Current Advances in Ethyl Acetate and Climate Action
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate, a versatile organic compound, has undergone significant evolution in its production methods and applications over the years. Initially synthesized in the 19th century, ethyl acetate's journey began with traditional esterification processes using ethanol and acetic acid. This method, while effective, was limited in scale and efficiency.
The mid-20th century saw a shift towards more industrial-scale production techniques. Catalytic dehydrogenation of ethanol emerged as a prominent method, offering improved yields and reduced waste. This period also marked the introduction of continuous flow reactors, which revolutionized the production process by allowing for higher throughput and better quality control.
As environmental concerns gained prominence in the late 20th century, the focus shifted towards developing greener production methods. The use of biocatalysts, particularly lipases, began to gain traction. These enzymes offered a more environmentally friendly alternative to traditional chemical catalysts, operating under milder conditions and producing fewer by-products.
The turn of the millennium brought about a renewed interest in ethyl acetate as a potential bio-based solvent. Research into the production of ethyl acetate from renewable resources intensified. Fermentation processes using various microorganisms capable of producing ethyl acetate directly from biomass feedstocks became a subject of extensive study.
Recent years have seen significant advancements in process intensification techniques for ethyl acetate production. Reactive distillation, which combines reaction and separation in a single unit, has emerged as a promising approach. This technology offers potential energy savings and reduced equipment costs compared to conventional processes.
The latest frontier in ethyl acetate evolution involves the integration of artificial intelligence and machine learning in process optimization. These technologies are being employed to fine-tune reaction conditions, predict product quality, and enhance overall production efficiency. Additionally, there's growing interest in developing novel catalysts, including metal-organic frameworks and nanostructured materials, which promise higher selectivity and improved yields.
In the context of climate action, recent research has focused on carbon capture and utilization (CCU) technologies that incorporate ethyl acetate production. These innovative approaches aim to convert captured CO2 into value-added products, including ethyl acetate, thereby contributing to circular economy principles and greenhouse gas reduction efforts.
As we look to the future, the evolution of ethyl acetate production is likely to continue along the path of sustainability and efficiency. Emerging technologies such as electrochemical synthesis and photocatalytic processes are being explored as potential next-generation production methods. These advancements not only promise to reduce the environmental footprint of ethyl acetate production but also to open up new applications in various industries, from pharmaceuticals to advanced materials.
The mid-20th century saw a shift towards more industrial-scale production techniques. Catalytic dehydrogenation of ethanol emerged as a prominent method, offering improved yields and reduced waste. This period also marked the introduction of continuous flow reactors, which revolutionized the production process by allowing for higher throughput and better quality control.
As environmental concerns gained prominence in the late 20th century, the focus shifted towards developing greener production methods. The use of biocatalysts, particularly lipases, began to gain traction. These enzymes offered a more environmentally friendly alternative to traditional chemical catalysts, operating under milder conditions and producing fewer by-products.
The turn of the millennium brought about a renewed interest in ethyl acetate as a potential bio-based solvent. Research into the production of ethyl acetate from renewable resources intensified. Fermentation processes using various microorganisms capable of producing ethyl acetate directly from biomass feedstocks became a subject of extensive study.
Recent years have seen significant advancements in process intensification techniques for ethyl acetate production. Reactive distillation, which combines reaction and separation in a single unit, has emerged as a promising approach. This technology offers potential energy savings and reduced equipment costs compared to conventional processes.
The latest frontier in ethyl acetate evolution involves the integration of artificial intelligence and machine learning in process optimization. These technologies are being employed to fine-tune reaction conditions, predict product quality, and enhance overall production efficiency. Additionally, there's growing interest in developing novel catalysts, including metal-organic frameworks and nanostructured materials, which promise higher selectivity and improved yields.
In the context of climate action, recent research has focused on carbon capture and utilization (CCU) technologies that incorporate ethyl acetate production. These innovative approaches aim to convert captured CO2 into value-added products, including ethyl acetate, thereby contributing to circular economy principles and greenhouse gas reduction efforts.
As we look to the future, the evolution of ethyl acetate production is likely to continue along the path of sustainability and efficiency. Emerging technologies such as electrochemical synthesis and photocatalytic processes are being explored as potential next-generation production methods. These advancements not only promise to reduce the environmental footprint of ethyl acetate production but also to open up new applications in various industries, from pharmaceuticals to advanced materials.
Market Demand Analysis
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. As a key solvent and intermediate in chemical processes, ethyl acetate's demand is closely tied to industrial production and consumer goods manufacturing. The market size for ethyl acetate was valued at approximately 3.3 million tons in 2020, with projections indicating a compound annual growth rate (CAGR) of around 4.5% through 2027.
The paint and coating industry remains the largest consumer of ethyl acetate, accounting for nearly 40% of the total market share. This sector's growth is fueled by increasing construction activities and automotive production in emerging economies. The adhesives industry follows closely, with ethyl acetate being a crucial component in the formulation of various adhesive products used in packaging, woodworking, and consumer goods.
In recent years, the pharmaceutical industry has emerged as a significant driver of ethyl acetate demand. Its use as a solvent in drug manufacturing and as an excipient in pharmaceutical formulations has led to increased consumption. The growing emphasis on healthcare and the expansion of pharmaceutical production in developing countries are expected to further boost this trend.
The food and beverage industry also contributes substantially to ethyl acetate demand, particularly in flavor and fragrance applications. As consumer preferences shift towards natural and organic products, the use of ethyl acetate as a natural solvent in food processing has gained traction. This trend aligns with the broader movement towards sustainable and eco-friendly practices across industries.
However, the market for ethyl acetate faces challenges in the context of climate action and environmental concerns. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and smog formation. This has led to increased regulatory scrutiny and a push for more sustainable alternatives in certain applications. The industry is responding by developing bio-based ethyl acetate and exploring greener production methods to address these environmental concerns.
The Asia-Pacific region dominates the global ethyl acetate market, accounting for over 40% of the total consumption. China and India are the key growth drivers in this region, with their rapidly expanding industrial sectors and increasing domestic demand. North America and Europe follow, with mature markets focusing on high-value applications and sustainable production methods.
In conclusion, the market demand for ethyl acetate remains robust, driven by its diverse applications and the growth of key end-use industries. However, the industry must navigate the challenges posed by environmental regulations and the need for sustainable practices. The future market trajectory will likely be shaped by innovations in green chemistry, bio-based production, and the development of environmentally friendly alternatives in certain applications.
The paint and coating industry remains the largest consumer of ethyl acetate, accounting for nearly 40% of the total market share. This sector's growth is fueled by increasing construction activities and automotive production in emerging economies. The adhesives industry follows closely, with ethyl acetate being a crucial component in the formulation of various adhesive products used in packaging, woodworking, and consumer goods.
In recent years, the pharmaceutical industry has emerged as a significant driver of ethyl acetate demand. Its use as a solvent in drug manufacturing and as an excipient in pharmaceutical formulations has led to increased consumption. The growing emphasis on healthcare and the expansion of pharmaceutical production in developing countries are expected to further boost this trend.
The food and beverage industry also contributes substantially to ethyl acetate demand, particularly in flavor and fragrance applications. As consumer preferences shift towards natural and organic products, the use of ethyl acetate as a natural solvent in food processing has gained traction. This trend aligns with the broader movement towards sustainable and eco-friendly practices across industries.
However, the market for ethyl acetate faces challenges in the context of climate action and environmental concerns. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and smog formation. This has led to increased regulatory scrutiny and a push for more sustainable alternatives in certain applications. The industry is responding by developing bio-based ethyl acetate and exploring greener production methods to address these environmental concerns.
The Asia-Pacific region dominates the global ethyl acetate market, accounting for over 40% of the total consumption. China and India are the key growth drivers in this region, with their rapidly expanding industrial sectors and increasing domestic demand. North America and Europe follow, with mature markets focusing on high-value applications and sustainable production methods.
In conclusion, the market demand for ethyl acetate remains robust, driven by its diverse applications and the growth of key end-use industries. However, the industry must navigate the challenges posed by environmental regulations and the need for sustainable practices. The future market trajectory will likely be shaped by innovations in green chemistry, bio-based production, and the development of environmentally friendly alternatives in certain applications.
Technical Challenges
The production and use of ethyl acetate face several technical challenges in the context of climate action. One of the primary issues is the carbon footprint associated with its traditional manufacturing process. The conventional method relies heavily on fossil fuel-based feedstocks and energy-intensive processes, contributing significantly to greenhouse gas emissions. This presents a major hurdle in aligning ethyl acetate production with global climate goals.
Another challenge lies in the optimization of catalytic processes for ethyl acetate synthesis. While advancements have been made in developing more efficient catalysts, there is still room for improvement in terms of selectivity, yield, and energy consumption. The search for catalysts that can operate at lower temperatures and pressures while maintaining high conversion rates remains an active area of research.
The purification and separation of ethyl acetate from reaction mixtures also pose technical difficulties. Current distillation methods are energy-intensive and often require multiple stages to achieve high purity levels. This not only increases production costs but also contributes to the overall environmental impact of the manufacturing process. Developing more efficient separation techniques that consume less energy and reduce waste is crucial for improving the sustainability of ethyl acetate production.
Recycling and circular economy approaches present another set of challenges. While ethyl acetate can be recovered and reused in some applications, the processes for doing so are not always economically viable or environmentally friendly. Improving recycling technologies and establishing effective collection systems are necessary steps towards reducing the environmental footprint of ethyl acetate throughout its lifecycle.
The volatility of ethyl acetate also raises concerns regarding its atmospheric impact. As a volatile organic compound (VOC), it can contribute to the formation of ground-level ozone and smog when released into the environment. Developing containment strategies and emission control technologies for industrial applications of ethyl acetate is essential for mitigating its potential negative effects on air quality and climate.
Lastly, the transition to bio-based or renewable sources for ethyl acetate production faces its own set of technical hurdles. While promising, the use of biomass feedstocks often requires complex pretreatment processes and faces challenges in terms of scalability and consistency of supply. Optimizing these processes to make them economically competitive with traditional petrochemical routes while ensuring a reduced environmental impact remains a significant challenge for researchers and industry professionals alike.
Another challenge lies in the optimization of catalytic processes for ethyl acetate synthesis. While advancements have been made in developing more efficient catalysts, there is still room for improvement in terms of selectivity, yield, and energy consumption. The search for catalysts that can operate at lower temperatures and pressures while maintaining high conversion rates remains an active area of research.
The purification and separation of ethyl acetate from reaction mixtures also pose technical difficulties. Current distillation methods are energy-intensive and often require multiple stages to achieve high purity levels. This not only increases production costs but also contributes to the overall environmental impact of the manufacturing process. Developing more efficient separation techniques that consume less energy and reduce waste is crucial for improving the sustainability of ethyl acetate production.
Recycling and circular economy approaches present another set of challenges. While ethyl acetate can be recovered and reused in some applications, the processes for doing so are not always economically viable or environmentally friendly. Improving recycling technologies and establishing effective collection systems are necessary steps towards reducing the environmental footprint of ethyl acetate throughout its lifecycle.
The volatility of ethyl acetate also raises concerns regarding its atmospheric impact. As a volatile organic compound (VOC), it can contribute to the formation of ground-level ozone and smog when released into the environment. Developing containment strategies and emission control technologies for industrial applications of ethyl acetate is essential for mitigating its potential negative effects on air quality and climate.
Lastly, the transition to bio-based or renewable sources for ethyl acetate production faces its own set of technical hurdles. While promising, the use of biomass feedstocks often requires complex pretreatment processes and faces challenges in terms of scalability and consistency of supply. Optimizing these processes to make them economically competitive with traditional petrochemical routes while ensuring a reduced environmental impact remains a significant challenge for researchers and industry professionals alike.
Current Solutions
01 Production and purification of ethyl acetate
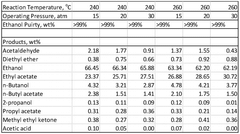
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the reaction conditions and separation processes to obtain high-quality ethyl acetate efficiently.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also employed in extraction processes, as a reaction medium, and in the production of other chemicals. Its versatility makes it a valuable component in many manufacturing processes.
- Ethyl acetate in green chemistry and sustainable processes: Research focuses on developing environmentally friendly methods for producing and using ethyl acetate. This includes the use of renewable resources as feedstock, the development of bio-based production methods, and the implementation of energy-efficient processes. These approaches aim to reduce the environmental impact of ethyl acetate production and utilization.
- Recovery and recycling of ethyl acetate: Methods for recovering and recycling ethyl acetate from industrial processes are described. These include adsorption techniques, membrane separation, and advanced distillation processes. The recovery and recycling of ethyl acetate contribute to reducing waste and improving the overall efficiency of industrial processes that use this solvent.
- Ethyl acetate derivatives and related compounds: Research on ethyl acetate derivatives and related compounds is presented. This includes the synthesis of novel compounds based on ethyl acetate, the study of their properties, and potential applications. The development of these derivatives aims to expand the range of applications for ethyl acetate-based compounds in various industries.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. The versatility of ethyl acetate in different chemical reactions and industrial processes is highlighted.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its use as a solvent in liquid-liquid extraction, chromatography, and other separation techniques is described. The effectiveness of ethyl acetate in isolating specific compounds from complex mixtures is emphasized.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The environmental impact and safety aspects of ethyl acetate production and use are addressed. This includes methods for reducing emissions, improving process safety, and developing more sustainable production techniques. Strategies for handling, storing, and disposing of ethyl acetate in an environmentally friendly manner are discussed.Expand Specific Solutions05 Novel derivatives and modifications of ethyl acetate
Research into novel derivatives and modifications of ethyl acetate is presented. This includes the development of new compounds based on ethyl acetate, as well as modifications to its structure to enhance its properties or create new functionalities. The potential applications of these novel derivatives in various industries are explored.Expand Specific Solutions
Industry Leaders
The current landscape of ethyl acetate production and climate action is characterized by a competitive and evolving market. The industry is in a transitional phase, with growing emphasis on sustainable practices and carbon reduction. Market size is expanding due to increased demand in various sectors, including coatings, adhesives, and pharmaceuticals. Technological maturity varies, with established players like Celanese International Corp. and China Petroleum & Chemical Corp. leading in traditional production methods. However, emerging companies and research institutions, such as Zhejiang University and Cornell University, are driving innovation in greener production processes and carbon capture technologies. This dynamic environment is fostering collaborations between industry giants and academic institutions, accelerating the development of more environmentally friendly ethyl acetate production methods aligned with climate action goals.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production that significantly reduces carbon emissions. Their method utilizes bio-based feedstocks and employs a novel catalytic system that operates at lower temperatures and pressures compared to traditional processes[1]. This approach not only decreases energy consumption but also incorporates carbon capture and utilization (CCU) technology, where CO2 is captured and used as a raw material in the production process[2]. The company has also implemented advanced process control systems and heat integration techniques to further optimize energy efficiency[3].
Strengths: Reduced carbon footprint, utilization of renewable resources, and improved energy efficiency. Weaknesses: Potential higher initial investment costs and dependence on bio-based feedstock availability.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a green ethyl acetate production technology that utilizes ethanol derived from biomass fermentation. Their process incorporates a highly selective heterogeneous catalyst that enables direct ethanol dehydrogenation to ethyl acetate with high yields and minimal byproducts[4]. The company has also implemented an integrated biorefinery concept, where the ethanol production is coupled with ethyl acetate synthesis, maximizing resource utilization and reducing overall emissions[5]. Additionally, Sinopec has invested in advanced membrane separation technologies to purify the ethyl acetate product, reducing energy consumption in the distillation process[6].
Strengths: Integration of bio-based feedstocks, high selectivity catalyst, and energy-efficient separation. Weaknesses: Reliance on agricultural feedstocks and potential competition with food production.
Key Innovations
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
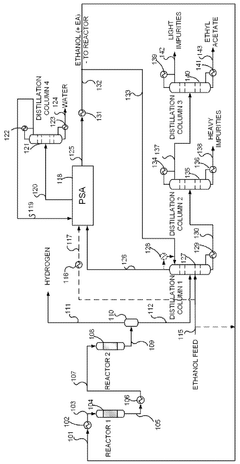
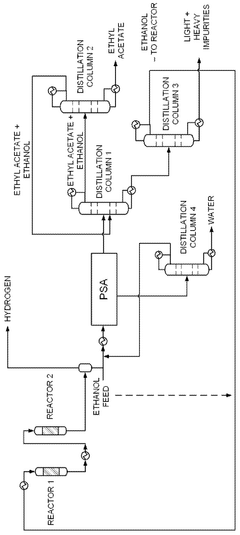
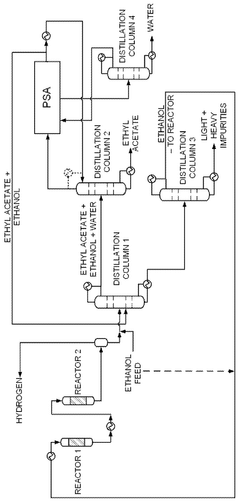
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
Environmental Impact
The production and use of ethyl acetate have significant environmental implications, particularly in the context of climate action. As a widely used solvent in various industries, ethyl acetate's lifecycle from production to disposal contributes to greenhouse gas emissions and other environmental concerns.
The manufacturing process of ethyl acetate traditionally involves the reaction of ethanol with acetic acid, often using sulfuric acid as a catalyst. This process is energy-intensive and relies heavily on fossil fuel-derived feedstocks, contributing to carbon dioxide emissions. However, recent advancements in green chemistry have led to the development of more sustainable production methods, such as the use of biocatalysts and renewable raw materials, which can significantly reduce the carbon footprint of ethyl acetate production.
In terms of usage, ethyl acetate's volatile nature means it can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. This is particularly relevant in industrial settings where large quantities are used, such as in the production of paints, coatings, and adhesives. Improved containment and recovery systems in these industries can help mitigate emissions and reduce environmental impact.
The disposal of ethyl acetate-containing products also poses environmental challenges. Improper disposal can lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. Implementing effective waste management strategies, including recycling and proper treatment of ethyl acetate-containing waste, is crucial for minimizing these risks.
On a positive note, ethyl acetate's biodegradability offers an advantage over some other solvents. Under appropriate conditions, it can break down relatively quickly in the environment, reducing long-term pollution risks. This property has led to increased interest in using ethyl acetate as a more environmentally friendly alternative to certain persistent organic solvents.
In the context of climate action, the focus on ethyl acetate has shifted towards developing cleaner production methods, improving energy efficiency in its use, and enhancing end-of-life management. Research into bio-based ethyl acetate production, using agricultural waste or other renewable sources, shows promise in reducing the reliance on fossil fuels and decreasing overall carbon emissions associated with its lifecycle.
Furthermore, the application of ethyl acetate in green technologies, such as in the production of biodegradable plastics or as a component in more environmentally friendly cleaning products, demonstrates its potential role in supporting broader climate action goals. By replacing more harmful chemicals in various applications, ethyl acetate can contribute to reducing the overall environmental footprint of multiple industries.
The manufacturing process of ethyl acetate traditionally involves the reaction of ethanol with acetic acid, often using sulfuric acid as a catalyst. This process is energy-intensive and relies heavily on fossil fuel-derived feedstocks, contributing to carbon dioxide emissions. However, recent advancements in green chemistry have led to the development of more sustainable production methods, such as the use of biocatalysts and renewable raw materials, which can significantly reduce the carbon footprint of ethyl acetate production.
In terms of usage, ethyl acetate's volatile nature means it can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. This is particularly relevant in industrial settings where large quantities are used, such as in the production of paints, coatings, and adhesives. Improved containment and recovery systems in these industries can help mitigate emissions and reduce environmental impact.
The disposal of ethyl acetate-containing products also poses environmental challenges. Improper disposal can lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. Implementing effective waste management strategies, including recycling and proper treatment of ethyl acetate-containing waste, is crucial for minimizing these risks.
On a positive note, ethyl acetate's biodegradability offers an advantage over some other solvents. Under appropriate conditions, it can break down relatively quickly in the environment, reducing long-term pollution risks. This property has led to increased interest in using ethyl acetate as a more environmentally friendly alternative to certain persistent organic solvents.
In the context of climate action, the focus on ethyl acetate has shifted towards developing cleaner production methods, improving energy efficiency in its use, and enhancing end-of-life management. Research into bio-based ethyl acetate production, using agricultural waste or other renewable sources, shows promise in reducing the reliance on fossil fuels and decreasing overall carbon emissions associated with its lifecycle.
Furthermore, the application of ethyl acetate in green technologies, such as in the production of biodegradable plastics or as a component in more environmentally friendly cleaning products, demonstrates its potential role in supporting broader climate action goals. By replacing more harmful chemicals in various applications, ethyl acetate can contribute to reducing the overall environmental footprint of multiple industries.
Regulatory Framework
The regulatory framework surrounding ethyl acetate production and use in the context of climate action is complex and evolving. At the international level, the Paris Agreement sets the overarching goal of limiting global temperature rise to well below 2°C above pre-industrial levels. This has led to increased scrutiny of industrial processes and chemicals that contribute to greenhouse gas emissions.
In the European Union, ethyl acetate falls under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation. REACH requires manufacturers and importers to register chemicals and provide safety data. The EU has also implemented the Industrial Emissions Directive (IED), which sets strict emission limits for various industrial activities, including those involving organic solvents like ethyl acetate.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and processors of ethyl acetate. Additionally, the Clean Air Act classifies ethyl acetate as a volatile organic compound (VOC), subject to emission controls in certain applications.
Many countries have implemented or are considering carbon pricing mechanisms, such as carbon taxes or cap-and-trade systems. These policies indirectly affect ethyl acetate production by increasing the cost of energy-intensive processes and incentivizing the adoption of more sustainable practices.
The chemical industry has responded to regulatory pressures by developing voluntary initiatives. For instance, the Responsible Care program, adopted by chemical manufacturers worldwide, promotes environmental stewardship and continuous improvement in safety and sustainability practices.
Emerging regulations are focusing on the circular economy and extended producer responsibility. These concepts are likely to impact the ethyl acetate industry by encouraging recycling, reuse, and more efficient use of resources throughout the product lifecycle.
As climate action intensifies, regulatory bodies are expected to tighten controls on industrial emissions and promote the transition to low-carbon technologies. This may lead to stricter emission limits, increased reporting requirements, and potential phase-outs of certain production methods deemed incompatible with climate goals.
The regulatory landscape is also shifting towards promoting bio-based alternatives. Policies supporting the bioeconomy may offer incentives for the production of bio-ethyl acetate, potentially reshaping the market dynamics of this chemical.
In the European Union, ethyl acetate falls under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation. REACH requires manufacturers and importers to register chemicals and provide safety data. The EU has also implemented the Industrial Emissions Directive (IED), which sets strict emission limits for various industrial activities, including those involving organic solvents like ethyl acetate.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The EPA has established reporting requirements for manufacturers and processors of ethyl acetate. Additionally, the Clean Air Act classifies ethyl acetate as a volatile organic compound (VOC), subject to emission controls in certain applications.
Many countries have implemented or are considering carbon pricing mechanisms, such as carbon taxes or cap-and-trade systems. These policies indirectly affect ethyl acetate production by increasing the cost of energy-intensive processes and incentivizing the adoption of more sustainable practices.
The chemical industry has responded to regulatory pressures by developing voluntary initiatives. For instance, the Responsible Care program, adopted by chemical manufacturers worldwide, promotes environmental stewardship and continuous improvement in safety and sustainability practices.
Emerging regulations are focusing on the circular economy and extended producer responsibility. These concepts are likely to impact the ethyl acetate industry by encouraging recycling, reuse, and more efficient use of resources throughout the product lifecycle.
As climate action intensifies, regulatory bodies are expected to tighten controls on industrial emissions and promote the transition to low-carbon technologies. This may lead to stricter emission limits, increased reporting requirements, and potential phase-outs of certain production methods deemed incompatible with climate goals.
The regulatory landscape is also shifting towards promoting bio-based alternatives. Policies supporting the bioeconomy may offer incentives for the production of bio-ethyl acetate, potentially reshaping the market dynamics of this chemical.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!