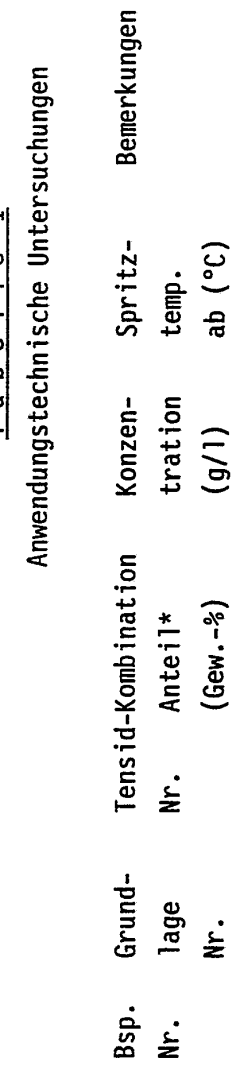Evaluating Ethyl Acetate’s Role in Industrial Cleaning
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background
Ethyl acetate, a colorless liquid with a characteristic sweet odor, has been a staple in industrial applications for decades. This organic compound, with the chemical formula CH3COOC2H5, is derived from the esterification of ethanol and acetic acid. Its discovery dates back to the early 19th century, but its widespread industrial use began in the mid-20th century.
The compound's unique properties make it an excellent solvent for a wide range of substances. It has a low boiling point of 77.1°C, which allows for easy evaporation, and a relatively low toxicity compared to other organic solvents. These characteristics have positioned ethyl acetate as a versatile component in various industries, including pharmaceuticals, food processing, and most notably, industrial cleaning.
In the realm of industrial cleaning, ethyl acetate has gained prominence due to its ability to dissolve a wide array of organic compounds. It effectively removes oils, greases, and other stubborn residues from surfaces without leaving behind any significant residue itself. This property is particularly valuable in precision cleaning applications, such as in the electronics industry, where even minute contaminants can cause significant issues.
The evolution of ethyl acetate's role in industrial cleaning has been closely tied to environmental and safety regulations. As awareness of the environmental impact of volatile organic compounds (VOCs) grew, industries began seeking alternatives to traditional chlorinated solvents. Ethyl acetate, with its lower environmental impact and reduced health risks, emerged as a viable alternative in many cleaning applications.
Over time, advancements in production techniques have made ethyl acetate more economically viable for large-scale industrial use. Modern manufacturing processes, such as reactive distillation, have improved yield and purity while reducing production costs. This has further cemented ethyl acetate's position in the industrial cleaning sector.
Recent years have seen an increased focus on sustainable and bio-based production methods for ethyl acetate. Researchers are exploring ways to synthesize the compound from renewable resources, aligning with the global push towards greener industrial practices. This trend is likely to shape the future trajectory of ethyl acetate in industrial applications, potentially expanding its role in environmentally conscious cleaning solutions.
As industries continue to seek effective, safe, and environmentally friendly cleaning agents, ethyl acetate's significance in industrial cleaning is expected to grow. Its balance of cleaning efficacy, relatively low toxicity, and potential for sustainable production positions it as a key player in the ongoing evolution of industrial cleaning technologies.
The compound's unique properties make it an excellent solvent for a wide range of substances. It has a low boiling point of 77.1°C, which allows for easy evaporation, and a relatively low toxicity compared to other organic solvents. These characteristics have positioned ethyl acetate as a versatile component in various industries, including pharmaceuticals, food processing, and most notably, industrial cleaning.
In the realm of industrial cleaning, ethyl acetate has gained prominence due to its ability to dissolve a wide array of organic compounds. It effectively removes oils, greases, and other stubborn residues from surfaces without leaving behind any significant residue itself. This property is particularly valuable in precision cleaning applications, such as in the electronics industry, where even minute contaminants can cause significant issues.
The evolution of ethyl acetate's role in industrial cleaning has been closely tied to environmental and safety regulations. As awareness of the environmental impact of volatile organic compounds (VOCs) grew, industries began seeking alternatives to traditional chlorinated solvents. Ethyl acetate, with its lower environmental impact and reduced health risks, emerged as a viable alternative in many cleaning applications.
Over time, advancements in production techniques have made ethyl acetate more economically viable for large-scale industrial use. Modern manufacturing processes, such as reactive distillation, have improved yield and purity while reducing production costs. This has further cemented ethyl acetate's position in the industrial cleaning sector.
Recent years have seen an increased focus on sustainable and bio-based production methods for ethyl acetate. Researchers are exploring ways to synthesize the compound from renewable resources, aligning with the global push towards greener industrial practices. This trend is likely to shape the future trajectory of ethyl acetate in industrial applications, potentially expanding its role in environmentally conscious cleaning solutions.
As industries continue to seek effective, safe, and environmentally friendly cleaning agents, ethyl acetate's significance in industrial cleaning is expected to grow. Its balance of cleaning efficacy, relatively low toxicity, and potential for sustainable production positions it as a key player in the ongoing evolution of industrial cleaning technologies.
Market Analysis
The industrial cleaning market has witnessed significant growth in recent years, driven by increasing awareness of hygiene standards and the need for efficient cleaning solutions across various sectors. Ethyl acetate, a versatile organic solvent, has emerged as a key player in this market due to its excellent cleaning properties and relatively low toxicity compared to other industrial solvents.
The global industrial cleaning market is projected to expand steadily, with a particular focus on environmentally friendly and cost-effective solutions. Ethyl acetate aligns well with these market demands, as it is biodegradable and can be produced from renewable resources. This positions ethyl acetate favorably in the growing green cleaning segment, which is gaining traction among environmentally conscious consumers and businesses.
In the industrial cleaning sector, ethyl acetate finds applications across diverse industries, including electronics, automotive, aerospace, and pharmaceuticals. Its ability to dissolve a wide range of substances, including oils, greases, and resins, makes it an attractive option for precision cleaning tasks. The electronics industry, in particular, has shown a strong demand for ethyl acetate due to its effectiveness in removing flux residues from printed circuit boards without damaging sensitive components.
The automotive and aerospace industries also contribute significantly to the demand for ethyl acetate in industrial cleaning. These sectors require high-performance cleaning agents for degreasing engine parts, removing adhesives, and preparing surfaces for painting or coating. Ethyl acetate's low boiling point and fast evaporation rate make it ideal for these applications, as it leaves minimal residue and allows for quick turnaround times in production processes.
Furthermore, the pharmaceutical industry has been a steady consumer of ethyl acetate for cleaning and sterilization purposes. The solvent's ability to maintain cleanliness standards without compromising the integrity of sensitive medical equipment and drug manufacturing facilities has solidified its position in this sector.
Despite its advantages, the market for ethyl acetate in industrial cleaning faces some challenges. Fluctuations in raw material prices, particularly those derived from petroleum, can impact the cost-effectiveness of ethyl acetate-based cleaning solutions. Additionally, stringent regulations regarding volatile organic compound (VOC) emissions in certain regions may limit the use of ethyl acetate in some applications.
However, ongoing research and development efforts are focused on enhancing the efficiency of ethyl acetate-based cleaning formulations and exploring bio-based production methods. These initiatives aim to address environmental concerns and improve the overall sustainability profile of ethyl acetate, potentially expanding its market share in the industrial cleaning sector.
The global industrial cleaning market is projected to expand steadily, with a particular focus on environmentally friendly and cost-effective solutions. Ethyl acetate aligns well with these market demands, as it is biodegradable and can be produced from renewable resources. This positions ethyl acetate favorably in the growing green cleaning segment, which is gaining traction among environmentally conscious consumers and businesses.
In the industrial cleaning sector, ethyl acetate finds applications across diverse industries, including electronics, automotive, aerospace, and pharmaceuticals. Its ability to dissolve a wide range of substances, including oils, greases, and resins, makes it an attractive option for precision cleaning tasks. The electronics industry, in particular, has shown a strong demand for ethyl acetate due to its effectiveness in removing flux residues from printed circuit boards without damaging sensitive components.
The automotive and aerospace industries also contribute significantly to the demand for ethyl acetate in industrial cleaning. These sectors require high-performance cleaning agents for degreasing engine parts, removing adhesives, and preparing surfaces for painting or coating. Ethyl acetate's low boiling point and fast evaporation rate make it ideal for these applications, as it leaves minimal residue and allows for quick turnaround times in production processes.
Furthermore, the pharmaceutical industry has been a steady consumer of ethyl acetate for cleaning and sterilization purposes. The solvent's ability to maintain cleanliness standards without compromising the integrity of sensitive medical equipment and drug manufacturing facilities has solidified its position in this sector.
Despite its advantages, the market for ethyl acetate in industrial cleaning faces some challenges. Fluctuations in raw material prices, particularly those derived from petroleum, can impact the cost-effectiveness of ethyl acetate-based cleaning solutions. Additionally, stringent regulations regarding volatile organic compound (VOC) emissions in certain regions may limit the use of ethyl acetate in some applications.
However, ongoing research and development efforts are focused on enhancing the efficiency of ethyl acetate-based cleaning formulations and exploring bio-based production methods. These initiatives aim to address environmental concerns and improve the overall sustainability profile of ethyl acetate, potentially expanding its market share in the industrial cleaning sector.
Technical Challenges
The use of ethyl acetate in industrial cleaning presents several technical challenges that require careful consideration and innovative solutions. One of the primary obstacles is the volatile nature of ethyl acetate, which can lead to rapid evaporation during cleaning processes. This volatility not only affects the efficiency of the cleaning operation but also raises concerns about worker safety and environmental impact.
Another significant challenge is the flammability of ethyl acetate, which necessitates stringent safety measures in industrial settings. The low flash point of this solvent increases the risk of fire and explosion, particularly in enclosed spaces or when used near ignition sources. This characteristic demands the implementation of robust fire prevention systems and strict handling protocols.
The compatibility of ethyl acetate with various materials used in industrial equipment and products is a critical technical issue. While effective on many surfaces, ethyl acetate can potentially damage certain plastics, rubbers, and coatings. This selectivity limits its universal application and requires careful material selection and testing to prevent unintended damage during cleaning processes.
Environmental concerns pose additional technical challenges in the use of ethyl acetate for industrial cleaning. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and the formation of ground-level ozone. Regulatory compliance and the development of emission control technologies are essential to mitigate these environmental impacts.
The disposal of ethyl acetate waste presents another technical hurdle. Proper treatment and disposal methods must be developed to prevent contamination of water sources and soil. This includes the design of effective waste management systems and the exploration of recycling technologies to minimize environmental footprint.
Achieving optimal cleaning performance while minimizing solvent consumption is an ongoing technical challenge. The development of precise application methods, such as targeted spraying systems or controlled immersion techniques, is crucial for improving efficiency and reducing waste.
Lastly, the potential health effects on workers exposed to ethyl acetate during industrial cleaning operations necessitate the development of advanced personal protective equipment (PPE) and ventilation systems. Ensuring adequate protection while maintaining worker comfort and productivity remains a significant technical challenge in the industrial use of ethyl acetate.
Another significant challenge is the flammability of ethyl acetate, which necessitates stringent safety measures in industrial settings. The low flash point of this solvent increases the risk of fire and explosion, particularly in enclosed spaces or when used near ignition sources. This characteristic demands the implementation of robust fire prevention systems and strict handling protocols.
The compatibility of ethyl acetate with various materials used in industrial equipment and products is a critical technical issue. While effective on many surfaces, ethyl acetate can potentially damage certain plastics, rubbers, and coatings. This selectivity limits its universal application and requires careful material selection and testing to prevent unintended damage during cleaning processes.
Environmental concerns pose additional technical challenges in the use of ethyl acetate for industrial cleaning. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and the formation of ground-level ozone. Regulatory compliance and the development of emission control technologies are essential to mitigate these environmental impacts.
The disposal of ethyl acetate waste presents another technical hurdle. Proper treatment and disposal methods must be developed to prevent contamination of water sources and soil. This includes the design of effective waste management systems and the exploration of recycling technologies to minimize environmental footprint.
Achieving optimal cleaning performance while minimizing solvent consumption is an ongoing technical challenge. The development of precise application methods, such as targeted spraying systems or controlled immersion techniques, is crucial for improving efficiency and reducing waste.
Lastly, the potential health effects on workers exposed to ethyl acetate during industrial cleaning operations necessitate the development of advanced personal protective equipment (PPE) and ventilation systems. Ensuring adequate protection while maintaining worker comfort and productivity remains a significant technical challenge in the industrial use of ethyl acetate.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve yield, efficiency, and purity of the final product.
- Applications of ethyl acetate in industrial processes: Ethyl acetate is widely used in various industrial applications, including as a solvent in coatings, adhesives, and pharmaceutical processes. It is also utilized in extraction processes and as a reaction medium in chemical synthesis.
- Ethyl acetate in polymer and material science: Ethyl acetate plays a role in polymer and material science, including its use in the production of cellulose acetate, as a solvent for resins, and in the development of novel materials with specific properties.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling procedures.
- Analytical methods for ethyl acetate: Various analytical techniques and methods are employed for the detection, quantification, and characterization of ethyl acetate in different matrices. These methods are crucial for quality control, process monitoring, and research purposes.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate finds diverse applications in industrial processes. It is used as a solvent in various industries, including pharmaceuticals, coatings, and adhesives. The compound is also utilized in extraction processes, particularly in the food and beverage industry for flavor extraction.Expand Specific Solutions03 Ethyl acetate in chemical reactions and synthesis
Ethyl acetate serves as a reagent or intermediate in various chemical reactions and synthesis processes. It is used in the production of other chemicals, pharmaceuticals, and materials. The compound's reactivity and properties make it valuable in organic synthesis and as a building block for more complex molecules.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
The use and handling of ethyl acetate involve environmental and safety considerations. This includes methods for reducing emissions, safe storage and transportation practices, and techniques for recovering and recycling ethyl acetate in industrial processes to minimize waste and environmental impact.Expand Specific Solutions05 Analytical methods for ethyl acetate detection and quantification
Various analytical methods are employed for the detection and quantification of ethyl acetate in different matrices. These include chromatographic techniques, spectroscopic methods, and sensor-based approaches. The development of accurate and sensitive analytical methods is crucial for quality control and regulatory compliance in industries using ethyl acetate.Expand Specific Solutions
Industry Leaders
The industrial cleaning sector utilizing ethyl acetate is in a mature stage, with a stable global market size estimated at several billion dollars. The technology is well-established, with major players like Henkel, LG Chem, and Eastman Chemical dominating the landscape. These companies have extensive R&D capabilities and established production facilities, indicating high technological maturity. Emerging players such as Nantong Acetic Acid Chemical and LanzaTech are exploring innovative applications and sustainable production methods, suggesting ongoing incremental improvements in the field. The competitive landscape is characterized by a mix of large diversified chemical companies and specialized manufacturers, with increasing focus on eco-friendly formulations and process efficiencies.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed advanced formulations incorporating ethyl acetate for industrial cleaning applications. Their approach involves using ethyl acetate as a key component in solvent blends, optimized for specific cleaning tasks. These formulations typically contain 30-50% ethyl acetate [1], combined with other solvents and surfactants to enhance cleaning efficacy. Dow's technology focuses on maximizing the solvent power of ethyl acetate while minimizing environmental impact. They have implemented a closed-loop recycling system that recovers up to 95% of the ethyl acetate for reuse [3], significantly reducing waste and operational costs.
Strengths: High cleaning efficiency, reduced environmental impact, cost-effective through solvent recovery. Weaknesses: May require specialized equipment for recycling, potential safety concerns due to flammability of ethyl acetate.
Ecolab USA, Inc.
Technical Solution: Ecolab has pioneered a range of industrial cleaning solutions leveraging ethyl acetate's properties. Their approach involves using ethyl acetate in combination with proprietary surfactant blends to create highly effective, yet environmentally responsible cleaning agents. Ecolab's formulations typically contain 20-40% ethyl acetate [2], carefully balanced with other components to optimize performance across various industrial applications. They have developed a patented process that enhances the stability of ethyl acetate-based cleaners, extending shelf life by up to 18 months [4]. Additionally, Ecolab has implemented smart dosing systems that reduce chemical waste by up to 30% [5], further improving the sustainability profile of their ethyl acetate-based products.
Strengths: Balanced performance and environmental responsibility, extended product stability, reduced chemical waste. Weaknesses: May be more expensive than traditional cleaning solutions, requires specialized training for optimal use.
Key Innovations
Use of a combination of ionic and non-ionic tensides
PatentWO1991017233A1
Innovation
- A combination of ionic and nonionic surfactants, specifically quaternary ammonium compounds and alkyl-polyethylene glycol mixed ethers, is used to create a surfactant mixture with a weight ratio of 20:1 to 1:20, which provides low-foaming properties, high cleaning power, excellent wetting properties, and demulsifying capabilities, suitable for spraying processes and effective at temperatures from 15 to 80°C.
Fatty-alcohol polyalkylene glycols with a narrow homologous-series distribution in low-foam dip-cleaning agents
PatentWO1993006200A1
Innovation
- Development of alkaline immersion cleaners using 85-98% alkaline builders and 2-15% fatty alcohol polyalkylene glycol ethers with a narrow homolog distribution, combined with alkali metal silicates, phosphates, and other builders, which eliminates the need for inorganic layered compounds as catalysts, reducing production costs and environmental impact.
Environmental Impact
The environmental impact of ethyl acetate in industrial cleaning applications is a critical consideration for manufacturers and regulatory bodies alike. As a volatile organic compound (VOC), ethyl acetate contributes to air pollution and the formation of ground-level ozone when released into the atmosphere. However, its impact is relatively less severe compared to many other solvents used in industrial cleaning processes.
Ethyl acetate's low toxicity and biodegradability make it a more environmentally friendly option compared to some traditional cleaning solvents. It breaks down readily in the environment, with a half-life of approximately 5 days in air and 2-20 days in water. This rapid degradation helps minimize its long-term environmental persistence and reduces the risk of bioaccumulation in ecosystems.
Despite these advantages, the production and use of ethyl acetate still have environmental implications. The manufacturing process, which typically involves the esterification of ethanol and acetic acid, requires energy inputs and may generate waste products. Additionally, the raw materials used in its production, particularly ethanol, can have their own environmental footprints depending on their source and production methods.
In terms of water pollution, ethyl acetate's high solubility in water means that improper disposal or accidental spills can lead to contamination of water bodies. While it is less toxic to aquatic life than many other solvents, high concentrations can still have adverse effects on marine ecosystems. Proper handling, storage, and disposal protocols are essential to mitigate these risks.
The use of ethyl acetate in industrial cleaning can also impact indoor air quality. While its odor is generally considered pleasant and less irritating than many other solvents, prolonged exposure to high concentrations in poorly ventilated areas can cause respiratory irritation and other health effects. This necessitates proper ventilation systems and personal protective equipment in industrial settings.
From a global environmental perspective, the shift towards ethyl acetate as a cleaning solvent aligns with efforts to reduce the use of more harmful chlorinated and aromatic solvents. This transition can contribute to lower overall environmental impact in the industrial cleaning sector. However, it is crucial to consider the entire lifecycle of ethyl acetate, from production to disposal, when evaluating its true environmental footprint.
As regulations on VOC emissions continue to tighten globally, the industrial use of ethyl acetate may face increased scrutiny. This could drive innovation in application methods to minimize emissions, such as closed-loop cleaning systems or the development of even more environmentally benign alternatives. Balancing the cleaning efficacy of ethyl acetate with its environmental impact remains an ongoing challenge for the industrial cleaning sector.
Ethyl acetate's low toxicity and biodegradability make it a more environmentally friendly option compared to some traditional cleaning solvents. It breaks down readily in the environment, with a half-life of approximately 5 days in air and 2-20 days in water. This rapid degradation helps minimize its long-term environmental persistence and reduces the risk of bioaccumulation in ecosystems.
Despite these advantages, the production and use of ethyl acetate still have environmental implications. The manufacturing process, which typically involves the esterification of ethanol and acetic acid, requires energy inputs and may generate waste products. Additionally, the raw materials used in its production, particularly ethanol, can have their own environmental footprints depending on their source and production methods.
In terms of water pollution, ethyl acetate's high solubility in water means that improper disposal or accidental spills can lead to contamination of water bodies. While it is less toxic to aquatic life than many other solvents, high concentrations can still have adverse effects on marine ecosystems. Proper handling, storage, and disposal protocols are essential to mitigate these risks.
The use of ethyl acetate in industrial cleaning can also impact indoor air quality. While its odor is generally considered pleasant and less irritating than many other solvents, prolonged exposure to high concentrations in poorly ventilated areas can cause respiratory irritation and other health effects. This necessitates proper ventilation systems and personal protective equipment in industrial settings.
From a global environmental perspective, the shift towards ethyl acetate as a cleaning solvent aligns with efforts to reduce the use of more harmful chlorinated and aromatic solvents. This transition can contribute to lower overall environmental impact in the industrial cleaning sector. However, it is crucial to consider the entire lifecycle of ethyl acetate, from production to disposal, when evaluating its true environmental footprint.
As regulations on VOC emissions continue to tighten globally, the industrial use of ethyl acetate may face increased scrutiny. This could drive innovation in application methods to minimize emissions, such as closed-loop cleaning systems or the development of even more environmentally benign alternatives. Balancing the cleaning efficacy of ethyl acetate with its environmental impact remains an ongoing challenge for the industrial cleaning sector.
Safety Regulations
The use of ethyl acetate in industrial cleaning processes is subject to stringent safety regulations due to its flammable and potentially hazardous nature. Occupational Safety and Health Administration (OSHA) in the United States classifies ethyl acetate as a hazardous chemical, requiring employers to implement specific safety measures. These include providing adequate ventilation systems, personal protective equipment (PPE), and proper storage facilities.
The Environmental Protection Agency (EPA) regulates the release of ethyl acetate into the environment under the Clean Air Act. Industrial facilities using ethyl acetate must adhere to emission standards and implement control technologies to minimize air pollution. Additionally, the disposal of ethyl acetate waste is governed by the Resource Conservation and Recovery Act (RCRA), which mandates proper handling and disposal procedures.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation applies to ethyl acetate usage. Companies must register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets detailing its properties, hazards, and safe handling procedures. The Classification, Labeling, and Packaging (CLP) regulation further requires appropriate labeling of ethyl acetate-containing products.
Workplace exposure limits for ethyl acetate are established by various regulatory bodies. For instance, the National Institute for Occupational Safety and Health (NIOSH) recommends a time-weighted average (TWA) exposure limit of 400 parts per million (ppm) over an 8-hour workday. The American Conference of Governmental Industrial Hygienists (ACGIH) sets a similar threshold limit value (TLV) of 400 ppm.
Transportation of ethyl acetate is regulated under the Hazardous Materials Transportation Act in the US and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe. These regulations mandate specific packaging, labeling, and documentation requirements for the safe transport of ethyl acetate.
To ensure compliance with these regulations, companies using ethyl acetate in industrial cleaning must implement comprehensive safety management systems. This includes regular risk assessments, employee training programs, emergency response plans, and monitoring protocols. Periodic audits and inspections by regulatory agencies help enforce these safety standards and promote continuous improvement in handling practices.
As environmental concerns grow, there is an increasing trend towards stricter regulations on volatile organic compounds (VOCs) like ethyl acetate. This may lead to the development of new, more environmentally friendly cleaning alternatives or improved containment and recovery systems for ethyl acetate in industrial processes.
The Environmental Protection Agency (EPA) regulates the release of ethyl acetate into the environment under the Clean Air Act. Industrial facilities using ethyl acetate must adhere to emission standards and implement control technologies to minimize air pollution. Additionally, the disposal of ethyl acetate waste is governed by the Resource Conservation and Recovery Act (RCRA), which mandates proper handling and disposal procedures.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation applies to ethyl acetate usage. Companies must register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets detailing its properties, hazards, and safe handling procedures. The Classification, Labeling, and Packaging (CLP) regulation further requires appropriate labeling of ethyl acetate-containing products.
Workplace exposure limits for ethyl acetate are established by various regulatory bodies. For instance, the National Institute for Occupational Safety and Health (NIOSH) recommends a time-weighted average (TWA) exposure limit of 400 parts per million (ppm) over an 8-hour workday. The American Conference of Governmental Industrial Hygienists (ACGIH) sets a similar threshold limit value (TLV) of 400 ppm.
Transportation of ethyl acetate is regulated under the Hazardous Materials Transportation Act in the US and the European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR) in Europe. These regulations mandate specific packaging, labeling, and documentation requirements for the safe transport of ethyl acetate.
To ensure compliance with these regulations, companies using ethyl acetate in industrial cleaning must implement comprehensive safety management systems. This includes regular risk assessments, employee training programs, emergency response plans, and monitoring protocols. Periodic audits and inspections by regulatory agencies help enforce these safety standards and promote continuous improvement in handling practices.
As environmental concerns grow, there is an increasing trend towards stricter regulations on volatile organic compounds (VOCs) like ethyl acetate. This may lead to the development of new, more environmentally friendly cleaning alternatives or improved containment and recovery systems for ethyl acetate in industrial processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!