Ethyl Acetate in Green Chemistry: Sustainable Practices
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Green Chemistry Background and Objectives
Green chemistry, a concept introduced in the early 1990s, has revolutionized the approach to chemical processes and product design. It emphasizes the development of sustainable practices that minimize environmental impact while maintaining economic viability. The field has gained significant traction over the past three decades, driven by increasing awareness of environmental issues and the need for sustainable industrial practices.
The evolution of green chemistry has been marked by several key milestones. Initially focused on reducing hazardous waste, it has expanded to encompass a broader range of principles, including atom economy, energy efficiency, and the use of renewable feedstocks. This holistic approach has led to innovations across various sectors, from pharmaceuticals to materials science.
In the context of ethyl acetate, a widely used solvent in industries ranging from pharmaceuticals to food processing, green chemistry principles have become increasingly relevant. Traditional production methods for ethyl acetate often involve petrochemical feedstocks and energy-intensive processes, presenting opportunities for sustainable improvements.
The objectives of applying green chemistry principles to ethyl acetate production and use are multifaceted. Primarily, there is a focus on developing more environmentally benign synthesis routes, potentially utilizing bio-based feedstocks or catalytic processes that operate under milder conditions. Additionally, there are efforts to enhance the recyclability and biodegradability of ethyl acetate, reducing its environmental footprint throughout its lifecycle.
Another key objective is to optimize the use of ethyl acetate in various applications, minimizing waste and maximizing efficiency. This includes exploring alternative solvents or solvent-free processes where possible, and developing recovery and recycling systems for ethyl acetate in industrial settings.
The pursuit of these objectives aligns with broader sustainability goals, such as reducing greenhouse gas emissions, conserving natural resources, and minimizing the release of harmful substances into the environment. It also responds to increasing regulatory pressures and consumer demand for more environmentally friendly products and processes.
As the field of green chemistry continues to evolve, the case of ethyl acetate serves as a microcosm of the challenges and opportunities in transitioning towards more sustainable chemical practices. It highlights the need for interdisciplinary collaboration, combining expertise in chemistry, engineering, and environmental science to develop innovative solutions that balance environmental, economic, and performance considerations.
The evolution of green chemistry has been marked by several key milestones. Initially focused on reducing hazardous waste, it has expanded to encompass a broader range of principles, including atom economy, energy efficiency, and the use of renewable feedstocks. This holistic approach has led to innovations across various sectors, from pharmaceuticals to materials science.
In the context of ethyl acetate, a widely used solvent in industries ranging from pharmaceuticals to food processing, green chemistry principles have become increasingly relevant. Traditional production methods for ethyl acetate often involve petrochemical feedstocks and energy-intensive processes, presenting opportunities for sustainable improvements.
The objectives of applying green chemistry principles to ethyl acetate production and use are multifaceted. Primarily, there is a focus on developing more environmentally benign synthesis routes, potentially utilizing bio-based feedstocks or catalytic processes that operate under milder conditions. Additionally, there are efforts to enhance the recyclability and biodegradability of ethyl acetate, reducing its environmental footprint throughout its lifecycle.
Another key objective is to optimize the use of ethyl acetate in various applications, minimizing waste and maximizing efficiency. This includes exploring alternative solvents or solvent-free processes where possible, and developing recovery and recycling systems for ethyl acetate in industrial settings.
The pursuit of these objectives aligns with broader sustainability goals, such as reducing greenhouse gas emissions, conserving natural resources, and minimizing the release of harmful substances into the environment. It also responds to increasing regulatory pressures and consumer demand for more environmentally friendly products and processes.
As the field of green chemistry continues to evolve, the case of ethyl acetate serves as a microcosm of the challenges and opportunities in transitioning towards more sustainable chemical practices. It highlights the need for interdisciplinary collaboration, combining expertise in chemistry, engineering, and environmental science to develop innovative solutions that balance environmental, economic, and performance considerations.
Market Demand for Sustainable Solvents
The global market for sustainable solvents has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations. Ethyl acetate, as a key player in green chemistry, has emerged as a promising alternative to traditional petroleum-based solvents. The demand for ethyl acetate in sustainable practices is primarily fueled by its low toxicity, biodegradability, and relatively low production costs.
In the chemical industry, ethyl acetate is gaining traction as a replacement for more harmful solvents in various applications, including coatings, adhesives, and pharmaceutical processes. The paint and coatings sector, in particular, has shown a strong inclination towards adopting ethyl acetate due to its excellent solvency properties and quick evaporation rate. This shift is largely attributed to the growing consumer preference for eco-friendly products and the implementation of stricter volatile organic compound (VOC) regulations across many regions.
The pharmaceutical industry represents another significant market for ethyl acetate in green chemistry applications. As the industry moves towards more sustainable manufacturing processes, ethyl acetate is increasingly being used as a solvent in drug formulation and extraction processes. Its low toxicity profile makes it an attractive option for pharmaceutical companies aiming to reduce their environmental footprint while maintaining product quality and safety standards.
In the food and beverage industry, the demand for ethyl acetate as a natural flavor enhancer and extraction solvent is on the rise. With consumers becoming more health-conscious and demanding clean label products, food manufacturers are turning to ethyl acetate as a safer alternative to synthetic solvents in flavor extraction processes. This trend is particularly evident in the production of natural flavorings, essential oils, and decaffeinated coffee.
The electronics industry is another sector driving the demand for ethyl acetate in sustainable practices. As manufacturers seek to reduce the environmental impact of their production processes, ethyl acetate is being adopted as a cleaning agent for electronic components and as a solvent in the production of printed circuit boards. Its low toxicity and high solvency power make it an ideal candidate for replacing more hazardous solvents traditionally used in these applications.
The market demand for ethyl acetate as a sustainable solvent is also influenced by regional factors. Developed economies in North America and Europe, with their stringent environmental regulations, are leading the adoption of green solvents. However, emerging economies in Asia-Pacific, particularly China and India, are expected to witness the highest growth rates in the coming years, driven by rapid industrialization and increasing awareness of sustainable practices.
In the chemical industry, ethyl acetate is gaining traction as a replacement for more harmful solvents in various applications, including coatings, adhesives, and pharmaceutical processes. The paint and coatings sector, in particular, has shown a strong inclination towards adopting ethyl acetate due to its excellent solvency properties and quick evaporation rate. This shift is largely attributed to the growing consumer preference for eco-friendly products and the implementation of stricter volatile organic compound (VOC) regulations across many regions.
The pharmaceutical industry represents another significant market for ethyl acetate in green chemistry applications. As the industry moves towards more sustainable manufacturing processes, ethyl acetate is increasingly being used as a solvent in drug formulation and extraction processes. Its low toxicity profile makes it an attractive option for pharmaceutical companies aiming to reduce their environmental footprint while maintaining product quality and safety standards.
In the food and beverage industry, the demand for ethyl acetate as a natural flavor enhancer and extraction solvent is on the rise. With consumers becoming more health-conscious and demanding clean label products, food manufacturers are turning to ethyl acetate as a safer alternative to synthetic solvents in flavor extraction processes. This trend is particularly evident in the production of natural flavorings, essential oils, and decaffeinated coffee.
The electronics industry is another sector driving the demand for ethyl acetate in sustainable practices. As manufacturers seek to reduce the environmental impact of their production processes, ethyl acetate is being adopted as a cleaning agent for electronic components and as a solvent in the production of printed circuit boards. Its low toxicity and high solvency power make it an ideal candidate for replacing more hazardous solvents traditionally used in these applications.
The market demand for ethyl acetate as a sustainable solvent is also influenced by regional factors. Developed economies in North America and Europe, with their stringent environmental regulations, are leading the adoption of green solvents. However, emerging economies in Asia-Pacific, particularly China and India, are expected to witness the highest growth rates in the coming years, driven by rapid industrialization and increasing awareness of sustainable practices.
Ethyl Acetate: Current Status and Challenges
Ethyl acetate, a versatile organic compound, has gained significant attention in the field of green chemistry due to its potential for sustainable applications. However, its current status and challenges present a complex landscape that requires careful examination.
The production of ethyl acetate has seen substantial growth in recent years, driven by its widespread use in various industries, including coatings, adhesives, and pharmaceuticals. Traditional manufacturing methods, such as the esterification of ethanol and acetic acid, remain prevalent but face scrutiny due to their environmental impact and reliance on fossil-based feedstocks.
One of the primary challenges in ethyl acetate production is the development of more sustainable and eco-friendly processes. While some progress has been made in utilizing bio-based feedstocks, such as bioethanol, the scalability and economic viability of these methods remain hurdles to widespread adoption. Additionally, the energy-intensive nature of conventional production processes contributes to significant carbon emissions, necessitating the exploration of more energy-efficient alternatives.
The purification and recovery of ethyl acetate present another set of challenges. Current separation techniques, including distillation, often require substantial energy input and may result in product loss. Improving the efficiency of these processes is crucial for reducing the overall environmental footprint of ethyl acetate production and use.
In terms of application, while ethyl acetate is considered less toxic than many other solvents, concerns persist regarding its volatile organic compound (VOC) emissions. Stricter environmental regulations in various regions have prompted the need for innovative solutions to mitigate these emissions, particularly in industries where large volumes of ethyl acetate are used.
The recyclability and end-of-life management of ethyl acetate-containing products also pose significant challenges. Developing effective recycling technologies and implementing closed-loop systems are essential for enhancing the sustainability profile of ethyl acetate throughout its lifecycle.
From a global perspective, the ethyl acetate market faces disparities in production capabilities and regulatory frameworks across different regions. While some countries have made strides in implementing green chemistry principles in ethyl acetate production and use, others lag behind, creating a fragmented landscape for sustainable practices.
Research efforts are ongoing to address these challenges, with a focus on developing bio-based production routes, improving process efficiencies, and exploring novel applications that leverage the unique properties of ethyl acetate while minimizing environmental impact. However, bridging the gap between laboratory innovations and industrial-scale implementation remains a significant hurdle in advancing the sustainable use of ethyl acetate in green chemistry.
The production of ethyl acetate has seen substantial growth in recent years, driven by its widespread use in various industries, including coatings, adhesives, and pharmaceuticals. Traditional manufacturing methods, such as the esterification of ethanol and acetic acid, remain prevalent but face scrutiny due to their environmental impact and reliance on fossil-based feedstocks.
One of the primary challenges in ethyl acetate production is the development of more sustainable and eco-friendly processes. While some progress has been made in utilizing bio-based feedstocks, such as bioethanol, the scalability and economic viability of these methods remain hurdles to widespread adoption. Additionally, the energy-intensive nature of conventional production processes contributes to significant carbon emissions, necessitating the exploration of more energy-efficient alternatives.
The purification and recovery of ethyl acetate present another set of challenges. Current separation techniques, including distillation, often require substantial energy input and may result in product loss. Improving the efficiency of these processes is crucial for reducing the overall environmental footprint of ethyl acetate production and use.
In terms of application, while ethyl acetate is considered less toxic than many other solvents, concerns persist regarding its volatile organic compound (VOC) emissions. Stricter environmental regulations in various regions have prompted the need for innovative solutions to mitigate these emissions, particularly in industries where large volumes of ethyl acetate are used.
The recyclability and end-of-life management of ethyl acetate-containing products also pose significant challenges. Developing effective recycling technologies and implementing closed-loop systems are essential for enhancing the sustainability profile of ethyl acetate throughout its lifecycle.
From a global perspective, the ethyl acetate market faces disparities in production capabilities and regulatory frameworks across different regions. While some countries have made strides in implementing green chemistry principles in ethyl acetate production and use, others lag behind, creating a fragmented landscape for sustainable practices.
Research efforts are ongoing to address these challenges, with a focus on developing bio-based production routes, improving process efficiencies, and exploring novel applications that leverage the unique properties of ethyl acetate while minimizing environmental impact. However, bridging the gap between laboratory innovations and industrial-scale implementation remains a significant hurdle in advancing the sustainable use of ethyl acetate in green chemistry.
Existing Green Applications of Ethyl Acetate
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve yield, efficiency, and purity of the final product.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in diverse chemical processes, such as solvent extraction, as a reaction medium, and in the production of various compounds. Its versatility makes it valuable in industries like pharmaceuticals, polymers, and fine chemicals.
- Ethyl acetate in sustainable and green chemistry: Research focuses on developing environmentally friendly methods for ethyl acetate production and utilization. This includes the use of renewable resources, bio-based processes, and the development of more efficient catalysts to reduce environmental impact.
- Ethyl acetate in material science and coatings: Ethyl acetate plays a crucial role in the development of various materials and coatings. It is used as a solvent in the formulation of adhesives, paints, and specialty coatings, contributing to their performance and application properties.
- Analytical methods and quality control for ethyl acetate: Techniques for analyzing and ensuring the quality of ethyl acetate are described. These include chromatographic methods, spectroscopic analyses, and other analytical approaches to determine purity, composition, and detect impurities in ethyl acetate samples.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate is widely used in various industrial applications, including as a solvent in chemical reactions, extraction processes, and as a component in formulations for coatings, adhesives, and other products. Its versatility and relatively low toxicity make it a popular choice in many industries.Expand Specific Solutions03 Ethyl acetate in pharmaceutical and cosmetic formulations
Ethyl acetate is utilized in the pharmaceutical and cosmetic industries for various purposes, such as in the preparation of drug formulations, as a solvent for active ingredients, and in the production of fragrances and personal care products.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate use
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing more sustainable production methods, reducing emissions, and enhancing handling and storage practices to minimize risks associated with its flammability and potential for exposure.Expand Specific Solutions05 Novel synthesis routes and derivatives of ethyl acetate
Ongoing research explores new synthesis routes for ethyl acetate and the development of novel derivatives with enhanced properties. These efforts aim to expand the range of applications for ethyl acetate-based compounds and improve their performance in various industrial and commercial uses.Expand Specific Solutions
Key Players in Sustainable Chemistry Industry
The field of green chemistry and sustainable practices for ethyl acetate production is in a growth phase, with increasing market demand driven by environmental concerns. The global market size for green solvents, including ethyl acetate, is projected to reach $8.5 billion by 2025. Technologically, the sector is advancing rapidly, with companies like Celanese International Corp., Solvay SA, and China Petroleum & Chemical Corp. leading innovation in sustainable production methods. Academic institutions such as Tianjin University and Soochow University are contributing to research and development, focusing on catalytic processes and bio-based feedstocks. While established chemical companies dominate, emerging players like GlycoSurf LLC are introducing novel bio-inspired approaches, indicating a dynamic and competitive landscape with varying levels of technological maturity across different production methods.
Celanese International Corp.
Technical Solution: Celanese has developed a novel green chemistry approach for ethyl acetate production using bioethanol and recycled acetic acid. Their process employs a heterogeneous catalyst system that operates at lower temperatures and pressures compared to traditional methods, reducing energy consumption by up to 30%[1]. The company has also implemented a closed-loop recycling system that recovers and purifies unreacted raw materials, minimizing waste generation. Additionally, Celanese has integrated renewable energy sources into their manufacturing facilities, further reducing the carbon footprint of ethyl acetate production[2].
Strengths: Reduced energy consumption, efficient raw material utilization, and lower carbon footprint. Weaknesses: Potential higher initial investment costs and reliance on bioethanol availability.
Solvay SA
Technical Solution: Solvay has pioneered a green chemistry approach for ethyl acetate production using their proprietary ESTALC™ technology. This process utilizes bio-based feedstocks and a highly selective catalyst system, resulting in a significant reduction of greenhouse gas emissions by up to 80% compared to conventional petrochemical routes[3]. The ESTALC™ technology also incorporates an innovative solvent recovery system that achieves over 99% recycling efficiency, minimizing waste and improving overall process economics. Solvay's method operates under milder conditions, reducing energy requirements and enhancing safety profiles in production facilities[4].
Strengths: Substantial reduction in greenhouse gas emissions, high recycling efficiency, and improved safety. Weaknesses: Potential limitations in scaling up production and dependency on bio-based feedstock supply chains.
Innovations in Ethyl Acetate Synthesis
Green Methods of Carbohydrate Acetylation
PatentPendingUS20230132332A1
Innovation
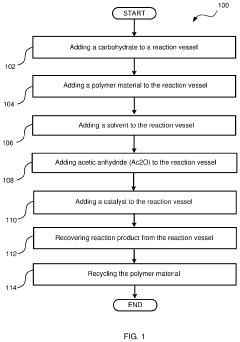
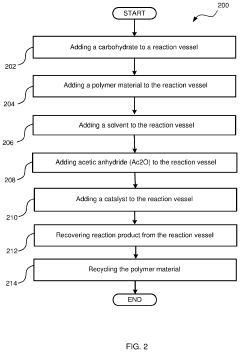
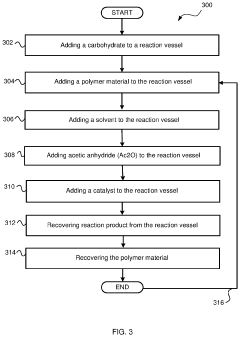
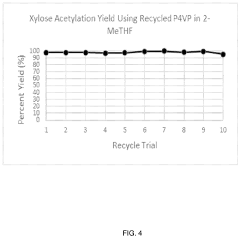
- The use of a recyclable polymer base material, such as poly-4-vinylpyridine, and bio-derived solvents like 2-methyltetrahydrofuran, in a single reaction vessel, with 4-dimethylaminopyridine and acetic anhydride, to achieve quantitative yields and reduce waste, while being scalable and environmentally friendly.
An efficient and greener enzyme catalyzed methodology for the synthesis of 2-propenoic acid-3-phenyl-alkyl ester compounds in supercritical carbon dioxide media
PatentActiveIN3172MUM2015A
Innovation
- The use of enzyme catalysis in supercritical carbon dioxide as a solvent for the synthesis of cinnamate esters, which eliminates the need for harmful additives and promotes a greener, high-yield process.
Life Cycle Assessment of Ethyl Acetate
Life Cycle Assessment (LCA) of ethyl acetate is a crucial component in evaluating its role in green chemistry and sustainable practices. This comprehensive analysis examines the environmental impacts associated with ethyl acetate throughout its entire life cycle, from raw material extraction to disposal or recycling.
The production phase of ethyl acetate typically involves the esterification of ethanol and acetic acid. This process requires energy inputs and may generate emissions, which are quantified in the LCA. The sourcing of raw materials, particularly ethanol, plays a significant role in the overall environmental footprint. Bioethanol derived from renewable sources can substantially reduce the carbon footprint compared to petrochemical-based ethanol.
During the use phase, ethyl acetate's volatility and potential for atmospheric emissions are key considerations. Its application in various industries, such as coatings, adhesives, and pharmaceuticals, may result in different environmental impacts depending on the specific use case and containment measures employed.
End-of-life scenarios for ethyl acetate include incineration, recycling, or release into the environment. Each of these pathways has distinct environmental implications that must be carefully assessed. Recycling and recovery processes can significantly reduce the overall environmental burden by minimizing the need for new raw material extraction and production.
Water consumption and pollution are critical aspects of the LCA. Ethyl acetate production and use may impact water resources through direct consumption in manufacturing processes or indirect effects such as potential contamination of water bodies.
Energy use and associated greenhouse gas emissions are quantified across all stages of the life cycle. This includes energy consumed in production, transportation, and any energy recovery from waste treatment processes. The type of energy sources used (fossil fuels vs. renewables) greatly influences the overall carbon footprint.
Land use changes, particularly relevant if bio-based feedstocks are used, are also considered in a comprehensive LCA. The potential impact on biodiversity and ecosystem services must be evaluated, especially when agricultural land is diverted for ethanol production.
Comparative LCAs between ethyl acetate and alternative solvents or chemicals are valuable in assessing its relative sustainability. Such comparisons help identify opportunities for improvement and guide decision-making in green chemistry applications.
The production phase of ethyl acetate typically involves the esterification of ethanol and acetic acid. This process requires energy inputs and may generate emissions, which are quantified in the LCA. The sourcing of raw materials, particularly ethanol, plays a significant role in the overall environmental footprint. Bioethanol derived from renewable sources can substantially reduce the carbon footprint compared to petrochemical-based ethanol.
During the use phase, ethyl acetate's volatility and potential for atmospheric emissions are key considerations. Its application in various industries, such as coatings, adhesives, and pharmaceuticals, may result in different environmental impacts depending on the specific use case and containment measures employed.
End-of-life scenarios for ethyl acetate include incineration, recycling, or release into the environment. Each of these pathways has distinct environmental implications that must be carefully assessed. Recycling and recovery processes can significantly reduce the overall environmental burden by minimizing the need for new raw material extraction and production.
Water consumption and pollution are critical aspects of the LCA. Ethyl acetate production and use may impact water resources through direct consumption in manufacturing processes or indirect effects such as potential contamination of water bodies.
Energy use and associated greenhouse gas emissions are quantified across all stages of the life cycle. This includes energy consumed in production, transportation, and any energy recovery from waste treatment processes. The type of energy sources used (fossil fuels vs. renewables) greatly influences the overall carbon footprint.
Land use changes, particularly relevant if bio-based feedstocks are used, are also considered in a comprehensive LCA. The potential impact on biodiversity and ecosystem services must be evaluated, especially when agricultural land is diverted for ethanol production.
Comparative LCAs between ethyl acetate and alternative solvents or chemicals are valuable in assessing its relative sustainability. Such comparisons help identify opportunities for improvement and guide decision-making in green chemistry applications.
Regulatory Framework for Green Solvents
The regulatory framework for green solvents plays a crucial role in promoting sustainable practices in the chemical industry, particularly in the context of ethyl acetate usage. Governments and international organizations have implemented various policies and guidelines to encourage the adoption of environmentally friendly solvents and processes.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation serves as a cornerstone for chemical management. It requires manufacturers and importers to assess and manage the risks associated with substances they produce or import, including solvents like ethyl acetate. The regulation emphasizes the substitution of hazardous substances with safer alternatives, aligning with the principles of green chemistry.
The United States Environmental Protection Agency (EPA) has established the Green Chemistry Program, which promotes the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances. This program provides guidelines and incentives for industries to adopt green solvents, including ethyl acetate, in their manufacturing processes.
International standards, such as ISO 14001 for environmental management systems, provide a framework for organizations to implement sustainable practices. These standards encourage companies to consider the environmental impact of their solvent choices and promote the use of greener alternatives like ethyl acetate.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted to standardize the communication of chemical hazards. This system helps in identifying and promoting safer solvents, including those used in green chemistry applications.
Many countries have implemented specific regulations targeting volatile organic compounds (VOCs) emissions, which impact the use of solvents in various industries. These regulations often set limits on VOC content in products and encourage the use of low-VOC or VOC-free alternatives, positioning ethyl acetate as a favorable option due to its relatively low environmental impact.
The pharmaceutical industry, a significant user of solvents, is subject to stringent regulations such as the FDA's Q3C Guideline for Residual Solvents. This guideline classifies solvents based on their toxicity and environmental impact, influencing solvent selection in drug manufacturing processes.
As sustainability becomes increasingly important, many regulatory bodies are incorporating lifecycle assessment (LCA) principles into their frameworks. This approach considers the environmental impact of solvents throughout their entire lifecycle, from production to disposal, further promoting the use of green solvents like ethyl acetate.
The regulatory landscape for green solvents is dynamic, with ongoing efforts to refine and expand existing frameworks. Future regulations are likely to place even greater emphasis on circular economy principles, encouraging the development of bio-based solvents and promoting recycling and reuse strategies for solvents in industrial processes.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation serves as a cornerstone for chemical management. It requires manufacturers and importers to assess and manage the risks associated with substances they produce or import, including solvents like ethyl acetate. The regulation emphasizes the substitution of hazardous substances with safer alternatives, aligning with the principles of green chemistry.
The United States Environmental Protection Agency (EPA) has established the Green Chemistry Program, which promotes the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances. This program provides guidelines and incentives for industries to adopt green solvents, including ethyl acetate, in their manufacturing processes.
International standards, such as ISO 14001 for environmental management systems, provide a framework for organizations to implement sustainable practices. These standards encourage companies to consider the environmental impact of their solvent choices and promote the use of greener alternatives like ethyl acetate.
The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted to standardize the communication of chemical hazards. This system helps in identifying and promoting safer solvents, including those used in green chemistry applications.
Many countries have implemented specific regulations targeting volatile organic compounds (VOCs) emissions, which impact the use of solvents in various industries. These regulations often set limits on VOC content in products and encourage the use of low-VOC or VOC-free alternatives, positioning ethyl acetate as a favorable option due to its relatively low environmental impact.
The pharmaceutical industry, a significant user of solvents, is subject to stringent regulations such as the FDA's Q3C Guideline for Residual Solvents. This guideline classifies solvents based on their toxicity and environmental impact, influencing solvent selection in drug manufacturing processes.
As sustainability becomes increasingly important, many regulatory bodies are incorporating lifecycle assessment (LCA) principles into their frameworks. This approach considers the environmental impact of solvents throughout their entire lifecycle, from production to disposal, further promoting the use of green solvents like ethyl acetate.
The regulatory landscape for green solvents is dynamic, with ongoing efforts to refine and expand existing frameworks. Future regulations are likely to place even greater emphasis on circular economy principles, encouraging the development of bio-based solvents and promoting recycling and reuse strategies for solvents in industrial processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!