Engineering perfusion filters and cell retention devices to enable continuous cell production modes
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Continuous Bioprocessing Technology Evolution and Objectives
Continuous bioprocessing represents a significant paradigm shift in biopharmaceutical manufacturing, evolving from traditional batch processing to more efficient, cost-effective continuous operations. This technological evolution began in the early 2000s with rudimentary perfusion systems and has accelerated dramatically over the past decade. The trajectory has been driven by increasing demands for biologics production capacity, cost pressures, and regulatory encouragement for innovative manufacturing approaches that enhance product quality consistency.
The evolution of continuous bioprocessing can be traced through several key technological milestones. Early perfusion systems relied on simple filtration methods with limited cell retention capabilities. These evolved into more sophisticated tangential flow filtration (TFF) systems in the mid-2000s, followed by alternating tangential flow (ATF) technologies that significantly improved cell viability and productivity. Recent advancements include acoustic wave separation, centrifugation-based systems, and hybrid approaches that combine multiple cell retention mechanisms.
The primary objective of continuous bioprocessing technology development is to establish robust, scalable manufacturing platforms that maintain consistent product quality while significantly reducing production costs. Specific goals include achieving higher cell densities (>100 million cells/mL compared to 10-15 million cells/mL in batch processes), extending culture durations from weeks to months, and minimizing process-related impurities through real-time monitoring and control.
Another critical objective is the development of integrated continuous processing lines that connect upstream cell culture with downstream purification operations. This integration aims to eliminate hold steps, reduce facility footprint, and enable real-time release testing—ultimately transforming manufacturing facilities into more agile, responsive production environments.
Regulatory considerations have also shaped technology objectives, with agencies like the FDA encouraging continuous manufacturing through initiatives such as the Process Analytical Technology (PAT) framework. This has driven development of advanced monitoring technologies, predictive modeling capabilities, and control strategies that ensure process robustness despite the inherent complexities of continuous operations.
Looking forward, the field is moving toward fully automated, closed systems with sophisticated in-line monitoring capabilities. The ultimate objective is to create manufacturing platforms that can rapidly adjust to changing market demands, support personalized medicine production requirements, and enable distributed manufacturing models that bring production closer to patients.
The evolution of continuous bioprocessing can be traced through several key technological milestones. Early perfusion systems relied on simple filtration methods with limited cell retention capabilities. These evolved into more sophisticated tangential flow filtration (TFF) systems in the mid-2000s, followed by alternating tangential flow (ATF) technologies that significantly improved cell viability and productivity. Recent advancements include acoustic wave separation, centrifugation-based systems, and hybrid approaches that combine multiple cell retention mechanisms.
The primary objective of continuous bioprocessing technology development is to establish robust, scalable manufacturing platforms that maintain consistent product quality while significantly reducing production costs. Specific goals include achieving higher cell densities (>100 million cells/mL compared to 10-15 million cells/mL in batch processes), extending culture durations from weeks to months, and minimizing process-related impurities through real-time monitoring and control.
Another critical objective is the development of integrated continuous processing lines that connect upstream cell culture with downstream purification operations. This integration aims to eliminate hold steps, reduce facility footprint, and enable real-time release testing—ultimately transforming manufacturing facilities into more agile, responsive production environments.
Regulatory considerations have also shaped technology objectives, with agencies like the FDA encouraging continuous manufacturing through initiatives such as the Process Analytical Technology (PAT) framework. This has driven development of advanced monitoring technologies, predictive modeling capabilities, and control strategies that ensure process robustness despite the inherent complexities of continuous operations.
Looking forward, the field is moving toward fully automated, closed systems with sophisticated in-line monitoring capabilities. The ultimate objective is to create manufacturing platforms that can rapidly adjust to changing market demands, support personalized medicine production requirements, and enable distributed manufacturing models that bring production closer to patients.
Market Demand Analysis for Continuous Cell Production Systems
The continuous bioprocessing market for cell production is experiencing robust growth, driven by increasing demand for biopharmaceuticals and cell therapies. Current market valuations indicate the global continuous bioprocessing market reached approximately $7.5 billion in 2022 and is projected to grow at a CAGR of 23.0% through 2030, with perfusion systems representing a significant segment of this expansion.
Biopharmaceutical manufacturers are increasingly adopting continuous cell production technologies due to their substantial economic advantages. Studies demonstrate that implementing perfusion-based manufacturing can reduce capital expenditure by 50-80% compared to traditional batch processing, while simultaneously decreasing operating costs by 20-30%. These economic benefits stem from higher volumetric productivity, reduced facility footprint, and improved product quality consistency.
The cell therapy sector represents a particularly promising market segment for continuous cell production technologies. With over 2,000 ongoing clinical trials for cell-based therapies globally, the demand for scalable, cost-effective manufacturing solutions is accelerating. Industry analysts project the cell therapy manufacturing market to reach $18 billion by 2028, with continuous processing technologies playing an increasingly central role.
Regulatory agencies have demonstrated growing support for continuous manufacturing approaches. The FDA's Emerging Technology Program and similar initiatives from the EMA have created pathways for accelerated adoption of continuous processing technologies. This regulatory encouragement has significantly reduced market entry barriers for novel perfusion filters and cell retention devices.
Regional market analysis reveals North America currently dominates the continuous bioprocessing market with approximately 40% share, followed by Europe at 30% and Asia-Pacific showing the fastest growth rate at 27% annually. China and India are emerging as significant markets due to expanding biopharmaceutical manufacturing capabilities and government initiatives supporting advanced manufacturing technologies.
Key customer segments include large biopharmaceutical companies seeking manufacturing efficiency improvements, contract development and manufacturing organizations (CDMOs) expanding their service offerings, and cell therapy developers requiring scalable production platforms. Each segment presents distinct requirements for perfusion filter and cell retention technologies, from high-throughput industrial applications to flexible, modular systems for personalized therapies.
Market research indicates growing demand for integrated continuous bioprocessing solutions that combine perfusion filters, cell retention devices, and automated control systems. End users increasingly prioritize technologies offering real-time monitoring capabilities, reduced contamination risk, and compatibility with existing manufacturing infrastructure. The ability to maintain consistent cell viability above 90% during extended culture periods represents a critical performance benchmark driving purchasing decisions.
Biopharmaceutical manufacturers are increasingly adopting continuous cell production technologies due to their substantial economic advantages. Studies demonstrate that implementing perfusion-based manufacturing can reduce capital expenditure by 50-80% compared to traditional batch processing, while simultaneously decreasing operating costs by 20-30%. These economic benefits stem from higher volumetric productivity, reduced facility footprint, and improved product quality consistency.
The cell therapy sector represents a particularly promising market segment for continuous cell production technologies. With over 2,000 ongoing clinical trials for cell-based therapies globally, the demand for scalable, cost-effective manufacturing solutions is accelerating. Industry analysts project the cell therapy manufacturing market to reach $18 billion by 2028, with continuous processing technologies playing an increasingly central role.
Regulatory agencies have demonstrated growing support for continuous manufacturing approaches. The FDA's Emerging Technology Program and similar initiatives from the EMA have created pathways for accelerated adoption of continuous processing technologies. This regulatory encouragement has significantly reduced market entry barriers for novel perfusion filters and cell retention devices.
Regional market analysis reveals North America currently dominates the continuous bioprocessing market with approximately 40% share, followed by Europe at 30% and Asia-Pacific showing the fastest growth rate at 27% annually. China and India are emerging as significant markets due to expanding biopharmaceutical manufacturing capabilities and government initiatives supporting advanced manufacturing technologies.
Key customer segments include large biopharmaceutical companies seeking manufacturing efficiency improvements, contract development and manufacturing organizations (CDMOs) expanding their service offerings, and cell therapy developers requiring scalable production platforms. Each segment presents distinct requirements for perfusion filter and cell retention technologies, from high-throughput industrial applications to flexible, modular systems for personalized therapies.
Market research indicates growing demand for integrated continuous bioprocessing solutions that combine perfusion filters, cell retention devices, and automated control systems. End users increasingly prioritize technologies offering real-time monitoring capabilities, reduced contamination risk, and compatibility with existing manufacturing infrastructure. The ability to maintain consistent cell viability above 90% during extended culture periods represents a critical performance benchmark driving purchasing decisions.
Current Challenges in Perfusion Filtration Technology
Despite significant advancements in perfusion filtration technology for continuous cell production, several critical challenges persist that limit widespread industrial adoption. The primary obstacle remains membrane fouling, where cell debris, proteins, and other biological materials accumulate on filter surfaces, progressively reducing filtration efficiency and necessitating frequent replacements. This not only increases operational costs but also introduces process interruptions that compromise the continuous nature of production.
Scale-up difficulties represent another substantial hurdle. Laboratory-scale perfusion systems often demonstrate excellent performance, but translating these results to industrial production volumes introduces complex fluid dynamics and uneven flow distribution that can significantly impact filtration efficiency and cell retention rates. The non-linear scaling behavior of these systems requires extensive re-engineering when moving from pilot to commercial scale.
Filter selectivity remains problematic in many applications, particularly when dealing with high-density cell cultures. Current technologies struggle to effectively discriminate between viable cells (which should be retained) and cell debris or dead cells (which should be removed). This limitation can lead to accumulation of non-viable material in the bioreactor, potentially affecting product quality and process stability.
Shear stress induced by filtration systems presents a significant challenge for sensitive cell lines. The mechanical forces exerted on cells during the filtration process can trigger cell damage, alter cellular metabolism, or induce apoptosis, ultimately affecting product yield and quality. This is particularly problematic for fragile mammalian cell lines commonly used in biopharmaceutical production.
Control system integration poses technical difficulties, as real-time monitoring of filter performance parameters (pressure differentials, flow rates, clogging indicators) requires sophisticated sensor arrays and control algorithms. Current systems often lack the precision needed for proactive maintenance interventions before filtration efficiency declines significantly.
Regulatory compliance adds another layer of complexity, with stringent requirements for materials compatibility, leachables/extractables profiles, and validation protocols. Many promising filtration technologies face extended development timelines due to these regulatory hurdles, particularly for applications in therapeutic protein or vaccine production.
Cost-effectiveness remains a persistent challenge, as advanced filtration technologies with improved performance characteristics often come with significantly higher capital and operational expenses. The industry continues to seek economically viable solutions that balance performance with affordability to make continuous processing financially attractive compared to traditional batch methods.
Scale-up difficulties represent another substantial hurdle. Laboratory-scale perfusion systems often demonstrate excellent performance, but translating these results to industrial production volumes introduces complex fluid dynamics and uneven flow distribution that can significantly impact filtration efficiency and cell retention rates. The non-linear scaling behavior of these systems requires extensive re-engineering when moving from pilot to commercial scale.
Filter selectivity remains problematic in many applications, particularly when dealing with high-density cell cultures. Current technologies struggle to effectively discriminate between viable cells (which should be retained) and cell debris or dead cells (which should be removed). This limitation can lead to accumulation of non-viable material in the bioreactor, potentially affecting product quality and process stability.
Shear stress induced by filtration systems presents a significant challenge for sensitive cell lines. The mechanical forces exerted on cells during the filtration process can trigger cell damage, alter cellular metabolism, or induce apoptosis, ultimately affecting product yield and quality. This is particularly problematic for fragile mammalian cell lines commonly used in biopharmaceutical production.
Control system integration poses technical difficulties, as real-time monitoring of filter performance parameters (pressure differentials, flow rates, clogging indicators) requires sophisticated sensor arrays and control algorithms. Current systems often lack the precision needed for proactive maintenance interventions before filtration efficiency declines significantly.
Regulatory compliance adds another layer of complexity, with stringent requirements for materials compatibility, leachables/extractables profiles, and validation protocols. Many promising filtration technologies face extended development timelines due to these regulatory hurdles, particularly for applications in therapeutic protein or vaccine production.
Cost-effectiveness remains a persistent challenge, as advanced filtration technologies with improved performance characteristics often come with significantly higher capital and operational expenses. The industry continues to seek economically viable solutions that balance performance with affordability to make continuous processing financially attractive compared to traditional batch methods.
Current Engineering Solutions for Cell Retention Systems
01 Tangential flow filtration systems for cell retention
Tangential flow filtration (TFF) systems are widely used for cell retention in perfusion bioreactors. These systems utilize a membrane where the feed flow runs parallel to the membrane surface, reducing fouling and allowing for continuous operation. TFF systems can be designed with various membrane configurations and pore sizes to efficiently retain cells while allowing nutrients and metabolites to pass through. These systems are particularly effective for high-density cell cultures and can be integrated with automated control systems for optimal performance.- Tangential flow filtration systems for cell retention: Tangential flow filtration (TFF) systems are used for cell retention in perfusion bioreactors. These systems allow for the continuous removal of cell-free media while retaining cells within the bioreactor. The filtration occurs parallel to the membrane surface, which reduces fouling and extends filter life. These systems can be optimized for various cell types and culture conditions, providing efficient cell retention while maintaining cell viability and productivity.
- Hollow fiber filtration for cell separation: Hollow fiber filtration systems utilize bundles of semi-permeable membrane tubes for effective cell retention. The hollow fiber design provides a large surface area for filtration while maintaining gentle conditions for cells. These systems allow small molecules and proteins to pass through while retaining cells and larger particles. The configuration can be optimized for different flow rates and cell densities, making them suitable for various bioprocessing applications.
- Acoustic wave cell retention devices: Acoustic wave technology provides a non-contact method for cell retention in perfusion systems. These devices use ultrasonic standing waves to concentrate cells in pressure nodes, allowing cell-free media to be removed. The acoustic approach minimizes shear stress on cells compared to membrane-based systems, potentially improving cell viability and product quality. These systems can be tuned for different cell types and are less prone to clogging issues that affect traditional filters.
- Microcarrier-based cell retention systems: Microcarrier-based systems provide surfaces for adherent cell attachment while enabling efficient cell retention in perfusion processes. These systems combine the benefits of suspension culture with the high cell densities possible with adherent cells. The microcarriers can be designed with various surface properties to optimize cell attachment and growth. Specialized filtration devices are used to retain the cell-laden microcarriers while allowing harvesting of cell-free product-containing media.
- Integrated single-use perfusion filter technologies: Single-use perfusion filter technologies integrate cell retention capabilities into disposable bioreactor systems. These technologies eliminate the need for cleaning and validation between batches, reducing turnaround time and cross-contamination risks. The disposable nature of these systems provides flexibility in manufacturing processes and facility design. Advanced materials and designs are employed to optimize filtration performance while maintaining the benefits of single-use technology.
02 Hollow fiber filtration technology for cell separation
Hollow fiber filtration systems consist of bundles of semi-permeable membrane tubes that allow for efficient cell retention while permitting the exchange of media components. The hollow fiber design provides a large surface area for filtration in a compact format, making it suitable for various scales of operation. These systems can be operated in different modes, including inside-out or outside-in filtration, depending on the specific application requirements. Hollow fiber filters are particularly valuable for maintaining high cell viability during continuous perfusion processes.Expand Specific Solutions03 Acoustic wave separation devices for cell retention
Acoustic wave separation technology utilizes ultrasonic standing waves to separate cells from culture media without the use of physical barriers or membranes. This non-invasive approach reduces cell stress and eliminates membrane fouling issues associated with traditional filtration methods. Acoustic separators can be tuned to specific cell types based on their physical properties and can operate continuously with minimal maintenance. These systems are particularly advantageous for sensitive cell lines and can be integrated into existing bioprocessing workflows.Expand Specific Solutions04 Microcarrier-based retention systems
Microcarrier-based cell retention systems utilize small particles that provide surfaces for cell attachment and growth while being easily retained by simple filtration or settling methods. These systems allow for high cell densities in suspension cultures and can be designed with various materials to optimize cell growth and product expression. Microcarrier systems can be combined with other retention technologies to enhance overall performance and can be particularly useful for adherent cell lines in perfusion processes.Expand Specific Solutions05 Integrated perfusion bioreactor systems with cell retention
Integrated perfusion bioreactor systems combine cell culture vessels with built-in cell retention devices to create complete solutions for continuous bioprocessing. These systems incorporate sensors, control systems, and retention technologies in a single platform, allowing for automated operation and monitoring. The integration of multiple components enhances process reliability and reduces contamination risks. These systems can be designed at various scales, from benchtop to production, and can be optimized for specific cell lines and products.Expand Specific Solutions
Leading Companies in Continuous Bioprocessing Equipment
The continuous cell production market for perfusion filters and cell retention devices is in a growth phase, with increasing adoption driven by biopharmaceutical manufacturing demands. The market is expanding rapidly as companies seek more efficient production methods, with projections indicating significant growth over the next decade. Technologically, the field shows varying maturity levels, with established players like Cytiva, Repligen, and Merck leading innovation alongside newer entrants such as FloDesign Sonics with acoustic separation technology. WuXi Biologics, Roche, and Boehringer Ingelheim are implementing these technologies at scale, while academic institutions like MIT and Tsinghua University contribute fundamental research. The competitive landscape features both specialized filtration companies and integrated biopharmaceutical manufacturers developing proprietary solutions.
Global Life Sciences Solutions USA LLC
Technical Solution: Global Life Sciences Solutions (operating as Cytiva in the US market) has developed the HyClone™ Cell Retention System, which utilizes acoustic wave separation technology for label-free, continuous cell retention. This innovative approach leverages acoustic forces to separate cells from media without physical barriers or membranes, significantly reducing fouling issues common in traditional filtration methods. Their system creates standing acoustic waves that concentrate cells at pressure nodes while allowing proteins and metabolites to be continuously harvested. The technology maintains exceptional cell viability (>95%) even during extended continuous operations by minimizing mechanical stress on cells. The system scales linearly from small-scale R&D applications to manufacturing scale, with demonstrated performance in perfusion cultures lasting over 60 days. Their integrated control system automatically adjusts acoustic parameters based on cell density measurements, ensuring consistent separation efficiency throughout the bioprocess duration while maintaining sterility through a closed-system design.
Strengths: Membrane-free technology eliminates fouling concerns; gentle cell handling maintains high viability; scalable from laboratory to production; minimal maintenance requirements during extended runs. Weaknesses: Higher energy consumption compared to passive filtration systems; acoustic technology may have limitations with certain cell types or at extremely high cell densities; requires precise control of operating parameters.
Repligen Corp.
Technical Solution: Repligen has developed the XCell™ ATF (Alternating Tangential Flow) system, a leading cell retention technology for continuous bioprocessing. This filtration system uses hollow fiber membranes with alternating flow direction to prevent membrane fouling while efficiently separating cells from harvested product. The technology enables high cell density cultures (>100 million cells/mL) while maintaining cell viability through reduced shear stress compared to traditional TFF systems. Repligen's ATF technology integrates seamlessly with existing bioreactors, allowing for perfusion processes that can increase volumetric productivity by 3-10 fold compared to fed-batch cultures. Their latest ATF 10 system can process up to 1,000L bioreactors, making it suitable for commercial-scale manufacturing. The company has also developed single-use versions (ATF-SU) to address the growing demand for flexible manufacturing solutions in continuous bioprocessing.
Strengths: Industry-leading cell retention technology with proven scalability from lab to commercial production; reduced membrane fouling through alternating flow mechanism; supports extremely high cell densities while maintaining viability; seamless integration with existing bioreactor systems. Weaknesses: Higher capital investment compared to traditional batch processing; requires specialized training for operators; membrane replacement costs can impact operational expenses for long-duration continuous processes.
Key Patents and Innovations in Perfusion Filter Design
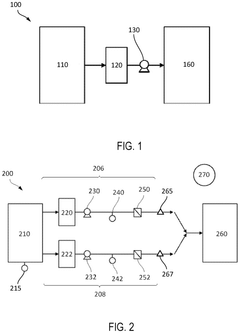
Perfusion bioreactor with filtration systems
PatentActiveUS12110483B2
Innovation
- A perfusion cell culture apparatus with dual filtration systems connected in parallel, equipped with sensors and automated controllers that detect filter failures and adjust media flow to maintain perfusion rates without shutting down the operation, ensuring continuous cell culture by isolating the malfunctioning filter and increasing flow through the operational one.
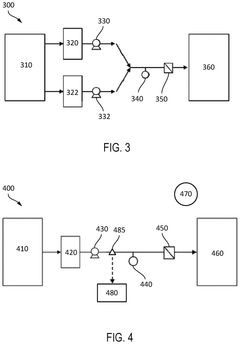
Cell retention device and method
PatentWO2017180814A1
Innovation
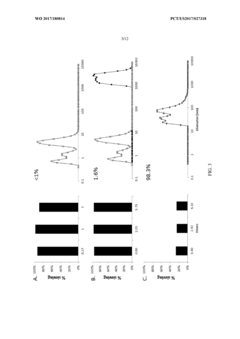
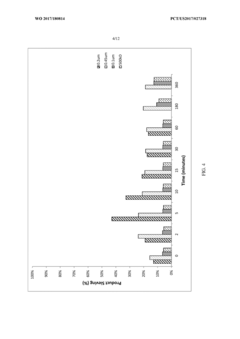
- Employing large pore size (5-10 μm) hollow-fiber filters operating in tangential flow filtration (TFF) mode, which maintains high product sieving capabilities for up to 35 days without special treatments or design modifications, allowing viable cells to be retained while dead cells and debris pass through.
Scale-up Considerations for Continuous Bioprocessing
Scaling up continuous bioprocessing systems requires careful engineering of perfusion filters and cell retention devices to maintain consistent cell production at larger volumes. The transition from laboratory-scale to production-scale operations introduces significant challenges that must be addressed through thoughtful design and implementation strategies.
When scaling up perfusion systems, filter capacity becomes a critical consideration. Laboratory-scale devices typically employ small membrane areas that cannot simply be proportionally increased for industrial applications. Instead, manufacturers must consider modular approaches that maintain optimal fluid dynamics while expanding filtration surface area. This often involves parallel configuration of multiple filter units rather than single larger units to ensure uniform flow distribution and prevent channeling effects.
Cell retention efficiency represents another crucial parameter that changes with scale. As bioreactor volumes increase, the pressure differential across retention devices may vary, potentially affecting cell viability and product quality. Engineers must implement robust pressure control systems that can accommodate the increased flow rates while maintaining gentle cell handling characteristics. Computational fluid dynamics modeling has emerged as an essential tool for predicting flow patterns and optimizing device geometry before physical implementation.
Material selection for filtration components requires additional scrutiny during scale-up. While laboratory systems may utilize expensive specialized materials, production-scale operations demand cost-effective alternatives that maintain performance specifications. Polymeric hollow fiber membranes have gained popularity due to their scalability and favorable economics, though ceramic and stainless steel options remain relevant for specific applications requiring enhanced durability or cleanability.
Fouling management strategies become increasingly important at larger scales. The accumulation of cells, cellular debris, and protein aggregates on filter surfaces can dramatically reduce operational efficiency over time. Advanced anti-fouling surface treatments, optimized tangential flow patterns, and automated back-flushing protocols must be incorporated into scaled-up designs to extend filter lifetime and maintain consistent performance.
Integration with process analytical technology (PAT) represents a significant consideration for scaled-up cell retention systems. Real-time monitoring of transmembrane pressure, filter integrity, and retention efficiency enables adaptive control strategies that can respond to changing conditions during extended continuous operations. This integration becomes more complex but also more valuable as process scale increases, allowing for predictive maintenance and quality assurance.
Regulatory compliance considerations also evolve with scale. Production-scale perfusion systems must demonstrate consistent performance across multiple batches while maintaining sterility and product quality. Design features that facilitate cleaning validation, sterilization-in-place capabilities, and non-invasive monitoring become essential elements of scaled-up retention devices.
When scaling up perfusion systems, filter capacity becomes a critical consideration. Laboratory-scale devices typically employ small membrane areas that cannot simply be proportionally increased for industrial applications. Instead, manufacturers must consider modular approaches that maintain optimal fluid dynamics while expanding filtration surface area. This often involves parallel configuration of multiple filter units rather than single larger units to ensure uniform flow distribution and prevent channeling effects.
Cell retention efficiency represents another crucial parameter that changes with scale. As bioreactor volumes increase, the pressure differential across retention devices may vary, potentially affecting cell viability and product quality. Engineers must implement robust pressure control systems that can accommodate the increased flow rates while maintaining gentle cell handling characteristics. Computational fluid dynamics modeling has emerged as an essential tool for predicting flow patterns and optimizing device geometry before physical implementation.
Material selection for filtration components requires additional scrutiny during scale-up. While laboratory systems may utilize expensive specialized materials, production-scale operations demand cost-effective alternatives that maintain performance specifications. Polymeric hollow fiber membranes have gained popularity due to their scalability and favorable economics, though ceramic and stainless steel options remain relevant for specific applications requiring enhanced durability or cleanability.
Fouling management strategies become increasingly important at larger scales. The accumulation of cells, cellular debris, and protein aggregates on filter surfaces can dramatically reduce operational efficiency over time. Advanced anti-fouling surface treatments, optimized tangential flow patterns, and automated back-flushing protocols must be incorporated into scaled-up designs to extend filter lifetime and maintain consistent performance.
Integration with process analytical technology (PAT) represents a significant consideration for scaled-up cell retention systems. Real-time monitoring of transmembrane pressure, filter integrity, and retention efficiency enables adaptive control strategies that can respond to changing conditions during extended continuous operations. This integration becomes more complex but also more valuable as process scale increases, allowing for predictive maintenance and quality assurance.
Regulatory compliance considerations also evolve with scale. Production-scale perfusion systems must demonstrate consistent performance across multiple batches while maintaining sterility and product quality. Design features that facilitate cleaning validation, sterilization-in-place capabilities, and non-invasive monitoring become essential elements of scaled-up retention devices.
Regulatory Framework for Continuous Manufacturing Processes
The regulatory landscape for continuous manufacturing processes in biopharmaceutical production, particularly for perfusion filters and cell retention devices, is evolving rapidly as these technologies gain wider adoption. The U.S. Food and Drug Administration (FDA) has demonstrated strong support for continuous manufacturing through its Emerging Technology Program, which provides a framework for early engagement between manufacturers and regulators to address potential challenges in implementing novel technologies.
Key regulatory considerations for continuous cell production systems include process validation, in-process controls, and real-time release testing. The FDA's Process Analytical Technology (PAT) initiative is particularly relevant, as it encourages manufacturers to design and develop processes that can ensure final product quality through timely measurements of critical quality attributes during processing.
The European Medicines Agency (EMA) has also published guidance on continuous manufacturing, emphasizing the importance of process understanding and control strategies. Their guidelines specifically address the need for robust cell retention technologies and filtration systems that can maintain consistent performance over extended production campaigns.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q8, Q9, Q10, and Q11, provide the quality-by-design principles that underpin regulatory expectations for continuous bioprocessing. These guidelines emphasize the need for thorough risk assessment and mitigation strategies when implementing novel manufacturing approaches.
Regulatory bodies increasingly recognize the potential benefits of continuous manufacturing, including improved product consistency, reduced batch-to-batch variability, and enhanced process control. However, they also acknowledge the unique challenges associated with defining batch identity, establishing appropriate sampling strategies, and managing process deviations in continuous operations.
For cell retention devices specifically, regulatory focus centers on demonstrating consistent performance over extended periods, validating cleaning and sterilization procedures for reusable components, and establishing appropriate monitoring strategies to detect potential filter fouling or performance degradation. Manufacturers must provide evidence that their cell retention technologies can maintain cell viability and productivity while preventing product contamination.
Looking forward, regulatory agencies are working to harmonize approaches to continuous manufacturing across different regions. The FDA's Framework for Regulatory Advanced Manufacturing Partnerships (RAMP) and similar initiatives aim to accelerate the development and implementation of advanced manufacturing technologies, including continuous processing systems for cell culture applications.
Key regulatory considerations for continuous cell production systems include process validation, in-process controls, and real-time release testing. The FDA's Process Analytical Technology (PAT) initiative is particularly relevant, as it encourages manufacturers to design and develop processes that can ensure final product quality through timely measurements of critical quality attributes during processing.
The European Medicines Agency (EMA) has also published guidance on continuous manufacturing, emphasizing the importance of process understanding and control strategies. Their guidelines specifically address the need for robust cell retention technologies and filtration systems that can maintain consistent performance over extended production campaigns.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q8, Q9, Q10, and Q11, provide the quality-by-design principles that underpin regulatory expectations for continuous bioprocessing. These guidelines emphasize the need for thorough risk assessment and mitigation strategies when implementing novel manufacturing approaches.
Regulatory bodies increasingly recognize the potential benefits of continuous manufacturing, including improved product consistency, reduced batch-to-batch variability, and enhanced process control. However, they also acknowledge the unique challenges associated with defining batch identity, establishing appropriate sampling strategies, and managing process deviations in continuous operations.
For cell retention devices specifically, regulatory focus centers on demonstrating consistent performance over extended periods, validating cleaning and sterilization procedures for reusable components, and establishing appropriate monitoring strategies to detect potential filter fouling or performance degradation. Manufacturers must provide evidence that their cell retention technologies can maintain cell viability and productivity while preventing product contamination.
Looking forward, regulatory agencies are working to harmonize approaches to continuous manufacturing across different regions. The FDA's Framework for Regulatory Advanced Manufacturing Partnerships (RAMP) and similar initiatives aim to accelerate the development and implementation of advanced manufacturing technologies, including continuous processing systems for cell culture applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!