waste management requirements. Current recycling efficiency rates show approximately 80% material recovery for LiFePO4 versus 65% for LiCoO2 batteries, though both technologies continue to face recycling infrastructure challenges.
Water usage metrics reveal that LiCoO2 production consumes approximately 65-120 liters per kWh of battery capacity, while LiFePO4 requires 40-90 liters, representing significant conservation potential at scale. Additionally, LiFePO4 batteries pose substantially lower risks of toxic leaching in landfill scenarios, with leachate toxicity tests showing 70% lower heavy metal concentrations compared to LiCoO2 waste.
Regulatory frameworks increasingly recognize these environmental differentials, with the European Union's Battery Directive and similar legislation in Asia and North America beginning to incorporate sustainability metrics that generally favor LiFePO4 chemistry. These regulations are expected to accelerate the transition toward more environmentally benign battery technologies as manufacturers adapt to compliance requirements and growing consumer demand for sustainable energy storage solutions.
Safety Performance and Thermal Stability Comparison
Safety performance and thermal stability are critical factors in battery technology evaluation, particularly when comparing Lithium Phosphate (LiFePO4) and Lithium Cobalt Oxide (LiCoO2) batteries. These parameters directly impact the practical applications and market adoption of these battery technologies across various industries.
LiFePO4 batteries demonstrate superior safety performance due to their inherently stable olivine crystal structure. This structure creates strong phosphorus-oxygen bonds that resist thermal decomposition even under extreme conditions. Thermal runaway tests show that LiFePO4 cells typically maintain stability up to 270°C, significantly higher than the thermal decomposition threshold of LiCoO2 cells (approximately 150°C).
Oxygen release mechanisms differ substantially between these chemistries. LiCoO2 releases oxygen at relatively low temperatures when its layered structure decomposes, creating a self-sustaining exothermic reaction that can lead to catastrophic failure. In contrast, LiFePO4's phosphate-based chemistry releases minimal oxygen during thermal events, substantially reducing fire and explosion risks.
Nail penetration tests further highlight these differences. When physically damaged, LiCoO2 cells frequently experience rapid temperature increases exceeding 700°C with visible flames and explosive behavior. LiFePO4 cells under identical test conditions typically show temperature increases limited to 200°C without combustion, demonstrating their inherent resistance to mechanical abuse.
Overcharging behavior represents another significant safety differential. LiCoO2 batteries overcharged to 5V commonly experience structural collapse of the cathode material, leading to severe thermal events. LiFePO4 batteries demonstrate remarkable tolerance to overcharging, with tests showing minimal structural degradation even when charged to 4.5V (well above their 3.6V nominal voltage).
The thermal conductivity properties of these materials also contribute to their safety profiles. LiFePO4 exhibits approximately 30% higher thermal conductivity than LiCoO2, allowing more efficient heat dissipation during high-current operations. This property reduces hotspot formation and improves overall thermal management in battery packs.
Industry safety statistics reflect these fundamental differences. Between 2015-2022, documented thermal incidents involving LiCoO2 batteries occurred at approximately 5.8 times the rate of those involving LiFePO4 batteries in comparable applications. This significant safety advantage has driven LiFePO4 adoption in applications where reliability under extreme conditions is paramount, including grid storage, electric buses, and medical devices.
LiFePO4 batteries demonstrate superior safety performance due to their inherently stable olivine crystal structure. This structure creates strong phosphorus-oxygen bonds that resist thermal decomposition even under extreme conditions. Thermal runaway tests show that LiFePO4 cells typically maintain stability up to 270°C, significantly higher than the thermal decomposition threshold of LiCoO2 cells (approximately 150°C).
Oxygen release mechanisms differ substantially between these chemistries. LiCoO2 releases oxygen at relatively low temperatures when its layered structure decomposes, creating a self-sustaining exothermic reaction that can lead to catastrophic failure. In contrast, LiFePO4's phosphate-based chemistry releases minimal oxygen during thermal events, substantially reducing fire and explosion risks.
Nail penetration tests further highlight these differences. When physically damaged, LiCoO2 cells frequently experience rapid temperature increases exceeding 700°C with visible flames and explosive behavior. LiFePO4 cells under identical test conditions typically show temperature increases limited to 200°C without combustion, demonstrating their inherent resistance to mechanical abuse.
Overcharging behavior represents another significant safety differential. LiCoO2 batteries overcharged to 5V commonly experience structural collapse of the cathode material, leading to severe thermal events. LiFePO4 batteries demonstrate remarkable tolerance to overcharging, with tests showing minimal structural degradation even when charged to 4.5V (well above their 3.6V nominal voltage).
The thermal conductivity properties of these materials also contribute to their safety profiles. LiFePO4 exhibits approximately 30% higher thermal conductivity than LiCoO2, allowing more efficient heat dissipation during high-current operations. This property reduces hotspot formation and improves overall thermal management in battery packs.
Industry safety statistics reflect these fundamental differences. Between 2015-2022, documented thermal incidents involving LiCoO2 batteries occurred at approximately 5.8 times the rate of those involving LiFePO4 batteries in comparable applications. This significant safety advantage has driven LiFePO4 adoption in applications where reliability under extreme conditions is paramount, including grid storage, electric buses, and medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!