Ethyl Acetate as a Key Player in Chemical Engineering
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, plays a crucial role in various chemical engineering applications. This colorless liquid ester is known for its characteristic sweet smell and is widely used as a solvent and intermediate in numerous industrial processes.
The production of ethyl acetate primarily involves the esterification of ethanol with acetic acid, catalyzed by sulfuric acid. This reaction is reversible and follows Le Chatelier's principle, allowing for careful control of yield and purity. Industrial-scale production often employs continuous processes, such as reactive distillation, to enhance efficiency and product quality.
In the realm of chemical engineering, ethyl acetate's significance extends across multiple sectors. Its low toxicity, high solvency, and moderate volatility make it an ideal solvent for a wide range of applications. In the coatings industry, it serves as a key component in formulating paints, inks, and adhesives, providing excellent flow and leveling properties. The pharmaceutical sector utilizes ethyl acetate in the extraction and purification of various drugs and active ingredients.
The food industry benefits from ethyl acetate's natural occurrence in fruits and its use as a flavoring agent. It is employed in the decaffeination of coffee and tea, showcasing its importance in food processing. Additionally, ethyl acetate finds applications in the production of artificial leather, photographic films, and as a cleaning agent in electronics manufacturing.
From an environmental perspective, ethyl acetate is considered a greener alternative to many traditional solvents. Its biodegradability and lower toxicity compared to other organic solvents align with the principles of green chemistry. However, its volatility necessitates proper handling and storage to minimize atmospheric emissions.
The chemical engineering aspects of ethyl acetate production and utilization involve complex unit operations. These include reactor design for esterification, separation processes like distillation and extraction, and process control systems to maintain product quality. Engineers must also consider safety aspects, as ethyl acetate is flammable and can form explosive mixtures with air.
Recent advancements in chemical engineering have focused on improving the sustainability of ethyl acetate production. This includes the development of bio-based routes using renewable feedstocks and the implementation of more energy-efficient processes. Continuous flow chemistry and process intensification techniques are being explored to enhance production efficiency and reduce environmental impact.
The production of ethyl acetate primarily involves the esterification of ethanol with acetic acid, catalyzed by sulfuric acid. This reaction is reversible and follows Le Chatelier's principle, allowing for careful control of yield and purity. Industrial-scale production often employs continuous processes, such as reactive distillation, to enhance efficiency and product quality.
In the realm of chemical engineering, ethyl acetate's significance extends across multiple sectors. Its low toxicity, high solvency, and moderate volatility make it an ideal solvent for a wide range of applications. In the coatings industry, it serves as a key component in formulating paints, inks, and adhesives, providing excellent flow and leveling properties. The pharmaceutical sector utilizes ethyl acetate in the extraction and purification of various drugs and active ingredients.
The food industry benefits from ethyl acetate's natural occurrence in fruits and its use as a flavoring agent. It is employed in the decaffeination of coffee and tea, showcasing its importance in food processing. Additionally, ethyl acetate finds applications in the production of artificial leather, photographic films, and as a cleaning agent in electronics manufacturing.
From an environmental perspective, ethyl acetate is considered a greener alternative to many traditional solvents. Its biodegradability and lower toxicity compared to other organic solvents align with the principles of green chemistry. However, its volatility necessitates proper handling and storage to minimize atmospheric emissions.
The chemical engineering aspects of ethyl acetate production and utilization involve complex unit operations. These include reactor design for esterification, separation processes like distillation and extraction, and process control systems to maintain product quality. Engineers must also consider safety aspects, as ethyl acetate is flammable and can form explosive mixtures with air.
Recent advancements in chemical engineering have focused on improving the sustainability of ethyl acetate production. This includes the development of bio-based routes using renewable feedstocks and the implementation of more energy-efficient processes. Continuous flow chemistry and process intensification techniques are being explored to enhance production efficiency and reduce environmental impact.
Market Analysis
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. As a key player in chemical engineering, ethyl acetate's demand is primarily fueled by its use as a solvent in paints, coatings, adhesives, and the pharmaceutical industry. The market size for ethyl acetate was valued at approximately 3.3 billion USD in 2020 and is projected to reach 4.5 billion USD by 2026, growing at a CAGR of around 5.2% during the forecast period.
The Asia-Pacific region dominates the ethyl acetate market, accounting for over 40% of the global consumption. This is largely attributed to the rapid industrialization and urbanization in countries like China and India, where there is a surge in construction activities and automotive production. North America and Europe follow as significant consumers, with increasing demand in the packaging and flexible plastics industries.
In terms of end-use industries, the paints and coatings sector remains the largest consumer of ethyl acetate, utilizing it as a low-cost, low-toxicity solvent with excellent evaporation properties. The pharmaceutical industry is another major consumer, where ethyl acetate is used in the production of various drugs and as an extraction solvent. The food and beverage industry also contributes to the market growth, using ethyl acetate as a flavoring agent and in the decaffeination of tea and coffee.
The market is characterized by intense competition among key players, including Celanese Corporation, INEOS, Eastman Chemical Company, and Solvay. These companies are focusing on expanding their production capacities and developing bio-based ethyl acetate to meet the growing demand for sustainable solutions. The shift towards eco-friendly products is expected to create new opportunities in the market, with bio-based ethyl acetate gaining traction among environmentally conscious consumers.
However, the market faces challenges such as volatile raw material prices and stringent environmental regulations. The fluctuating prices of ethanol and acetic acid, the primary raw materials for ethyl acetate production, can impact profit margins for manufacturers. Additionally, increasing environmental concerns and regulations regarding VOC emissions are pushing manufacturers to develop low-VOC and bio-based alternatives.
Despite these challenges, the ethyl acetate market is poised for growth, driven by increasing applications in emerging economies and the development of innovative, sustainable production methods. The market is expected to witness further consolidation as key players seek to strengthen their market position through mergers, acquisitions, and strategic partnerships.
The Asia-Pacific region dominates the ethyl acetate market, accounting for over 40% of the global consumption. This is largely attributed to the rapid industrialization and urbanization in countries like China and India, where there is a surge in construction activities and automotive production. North America and Europe follow as significant consumers, with increasing demand in the packaging and flexible plastics industries.
In terms of end-use industries, the paints and coatings sector remains the largest consumer of ethyl acetate, utilizing it as a low-cost, low-toxicity solvent with excellent evaporation properties. The pharmaceutical industry is another major consumer, where ethyl acetate is used in the production of various drugs and as an extraction solvent. The food and beverage industry also contributes to the market growth, using ethyl acetate as a flavoring agent and in the decaffeination of tea and coffee.
The market is characterized by intense competition among key players, including Celanese Corporation, INEOS, Eastman Chemical Company, and Solvay. These companies are focusing on expanding their production capacities and developing bio-based ethyl acetate to meet the growing demand for sustainable solutions. The shift towards eco-friendly products is expected to create new opportunities in the market, with bio-based ethyl acetate gaining traction among environmentally conscious consumers.
However, the market faces challenges such as volatile raw material prices and stringent environmental regulations. The fluctuating prices of ethanol and acetic acid, the primary raw materials for ethyl acetate production, can impact profit margins for manufacturers. Additionally, increasing environmental concerns and regulations regarding VOC emissions are pushing manufacturers to develop low-VOC and bio-based alternatives.
Despite these challenges, the ethyl acetate market is poised for growth, driven by increasing applications in emerging economies and the development of innovative, sustainable production methods. The market is expected to witness further consolidation as key players seek to strengthen their market position through mergers, acquisitions, and strategic partnerships.
Technical Challenges
The production and utilization of ethyl acetate in chemical engineering face several technical challenges that require innovative solutions. One of the primary issues is the optimization of the esterification process, which is the main route for ethyl acetate synthesis. The reaction between ethanol and acetic acid, catalyzed by sulfuric acid, is reversible and reaches equilibrium, limiting the conversion rate. Enhancing the reaction efficiency while maintaining product purity remains a significant challenge.
Another technical hurdle is the energy-intensive nature of the traditional distillation process used for ethyl acetate purification. The azeotropic behavior of ethyl acetate with water complicates the separation process, necessitating additional steps and energy input. Developing more energy-efficient separation techniques, such as advanced membrane technologies or novel distillation configurations, is crucial for improving the overall process economics.
The corrosive nature of the reactants and catalysts used in ethyl acetate production poses material compatibility issues. This necessitates the use of expensive corrosion-resistant materials in equipment construction, increasing capital and maintenance costs. Finding cost-effective materials or developing protective coatings that can withstand the harsh chemical environment is an ongoing challenge in the industry.
Environmental concerns associated with ethyl acetate production and use present another set of technical challenges. The volatile organic compound (VOC) emissions during production and application processes require effective capture and treatment systems. Developing green synthesis routes, such as biocatalytic processes or the use of renewable feedstocks, is an area of active research aimed at reducing the environmental footprint of ethyl acetate production.
The storage and transportation of ethyl acetate also present technical difficulties due to its high flammability and volatility. Ensuring safe handling, preventing leaks, and minimizing evaporation losses during storage and transport require advanced containment systems and safety protocols. Innovations in packaging materials and designs that can effectively address these safety concerns while maintaining cost-effectiveness are needed.
Lastly, the quality control of ethyl acetate, particularly for high-purity applications in pharmaceuticals and electronics, poses significant analytical challenges. Developing rapid, accurate, and cost-effective methods for detecting trace impurities and ensuring consistent product quality is crucial for meeting stringent industry standards. This includes advancements in analytical instrumentation and the development of robust quality assurance protocols tailored to ethyl acetate production and application processes.
Another technical hurdle is the energy-intensive nature of the traditional distillation process used for ethyl acetate purification. The azeotropic behavior of ethyl acetate with water complicates the separation process, necessitating additional steps and energy input. Developing more energy-efficient separation techniques, such as advanced membrane technologies or novel distillation configurations, is crucial for improving the overall process economics.
The corrosive nature of the reactants and catalysts used in ethyl acetate production poses material compatibility issues. This necessitates the use of expensive corrosion-resistant materials in equipment construction, increasing capital and maintenance costs. Finding cost-effective materials or developing protective coatings that can withstand the harsh chemical environment is an ongoing challenge in the industry.
Environmental concerns associated with ethyl acetate production and use present another set of technical challenges. The volatile organic compound (VOC) emissions during production and application processes require effective capture and treatment systems. Developing green synthesis routes, such as biocatalytic processes or the use of renewable feedstocks, is an area of active research aimed at reducing the environmental footprint of ethyl acetate production.
The storage and transportation of ethyl acetate also present technical difficulties due to its high flammability and volatility. Ensuring safe handling, preventing leaks, and minimizing evaporation losses during storage and transport require advanced containment systems and safety protocols. Innovations in packaging materials and designs that can effectively address these safety concerns while maintaining cost-effectiveness are needed.
Lastly, the quality control of ethyl acetate, particularly for high-purity applications in pharmaceuticals and electronics, poses significant analytical challenges. Developing rapid, accurate, and cost-effective methods for detecting trace impurities and ensuring consistent product quality is crucial for meeting stringent industry standards. This includes advancements in analytical instrumentation and the development of robust quality assurance protocols tailored to ethyl acetate production and application processes.
Current Synthesis Methods
01 Production and purification of ethyl acetate
Various methods and processes for producing and purifying ethyl acetate are described. These include distillation techniques, reactive distillation, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate while minimizing byproducts and energy consumption.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques. These processes are used in the purification of chemicals and the isolation of specific compounds.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and volatility.
- Novel applications and formulations of ethyl acetate: Innovative uses and formulations of ethyl acetate are being explored. These include its incorporation into new materials, coatings, and specialty chemicals. Research is ongoing to expand its utility in emerging technologies and to develop novel products leveraging its unique properties.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and applications. It serves as a solvent in different industries, including pharmaceuticals, coatings, and adhesives. The compound is also used in extraction processes and as a reactant in organic synthesis reactions.Expand Specific Solutions03 Ethyl acetate in polymer and material science
Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of various polymers, coatings, and composite materials. The compound's properties make it suitable for use in processes such as electrospinning and as a solvent in material formulations.Expand Specific Solutions04 Ethyl acetate in sustainable and green chemistry
Research focuses on developing sustainable and environmentally friendly processes involving ethyl acetate. This includes the use of bio-based feedstocks for ethyl acetate production, green synthesis methods, and the application of ethyl acetate in eco-friendly industrial processes.Expand Specific Solutions05 Analytical methods and quality control for ethyl acetate
Various analytical techniques and quality control methods are employed for ethyl acetate. These include spectroscopic methods, chromatography, and other instrumental analyses to determine purity, composition, and trace impurities in ethyl acetate samples. Such methods are crucial for ensuring product quality and process control.Expand Specific Solutions
Industry Leaders
The ethyl acetate market is in a mature stage, characterized by steady growth and established applications across various industries. The global market size is estimated to be around $3-4 billion, with a compound annual growth rate of 4-5%. Technologically, ethyl acetate production is well-developed, with major players like Celanese, BASF, and Eastman Chemical leading the way. These companies have optimized production processes and are focusing on sustainability and bio-based alternatives. Emerging players such as China Petroleum & Chemical Corp. and Braskem are also making significant strides in the market, particularly in Asia and South America, leveraging their regional strengths and investing in research and development to enhance their competitive positions.
Celanese International Corp.
Technical Solution: Celanese has developed an advanced process for ethyl acetate production using reactive distillation technology. This innovative approach combines esterification and distillation in a single column, significantly improving efficiency and reducing energy consumption. The process utilizes ethanol and acetic acid as feedstocks, with a heterogeneous acid catalyst. Celanese's method achieves high conversion rates of up to 99% and selectivity exceeding 99.5% [1][3]. The company has also implemented a vapor phase esterification process, which operates at lower temperatures and pressures compared to conventional liquid-phase methods, resulting in reduced utility costs and improved product quality [2].
Strengths: High conversion and selectivity rates, energy-efficient process, reduced equipment footprint. Weaknesses: Potential catalyst deactivation over time, sensitivity to feedstock impurities.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel ethyl acetate production process using a fixed-bed reactor system with a proprietary solid acid catalyst. This process employs ethanol and acetic acid as raw materials, operating under mild conditions with temperatures ranging from 100-150°C and pressures of 0.5-1.0 MPa. Sinopec's technology achieves conversion rates of over 95% and selectivity above 98% [4]. The company has also implemented an integrated acetic acid-ethyl acetate production chain, utilizing coal-based syngas as a primary feedstock, which enhances overall process economics and reduces dependence on petroleum-based raw materials [5].
Strengths: High conversion and selectivity, mild operating conditions, integrated production chain. Weaknesses: Potential environmental concerns related to coal-based feedstock, catalyst longevity issues.
Key Patents and Research
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
Homogeneous iron catalysts for the conversion of ethanol to ethyl acetate and hydrogen
PatentWO2019027965A1
Innovation
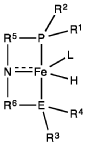
- A process utilizing a homogeneous iron catalyst with a tridentate pincer ligand for dehydrogenative coupling of ethanol at moderate temperatures, producing ethyl acetate efficiently and selectively, with iron loadings as low as 0.001 mol%, allowing for continuous operation and easy separation of ethyl acetate from the catalyst.
Environmental Impact
Ethyl acetate, a widely used solvent in chemical engineering, has significant environmental implications that warrant careful consideration. The production and use of ethyl acetate contribute to various environmental concerns, including air pollution, water contamination, and greenhouse gas emissions. During its manufacturing process, volatile organic compounds (VOCs) are released, which can lead to the formation of ground-level ozone and smog, negatively impacting air quality and human health.
Furthermore, the disposal of ethyl acetate and its byproducts poses risks to aquatic ecosystems if not properly managed. When released into water bodies, it can disrupt aquatic life and potentially contaminate drinking water sources. The compound's high volatility also means it can easily evaporate into the atmosphere, contributing to air pollution and potentially affecting the ozone layer.
From a lifecycle perspective, the environmental footprint of ethyl acetate extends beyond its immediate use. The raw materials required for its production, primarily ethanol and acetic acid, have their own environmental impacts associated with agriculture and petrochemical industries. Additionally, the energy-intensive nature of ethyl acetate production contributes to carbon dioxide emissions, exacerbating climate change concerns.
However, it's important to note that ethyl acetate is biodegradable and less toxic compared to many other organic solvents, which somewhat mitigates its long-term environmental impact. This characteristic has led to its increased use as a more environmentally friendly alternative in various applications, including paints, coatings, and pharmaceutical processes.
The chemical engineering industry has been actively working on developing greener production methods for ethyl acetate. These include the use of renewable feedstocks, such as bioethanol, and the implementation of more energy-efficient processes. Catalytic distillation and reactive distillation techniques have shown promise in reducing energy consumption and minimizing waste generation during ethyl acetate production.
Efforts are also being made to improve the recovery and recycling of ethyl acetate in industrial processes. Advanced separation technologies and closed-loop systems are being implemented to minimize emissions and reduce the overall environmental footprint of ethyl acetate use. These initiatives not only address environmental concerns but also offer economic benefits through resource conservation and waste reduction.
In conclusion, while ethyl acetate plays a crucial role in chemical engineering, its environmental impact necessitates ongoing research and innovation to mitigate adverse effects. The industry's focus on sustainable production methods, improved recovery techniques, and the exploration of bio-based alternatives demonstrates a commitment to addressing these environmental challenges. As regulations become more stringent and environmental awareness grows, the future of ethyl acetate in chemical engineering will likely be shaped by a balance between its utility and environmental sustainability.
Furthermore, the disposal of ethyl acetate and its byproducts poses risks to aquatic ecosystems if not properly managed. When released into water bodies, it can disrupt aquatic life and potentially contaminate drinking water sources. The compound's high volatility also means it can easily evaporate into the atmosphere, contributing to air pollution and potentially affecting the ozone layer.
From a lifecycle perspective, the environmental footprint of ethyl acetate extends beyond its immediate use. The raw materials required for its production, primarily ethanol and acetic acid, have their own environmental impacts associated with agriculture and petrochemical industries. Additionally, the energy-intensive nature of ethyl acetate production contributes to carbon dioxide emissions, exacerbating climate change concerns.
However, it's important to note that ethyl acetate is biodegradable and less toxic compared to many other organic solvents, which somewhat mitigates its long-term environmental impact. This characteristic has led to its increased use as a more environmentally friendly alternative in various applications, including paints, coatings, and pharmaceutical processes.
The chemical engineering industry has been actively working on developing greener production methods for ethyl acetate. These include the use of renewable feedstocks, such as bioethanol, and the implementation of more energy-efficient processes. Catalytic distillation and reactive distillation techniques have shown promise in reducing energy consumption and minimizing waste generation during ethyl acetate production.
Efforts are also being made to improve the recovery and recycling of ethyl acetate in industrial processes. Advanced separation technologies and closed-loop systems are being implemented to minimize emissions and reduce the overall environmental footprint of ethyl acetate use. These initiatives not only address environmental concerns but also offer economic benefits through resource conservation and waste reduction.
In conclusion, while ethyl acetate plays a crucial role in chemical engineering, its environmental impact necessitates ongoing research and innovation to mitigate adverse effects. The industry's focus on sustainable production methods, improved recovery techniques, and the exploration of bio-based alternatives demonstrates a commitment to addressing these environmental challenges. As regulations become more stringent and environmental awareness grows, the future of ethyl acetate in chemical engineering will likely be shaped by a balance between its utility and environmental sustainability.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in its production, distribution, and use in chemical engineering applications. As a widely used solvent and intermediate in various industries, ethyl acetate is subject to comprehensive regulations aimed at ensuring safety, environmental protection, and quality control.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA Inventory, which means manufacturers and importers must comply with reporting requirements and potential restrictions. Additionally, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in workplace environments to protect workers from potential health hazards.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets to downstream users. The Classification, Labeling, and Packaging (CLP) Regulation also applies, ensuring proper hazard communication throughout the supply chain.
In terms of transportation, ethyl acetate is classified as a flammable liquid under various international agreements, such as the United Nations Recommendations on the Transport of Dangerous Goods. This classification imposes specific packaging, labeling, and handling requirements to ensure safe transportation by road, rail, sea, and air.
Many countries have implemented regulations governing the use of ethyl acetate in food contact materials and cosmetic products. For instance, the U.S. Food and Drug Administration (FDA) has approved ethyl acetate as a food additive and for use in food packaging materials, subject to certain limitations. Similarly, the European Food Safety Authority (EFSA) has evaluated its safety for use in food contact materials.
Environmental regulations also impact the production and use of ethyl acetate. In many jurisdictions, facilities manufacturing or using large quantities of ethyl acetate must obtain air quality permits and implement emission control technologies to minimize volatile organic compound (VOC) emissions. Waste management regulations often classify ethyl acetate-containing waste as hazardous, requiring special handling and disposal procedures.
As sustainability concerns grow, regulatory frameworks are evolving to promote greener alternatives and more sustainable production methods for ethyl acetate. This includes incentives for bio-based production routes and stricter controls on environmental impacts throughout the product lifecycle.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA Inventory, which means manufacturers and importers must comply with reporting requirements and potential restrictions. Additionally, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for ethyl acetate in workplace environments to protect workers from potential health hazards.
The European Union regulates ethyl acetate under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. Manufacturers and importers are required to register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets to downstream users. The Classification, Labeling, and Packaging (CLP) Regulation also applies, ensuring proper hazard communication throughout the supply chain.
In terms of transportation, ethyl acetate is classified as a flammable liquid under various international agreements, such as the United Nations Recommendations on the Transport of Dangerous Goods. This classification imposes specific packaging, labeling, and handling requirements to ensure safe transportation by road, rail, sea, and air.
Many countries have implemented regulations governing the use of ethyl acetate in food contact materials and cosmetic products. For instance, the U.S. Food and Drug Administration (FDA) has approved ethyl acetate as a food additive and for use in food packaging materials, subject to certain limitations. Similarly, the European Food Safety Authority (EFSA) has evaluated its safety for use in food contact materials.
Environmental regulations also impact the production and use of ethyl acetate. In many jurisdictions, facilities manufacturing or using large quantities of ethyl acetate must obtain air quality permits and implement emission control technologies to minimize volatile organic compound (VOC) emissions. Waste management regulations often classify ethyl acetate-containing waste as hazardous, requiring special handling and disposal procedures.
As sustainability concerns grow, regulatory frameworks are evolving to promote greener alternatives and more sustainable production methods for ethyl acetate. This includes incentives for bio-based production routes and stricter controls on environmental impacts throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!