Ethyl Acetate’s Potential in Nanotechnology Applications
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate in Nanotech: Background and Objectives
Ethyl acetate, a versatile organic compound, has emerged as a promising candidate in the rapidly evolving field of nanotechnology. This colorless liquid, commonly used as a solvent in various industries, is now being explored for its potential applications at the nanoscale. The journey of ethyl acetate in nanotechnology began with the recognition of its unique properties, including low toxicity, high volatility, and excellent solvency for a wide range of materials.
The evolution of nanotechnology has been marked by continuous efforts to manipulate matter at the atomic and molecular scale. In this context, ethyl acetate's role has gradually shifted from a mere solvent to a potential enabler of novel nanostructures and processes. Its ability to dissolve both polar and non-polar substances makes it particularly interesting for the synthesis and modification of nanomaterials.
One of the key drivers behind the exploration of ethyl acetate in nanotechnology is the growing demand for sustainable and environmentally friendly processes. As a bio-based solvent, ethyl acetate aligns well with the principles of green chemistry, offering a potentially less harmful alternative to traditional petroleum-derived solvents in nanomaterial production.
The primary objective of investigating ethyl acetate's potential in nanotechnology applications is to develop innovative methods for synthesizing, processing, and functionalizing nanomaterials. Researchers aim to leverage its unique properties to create more efficient and sustainable nanofabrication techniques, potentially revolutionizing areas such as drug delivery, energy storage, and advanced materials.
Another significant goal is to explore ethyl acetate's role in the self-assembly of nanostructures. Its controlled evaporation characteristics and interaction with various materials could lead to the development of novel bottom-up fabrication methods for complex nanoarchitectures. This approach holds promise for creating highly ordered and functional nanostructures with applications in electronics, photonics, and sensing technologies.
Furthermore, the research community is focusing on understanding the fundamental interactions between ethyl acetate and nanomaterials. This knowledge is crucial for optimizing existing processes and discovering new applications. By elucidating these mechanisms, scientists hope to unlock new possibilities in areas such as nanoparticle surface modification, nanocomposite formation, and the development of smart materials responsive to environmental stimuli.
As the field progresses, there is a growing emphasis on integrating ethyl acetate-based nanotechnology solutions into practical applications. This includes exploring its potential in enhancing the performance of nanoscale electronic devices, improving the efficiency of energy conversion and storage systems, and developing advanced coatings with unique properties.
The evolution of nanotechnology has been marked by continuous efforts to manipulate matter at the atomic and molecular scale. In this context, ethyl acetate's role has gradually shifted from a mere solvent to a potential enabler of novel nanostructures and processes. Its ability to dissolve both polar and non-polar substances makes it particularly interesting for the synthesis and modification of nanomaterials.
One of the key drivers behind the exploration of ethyl acetate in nanotechnology is the growing demand for sustainable and environmentally friendly processes. As a bio-based solvent, ethyl acetate aligns well with the principles of green chemistry, offering a potentially less harmful alternative to traditional petroleum-derived solvents in nanomaterial production.
The primary objective of investigating ethyl acetate's potential in nanotechnology applications is to develop innovative methods for synthesizing, processing, and functionalizing nanomaterials. Researchers aim to leverage its unique properties to create more efficient and sustainable nanofabrication techniques, potentially revolutionizing areas such as drug delivery, energy storage, and advanced materials.
Another significant goal is to explore ethyl acetate's role in the self-assembly of nanostructures. Its controlled evaporation characteristics and interaction with various materials could lead to the development of novel bottom-up fabrication methods for complex nanoarchitectures. This approach holds promise for creating highly ordered and functional nanostructures with applications in electronics, photonics, and sensing technologies.
Furthermore, the research community is focusing on understanding the fundamental interactions between ethyl acetate and nanomaterials. This knowledge is crucial for optimizing existing processes and discovering new applications. By elucidating these mechanisms, scientists hope to unlock new possibilities in areas such as nanoparticle surface modification, nanocomposite formation, and the development of smart materials responsive to environmental stimuli.
As the field progresses, there is a growing emphasis on integrating ethyl acetate-based nanotechnology solutions into practical applications. This includes exploring its potential in enhancing the performance of nanoscale electronic devices, improving the efficiency of energy conversion and storage systems, and developing advanced coatings with unique properties.
Market Analysis for Ethyl Acetate-Based Nanomaterials
The market for ethyl acetate-based nanomaterials is experiencing significant growth, driven by the increasing demand for advanced materials in various industries. The global nanomaterials market is projected to reach $14.2 billion by 2025, with ethyl acetate-based nanomaterials playing a crucial role in this expansion. These materials offer unique properties such as enhanced mechanical strength, improved thermal conductivity, and superior chemical resistance, making them attractive for a wide range of applications.
In the electronics industry, ethyl acetate-based nanomaterials are gaining traction for use in flexible displays, conductive coatings, and energy storage devices. The miniaturization trend in consumer electronics is fueling the demand for these materials, as they enable the development of smaller, more efficient components. The automotive sector is another key market, with ethyl acetate-based nanomaterials being utilized in lightweight composites, anti-corrosion coatings, and advanced sensors for autonomous vehicles.
The healthcare and pharmaceutical industries are also driving market growth, with ethyl acetate-based nanomaterials finding applications in drug delivery systems, medical imaging, and biosensors. The ability of these materials to enhance drug efficacy and reduce side effects is particularly valuable in targeted cancer therapies and personalized medicine.
Environmental concerns and sustainability initiatives are creating new opportunities for ethyl acetate-based nanomaterials in the energy sector. These materials are being explored for use in solar cells, fuel cells, and energy-efficient coatings, contributing to the development of cleaner energy technologies.
Geographically, North America and Europe are currently the largest markets for ethyl acetate-based nanomaterials, owing to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing R&D investments, and government support for nanotechnology development.
Despite the promising outlook, challenges such as high production costs, scalability issues, and regulatory uncertainties remain. Addressing these challenges will be crucial for realizing the full market potential of ethyl acetate-based nanomaterials. As research and development efforts intensify, and manufacturing processes become more efficient, the market is expected to expand further, opening up new applications and opportunities across various industries.
In the electronics industry, ethyl acetate-based nanomaterials are gaining traction for use in flexible displays, conductive coatings, and energy storage devices. The miniaturization trend in consumer electronics is fueling the demand for these materials, as they enable the development of smaller, more efficient components. The automotive sector is another key market, with ethyl acetate-based nanomaterials being utilized in lightweight composites, anti-corrosion coatings, and advanced sensors for autonomous vehicles.
The healthcare and pharmaceutical industries are also driving market growth, with ethyl acetate-based nanomaterials finding applications in drug delivery systems, medical imaging, and biosensors. The ability of these materials to enhance drug efficacy and reduce side effects is particularly valuable in targeted cancer therapies and personalized medicine.
Environmental concerns and sustainability initiatives are creating new opportunities for ethyl acetate-based nanomaterials in the energy sector. These materials are being explored for use in solar cells, fuel cells, and energy-efficient coatings, contributing to the development of cleaner energy technologies.
Geographically, North America and Europe are currently the largest markets for ethyl acetate-based nanomaterials, owing to their advanced research infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the fastest growth, driven by rapid industrialization, increasing R&D investments, and government support for nanotechnology development.
Despite the promising outlook, challenges such as high production costs, scalability issues, and regulatory uncertainties remain. Addressing these challenges will be crucial for realizing the full market potential of ethyl acetate-based nanomaterials. As research and development efforts intensify, and manufacturing processes become more efficient, the market is expected to expand further, opening up new applications and opportunities across various industries.
Current Challenges in Ethyl Acetate Nanotech Applications
Despite the promising potential of ethyl acetate in nanotechnology applications, several significant challenges currently hinder its widespread adoption and optimal utilization. One of the primary obstacles is the difficulty in controlling the size and uniformity of nanoparticles produced using ethyl acetate as a solvent or reagent. The inherent volatility of ethyl acetate can lead to rapid evaporation during synthesis processes, resulting in inconsistent particle formation and aggregation.
Another challenge lies in the stability of ethyl acetate-based nanoformulations. The relatively low boiling point of ethyl acetate can cause issues with long-term storage and transportation of nanoparticle suspensions, potentially affecting their shelf life and performance in various applications. This instability may limit the practical use of ethyl acetate in certain nanotech products that require extended periods of storage or exposure to varying environmental conditions.
The scalability of ethyl acetate-based nanotech processes presents another hurdle. While laboratory-scale synthesis may yield promising results, translating these methods to industrial-scale production often encounters difficulties in maintaining consistent quality and properties of the nanomaterials. This scaling challenge is particularly evident in applications such as drug delivery systems and advanced materials manufacturing.
Environmental and safety concerns also pose challenges to the widespread use of ethyl acetate in nanotechnology. Although ethyl acetate is generally considered less toxic compared to many other organic solvents, its flammability and potential for forming explosive mixtures with air require stringent safety measures in handling and processing. Additionally, the environmental impact of large-scale ethyl acetate use in nanotech applications needs further assessment, particularly regarding waste management and potential atmospheric emissions.
The limited compatibility of ethyl acetate with certain materials and substrates used in nanotechnology applications presents another challenge. This incompatibility can restrict the range of potential applications and may necessitate the development of specialized processing techniques or protective measures to prevent undesired interactions between ethyl acetate and sensitive components.
Regulatory hurdles and standardization issues also contribute to the challenges faced in ethyl acetate nanotech applications. The rapidly evolving nature of nanotechnology, coupled with the unique properties of ethyl acetate-based nanomaterials, creates complexities in establishing comprehensive regulatory frameworks and industry standards. This regulatory uncertainty can impede investment and commercialization efforts in the field.
Addressing these challenges requires concerted efforts in research and development, focusing on enhancing the stability and control of ethyl acetate-based nanoformulations, improving scalability of production processes, and developing innovative solutions for environmental and safety concerns. Collaborative initiatives between academia, industry, and regulatory bodies are essential to overcome these obstacles and fully realize the potential of ethyl acetate in advancing nanotechnology applications.
Another challenge lies in the stability of ethyl acetate-based nanoformulations. The relatively low boiling point of ethyl acetate can cause issues with long-term storage and transportation of nanoparticle suspensions, potentially affecting their shelf life and performance in various applications. This instability may limit the practical use of ethyl acetate in certain nanotech products that require extended periods of storage or exposure to varying environmental conditions.
The scalability of ethyl acetate-based nanotech processes presents another hurdle. While laboratory-scale synthesis may yield promising results, translating these methods to industrial-scale production often encounters difficulties in maintaining consistent quality and properties of the nanomaterials. This scaling challenge is particularly evident in applications such as drug delivery systems and advanced materials manufacturing.
Environmental and safety concerns also pose challenges to the widespread use of ethyl acetate in nanotechnology. Although ethyl acetate is generally considered less toxic compared to many other organic solvents, its flammability and potential for forming explosive mixtures with air require stringent safety measures in handling and processing. Additionally, the environmental impact of large-scale ethyl acetate use in nanotech applications needs further assessment, particularly regarding waste management and potential atmospheric emissions.
The limited compatibility of ethyl acetate with certain materials and substrates used in nanotechnology applications presents another challenge. This incompatibility can restrict the range of potential applications and may necessitate the development of specialized processing techniques or protective measures to prevent undesired interactions between ethyl acetate and sensitive components.
Regulatory hurdles and standardization issues also contribute to the challenges faced in ethyl acetate nanotech applications. The rapidly evolving nature of nanotechnology, coupled with the unique properties of ethyl acetate-based nanomaterials, creates complexities in establishing comprehensive regulatory frameworks and industry standards. This regulatory uncertainty can impede investment and commercialization efforts in the field.
Addressing these challenges requires concerted efforts in research and development, focusing on enhancing the stability and control of ethyl acetate-based nanoformulations, improving scalability of production processes, and developing innovative solutions for environmental and safety concerns. Collaborative initiatives between academia, industry, and regulatory bodies are essential to overcome these obstacles and fully realize the potential of ethyl acetate in advancing nanotechnology applications.
Existing Ethyl Acetate Nanotech Solutions
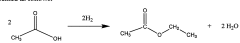
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the reaction conditions and separation processes to obtain high-quality ethyl acetate efficiently.- Production and purification of ethyl acetate: Various methods and processes for producing and purifying ethyl acetate are described. These include distillation techniques, reactive distillation, and the use of specific catalysts to improve yield and purity. The processes aim to enhance efficiency and reduce energy consumption in ethyl acetate production.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also utilized in extraction processes and as a reaction medium in chemical synthesis.
- Ethyl acetate in polymer and material science: Ethyl acetate plays a role in polymer and material science applications. It is used in the preparation of various polymers, as a solvent for resins, and in the development of composite materials. The compound's properties make it suitable for use in coating formulations and adhesive technologies.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on addressing environmental and safety concerns related to ethyl acetate use. This includes developing eco-friendly production methods, improving handling and storage practices, and exploring alternatives to reduce environmental impact and enhance worker safety.
- Novel synthesis routes and derivatives of ethyl acetate: Innovative approaches to synthesizing ethyl acetate and its derivatives are explored. These include the development of new catalysts, alternative feedstocks, and novel reaction pathways. Research also focuses on creating value-added products and functionalized derivatives of ethyl acetate for specialized applications.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also employed in extraction processes, as a reaction medium, and in the production of other chemicals. Its versatility makes it a valuable component in many manufacturing processes.Expand Specific Solutions03 Ethyl acetate in green chemistry and sustainable processes
Research focuses on developing environmentally friendly methods for ethyl acetate production and utilization. This includes the use of renewable resources, bio-based feedstocks, and sustainable catalysts. Efforts are made to reduce energy consumption and waste generation in ethyl acetate-related processes, aligning with green chemistry principles.Expand Specific Solutions04 Recovery and recycling of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes are developed to improve efficiency and reduce environmental impact. These include adsorption techniques, membrane separation, and advanced distillation processes. The aim is to minimize waste and maximize the reuse of ethyl acetate in various applications.Expand Specific Solutions05 Ethyl acetate derivatives and related compounds
Research extends to the synthesis and applications of ethyl acetate derivatives and related compounds. This includes the development of novel esters, modified versions of ethyl acetate, and compounds with similar properties. These derivatives are explored for their potential use in specialized applications across various industries.Expand Specific Solutions
Key Players in Ethyl Acetate Nanotechnology Research
The ethyl acetate nanotechnology market is in its early growth stage, characterized by increasing research and development activities. The market size is expanding as more applications are discovered, particularly in electronics, coatings, and drug delivery. While the technology is still evolving, several key players are driving innovation. Companies like Celanese International Corp., SABIC Global Technologies BV, and Eastman Chemical Co. are leveraging their expertise in chemical manufacturing to explore ethyl acetate's potential in nanotech applications. Academic institutions such as Nanjing University and the University of California are contributing to fundamental research. The involvement of major petrochemical firms like China Petroleum & Chemical Corp. indicates growing industrial interest, suggesting the technology is progressing towards commercial viability.
Celanese International Corp.
Technical Solution: Celanese has developed a novel approach for using ethyl acetate in nanotechnology applications, particularly in the production of high-performance nanocomposites. Their method involves using ethyl acetate as a solvent and dispersing agent for nanoparticles, enabling better integration into polymer matrices. This process results in nanocomposites with enhanced mechanical properties and improved barrier characteristics[1]. The company has also explored the use of ethyl acetate in the synthesis of carbon nanotubes, where it serves as both a carbon source and a reaction medium, leading to more controlled growth and higher purity nanotubes[2].
Strengths: Improved nanoparticle dispersion, enhanced nanocomposite properties, versatile application in carbon nanotube synthesis. Weaknesses: Potential volatility issues, may require specialized handling equipment.
SABIC Global Technologies BV
Technical Solution: SABIC has pioneered the use of ethyl acetate in nanotechnology for advanced polymer processing. Their innovative approach involves using ethyl acetate as a green solvent for the preparation of polymer nanocomposites. The company has developed a proprietary process that utilizes ethyl acetate's unique properties to create uniform dispersions of nanoparticles in polymer matrices, resulting in materials with significantly improved mechanical and thermal properties[3]. Additionally, SABIC has explored the use of ethyl acetate in the production of nanostructured coatings, where it acts as a carrier for nanoparticles, enabling the creation of thin, uniform films with enhanced durability and functionality[4].
Strengths: Green solvent approach, improved nanoparticle dispersion, versatile application in coatings. Weaknesses: May require process modifications for existing manufacturing lines.
Breakthrough Ethyl Acetate Nanotech Patents and Literature
Processes for making ethyl acetate from acetic acid
PatentWO2011053366A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with metals like tin or rhenium, which operate at specific temperature and pressure conditions to achieve high selectivity for ethyl acetate while minimizing by-product formation.
Applicator liquid containing ethyl lactate for preparation of nanotube films
PatentInactiveEP1754233A2
Innovation
- A nanotube composition in ethyl lactate with reduced metal and particulate impurities, homogeneously dispersed without precipitation, is developed for use in electronics fabrication, utilizing pretreatment and sonication to achieve stable dispersions compatible with semiconductor equipment.
Environmental Impact of Ethyl Acetate in Nanotechnology
The environmental impact of ethyl acetate in nanotechnology applications is a critical consideration as this solvent gains prominence in various nanomaterial synthesis and processing techniques. Ethyl acetate, known for its relatively low toxicity and high volatility, presents both advantages and challenges from an environmental perspective.
One of the primary environmental benefits of using ethyl acetate in nanotechnology is its potential to replace more harmful solvents. Compared to many traditional organic solvents, ethyl acetate has a lower environmental persistence and reduced toxicity to aquatic life. This makes it a more environmentally friendly option for large-scale nanomaterial production processes, potentially reducing the overall ecological footprint of nanotechnology manufacturing.
However, the increased use of ethyl acetate in nanotechnology applications also raises concerns about its atmospheric emissions. As a volatile organic compound (VOC), ethyl acetate can contribute to the formation of ground-level ozone and photochemical smog when released into the air. This necessitates proper handling and emission control measures in industrial settings to mitigate potential air quality impacts.
The biodegradability of ethyl acetate is another important environmental factor. While it degrades more readily than many other solvents, its rapid breakdown in the environment can lead to temporary increases in biochemical oxygen demand (BOD) in aquatic ecosystems if released in large quantities. This underscores the importance of proper waste management and treatment protocols in nanotechnology facilities using ethyl acetate.
In the context of nanomaterial production, the environmental impact of ethyl acetate is closely tied to the specific processes and materials involved. For instance, in the synthesis of carbon nanotubes or graphene, ethyl acetate may serve as a less hazardous alternative to more aggressive solvents, potentially reducing the environmental risks associated with these advanced materials.
The lifecycle assessment of ethyl acetate in nanotechnology applications reveals a complex environmental profile. While its production from renewable resources like ethanol can offer sustainability benefits, the energy-intensive nature of some nanomaterial synthesis processes may offset these advantages. This highlights the need for holistic environmental evaluations that consider the entire production chain, from raw material sourcing to end-product disposal.
As nanotechnology continues to evolve, ongoing research into the environmental fate and behavior of ethyl acetate in various nanotech applications is crucial. This includes studying its interactions with different nanomaterials, potential for nanoparticle dispersion in the environment, and long-term ecological effects. Such research will be vital in developing sustainable practices and regulations for the use of ethyl acetate in the rapidly advancing field of nanotechnology.
One of the primary environmental benefits of using ethyl acetate in nanotechnology is its potential to replace more harmful solvents. Compared to many traditional organic solvents, ethyl acetate has a lower environmental persistence and reduced toxicity to aquatic life. This makes it a more environmentally friendly option for large-scale nanomaterial production processes, potentially reducing the overall ecological footprint of nanotechnology manufacturing.
However, the increased use of ethyl acetate in nanotechnology applications also raises concerns about its atmospheric emissions. As a volatile organic compound (VOC), ethyl acetate can contribute to the formation of ground-level ozone and photochemical smog when released into the air. This necessitates proper handling and emission control measures in industrial settings to mitigate potential air quality impacts.
The biodegradability of ethyl acetate is another important environmental factor. While it degrades more readily than many other solvents, its rapid breakdown in the environment can lead to temporary increases in biochemical oxygen demand (BOD) in aquatic ecosystems if released in large quantities. This underscores the importance of proper waste management and treatment protocols in nanotechnology facilities using ethyl acetate.
In the context of nanomaterial production, the environmental impact of ethyl acetate is closely tied to the specific processes and materials involved. For instance, in the synthesis of carbon nanotubes or graphene, ethyl acetate may serve as a less hazardous alternative to more aggressive solvents, potentially reducing the environmental risks associated with these advanced materials.
The lifecycle assessment of ethyl acetate in nanotechnology applications reveals a complex environmental profile. While its production from renewable resources like ethanol can offer sustainability benefits, the energy-intensive nature of some nanomaterial synthesis processes may offset these advantages. This highlights the need for holistic environmental evaluations that consider the entire production chain, from raw material sourcing to end-product disposal.
As nanotechnology continues to evolve, ongoing research into the environmental fate and behavior of ethyl acetate in various nanotech applications is crucial. This includes studying its interactions with different nanomaterials, potential for nanoparticle dispersion in the environment, and long-term ecological effects. Such research will be vital in developing sustainable practices and regulations for the use of ethyl acetate in the rapidly advancing field of nanotechnology.
Scalability and Industrial Integration of Ethyl Acetate Nanotech
The scalability and industrial integration of ethyl acetate in nanotechnology applications present both significant opportunities and challenges. As the potential of ethyl acetate in nanotech becomes increasingly apparent, the focus shifts to developing efficient and cost-effective methods for large-scale production and seamless integration into existing industrial processes.
One of the primary considerations in scaling up ethyl acetate-based nanotechnology is the optimization of synthesis methods. Current laboratory-scale techniques often involve complex procedures that may not be directly transferable to industrial settings. Researchers are exploring continuous flow reactors and microfluidic systems to enhance production rates and maintain consistent quality at larger scales.
The integration of ethyl acetate nanotech into industrial processes requires careful consideration of compatibility with existing equipment and workflows. Many industries, such as electronics and pharmaceuticals, have established manufacturing protocols that may need modification to accommodate new nanomaterials. This adaptation process involves not only technical challenges but also regulatory compliance and worker safety considerations.
Cost-effectiveness is a crucial factor in the widespread adoption of ethyl acetate nanotechnology. While the material itself is relatively inexpensive, the processing and purification steps necessary for nanotech applications can significantly increase production costs. Developing more efficient purification methods and recycling processes is essential for making ethyl acetate nanotech economically viable on an industrial scale.
Environmental considerations play a vital role in the scalability of ethyl acetate nanotech. As production volumes increase, so does the potential environmental impact. Implementing closed-loop systems and developing green synthesis methods are becoming priorities to ensure sustainable large-scale production.
The integration of ethyl acetate nanotech into existing product lines presents opportunities for enhanced performance and new functionalities. However, it also requires extensive testing and validation to ensure that the incorporation of nanomaterials does not compromise the integrity or safety of the final products. This necessitates the development of standardized testing protocols and quality control measures specific to ethyl acetate nanotech applications.
Collaboration between academic research institutions and industry partners is crucial for addressing scalability challenges. Knowledge transfer and joint development projects can accelerate the transition from laboratory discoveries to industrial applications. Additionally, the establishment of pilot plants and demonstration facilities can provide valuable insights into the practical aspects of scaling up ethyl acetate nanotech production.
One of the primary considerations in scaling up ethyl acetate-based nanotechnology is the optimization of synthesis methods. Current laboratory-scale techniques often involve complex procedures that may not be directly transferable to industrial settings. Researchers are exploring continuous flow reactors and microfluidic systems to enhance production rates and maintain consistent quality at larger scales.
The integration of ethyl acetate nanotech into industrial processes requires careful consideration of compatibility with existing equipment and workflows. Many industries, such as electronics and pharmaceuticals, have established manufacturing protocols that may need modification to accommodate new nanomaterials. This adaptation process involves not only technical challenges but also regulatory compliance and worker safety considerations.
Cost-effectiveness is a crucial factor in the widespread adoption of ethyl acetate nanotechnology. While the material itself is relatively inexpensive, the processing and purification steps necessary for nanotech applications can significantly increase production costs. Developing more efficient purification methods and recycling processes is essential for making ethyl acetate nanotech economically viable on an industrial scale.
Environmental considerations play a vital role in the scalability of ethyl acetate nanotech. As production volumes increase, so does the potential environmental impact. Implementing closed-loop systems and developing green synthesis methods are becoming priorities to ensure sustainable large-scale production.
The integration of ethyl acetate nanotech into existing product lines presents opportunities for enhanced performance and new functionalities. However, it also requires extensive testing and validation to ensure that the incorporation of nanomaterials does not compromise the integrity or safety of the final products. This necessitates the development of standardized testing protocols and quality control measures specific to ethyl acetate nanotech applications.
Collaboration between academic research institutions and industry partners is crucial for addressing scalability challenges. Knowledge transfer and joint development projects can accelerate the transition from laboratory discoveries to industrial applications. Additionally, the establishment of pilot plants and demonstration facilities can provide valuable insights into the practical aspects of scaling up ethyl acetate nanotech production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!