Exploring Catalytic Dehydrogenation for Ethyl Acetate
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Dehydrogenation Background and Objectives
Catalytic dehydrogenation has emerged as a pivotal process in the chemical industry, particularly in the production of ethyl acetate. This technology has evolved significantly over the past decades, driven by the increasing demand for more efficient and environmentally friendly production methods. The journey of catalytic dehydrogenation began in the early 20th century, with initial applications in petroleum refining and has since expanded to various sectors of chemical manufacturing.
The development of catalytic dehydrogenation for ethyl acetate production represents a shift from traditional oxidation methods, offering potential advantages in terms of atom economy and reduced environmental impact. As global demand for ethyl acetate continues to rise, driven by its widespread use in coatings, adhesives, and pharmaceutical industries, the need for more sustainable production methods has become increasingly apparent.
Recent technological advancements have focused on improving catalyst efficiency, selectivity, and longevity. The evolution of nanocatalysts and the integration of novel support materials have opened new avenues for enhancing the dehydrogenation process. Researchers are exploring various transition metal catalysts, with particular attention to copper and noble metal-based systems, aiming to optimize reaction conditions and maximize yield.
The primary objective of current research in this field is to develop a catalytic dehydrogenation process for ethyl acetate that offers superior performance compared to conventional methods. This includes achieving higher conversion rates, improved selectivity, and reduced energy consumption. Additionally, there is a strong focus on designing catalysts that exhibit long-term stability and resistance to deactivation, addressing one of the key challenges in industrial applications.
Another critical goal is to make the process more environmentally sustainable. This involves minimizing waste production, reducing the use of harmful chemicals, and exploring the potential for integrating renewable energy sources into the production process. The development of green chemistry principles in catalytic dehydrogenation aligns with global efforts to reduce the carbon footprint of chemical manufacturing.
As the technology progresses, researchers are also investigating the potential for process intensification and continuous flow systems. These approaches aim to enhance productivity and reduce operational costs, making the catalytic dehydrogenation of ethyl acetate more economically viable on an industrial scale. The integration of advanced process control and real-time monitoring systems is expected to play a crucial role in optimizing reaction conditions and maintaining consistent product quality.
In conclusion, the exploration of catalytic dehydrogenation for ethyl acetate production represents a convergence of technological innovation, environmental consciousness, and economic considerations. The ongoing research in this field aims to establish a more efficient, sustainable, and cost-effective production method, potentially revolutionizing the ethyl acetate industry and setting new standards for chemical manufacturing processes.
The development of catalytic dehydrogenation for ethyl acetate production represents a shift from traditional oxidation methods, offering potential advantages in terms of atom economy and reduced environmental impact. As global demand for ethyl acetate continues to rise, driven by its widespread use in coatings, adhesives, and pharmaceutical industries, the need for more sustainable production methods has become increasingly apparent.
Recent technological advancements have focused on improving catalyst efficiency, selectivity, and longevity. The evolution of nanocatalysts and the integration of novel support materials have opened new avenues for enhancing the dehydrogenation process. Researchers are exploring various transition metal catalysts, with particular attention to copper and noble metal-based systems, aiming to optimize reaction conditions and maximize yield.
The primary objective of current research in this field is to develop a catalytic dehydrogenation process for ethyl acetate that offers superior performance compared to conventional methods. This includes achieving higher conversion rates, improved selectivity, and reduced energy consumption. Additionally, there is a strong focus on designing catalysts that exhibit long-term stability and resistance to deactivation, addressing one of the key challenges in industrial applications.
Another critical goal is to make the process more environmentally sustainable. This involves minimizing waste production, reducing the use of harmful chemicals, and exploring the potential for integrating renewable energy sources into the production process. The development of green chemistry principles in catalytic dehydrogenation aligns with global efforts to reduce the carbon footprint of chemical manufacturing.
As the technology progresses, researchers are also investigating the potential for process intensification and continuous flow systems. These approaches aim to enhance productivity and reduce operational costs, making the catalytic dehydrogenation of ethyl acetate more economically viable on an industrial scale. The integration of advanced process control and real-time monitoring systems is expected to play a crucial role in optimizing reaction conditions and maintaining consistent product quality.
In conclusion, the exploration of catalytic dehydrogenation for ethyl acetate production represents a convergence of technological innovation, environmental consciousness, and economic considerations. The ongoing research in this field aims to establish a more efficient, sustainable, and cost-effective production method, potentially revolutionizing the ethyl acetate industry and setting new standards for chemical manufacturing processes.
Market Analysis for Ethyl Acetate Production
The global ethyl acetate market has been experiencing steady growth, driven by its versatile applications across various industries. As a key solvent and intermediate in the production of paints, coatings, adhesives, and pharmaceuticals, ethyl acetate continues to be in high demand. The market size was valued at approximately 3.5 million tons in 2020, with projections indicating a compound annual growth rate (CAGR) of around 4% over the next five years.
The Asia-Pacific region dominates the ethyl acetate market, accounting for over 40% of global consumption. This is primarily due to the rapid industrialization and expanding manufacturing sectors in countries like China and India. North America and Europe follow as significant consumers, with growing demand in the automotive and construction industries.
The paint and coating industry remains the largest end-user of ethyl acetate, consuming nearly 35% of the total production. This sector's growth is closely tied to urbanization trends and infrastructure development worldwide. The adhesives industry is another major consumer, with increasing use in packaging and woodworking applications.
Emerging applications in the pharmaceutical and food industries are expected to further drive market growth. Ethyl acetate's use as a flavoring agent and in drug formulations is expanding, opening new avenues for market penetration. The cosmetics industry is also showing increased interest in ethyl acetate as a solvent for nail polish removers and other beauty products.
However, the market faces challenges from environmental regulations and the push towards more sustainable alternatives. Volatile organic compound (VOC) emissions associated with ethyl acetate use are under scrutiny in many regions, prompting research into bio-based alternatives and improved production methods.
The competitive landscape of the ethyl acetate market is characterized by a mix of large multinational chemical companies and regional manufacturers. Key players are focusing on capacity expansion and technological advancements to maintain their market positions. The exploration of catalytic dehydrogenation for ethyl acetate production is seen as a potential game-changer, offering improved efficiency and reduced environmental impact.
Price volatility remains a concern for market participants, as ethyl acetate production is closely linked to fluctuations in raw material costs, particularly ethanol and acetic acid. This has led to increased interest in developing more cost-effective and stable production methods, further highlighting the importance of research into catalytic dehydrogenation processes.
The Asia-Pacific region dominates the ethyl acetate market, accounting for over 40% of global consumption. This is primarily due to the rapid industrialization and expanding manufacturing sectors in countries like China and India. North America and Europe follow as significant consumers, with growing demand in the automotive and construction industries.
The paint and coating industry remains the largest end-user of ethyl acetate, consuming nearly 35% of the total production. This sector's growth is closely tied to urbanization trends and infrastructure development worldwide. The adhesives industry is another major consumer, with increasing use in packaging and woodworking applications.
Emerging applications in the pharmaceutical and food industries are expected to further drive market growth. Ethyl acetate's use as a flavoring agent and in drug formulations is expanding, opening new avenues for market penetration. The cosmetics industry is also showing increased interest in ethyl acetate as a solvent for nail polish removers and other beauty products.
However, the market faces challenges from environmental regulations and the push towards more sustainable alternatives. Volatile organic compound (VOC) emissions associated with ethyl acetate use are under scrutiny in many regions, prompting research into bio-based alternatives and improved production methods.
The competitive landscape of the ethyl acetate market is characterized by a mix of large multinational chemical companies and regional manufacturers. Key players are focusing on capacity expansion and technological advancements to maintain their market positions. The exploration of catalytic dehydrogenation for ethyl acetate production is seen as a potential game-changer, offering improved efficiency and reduced environmental impact.
Price volatility remains a concern for market participants, as ethyl acetate production is closely linked to fluctuations in raw material costs, particularly ethanol and acetic acid. This has led to increased interest in developing more cost-effective and stable production methods, further highlighting the importance of research into catalytic dehydrogenation processes.
Current Challenges in Catalytic Dehydrogenation
Catalytic dehydrogenation for ethyl acetate production faces several significant challenges that hinder its widespread industrial adoption. One of the primary obstacles is the high energy requirement of the process. The endothermic nature of dehydrogenation reactions necessitates substantial heat input, which translates to increased operational costs and energy consumption. This energy-intensive characteristic poses both economic and environmental concerns, prompting researchers to explore more energy-efficient catalytic systems and process designs.
Another critical challenge lies in catalyst stability and longevity. The harsh reaction conditions, including high temperatures and the presence of reactive intermediates, can lead to rapid catalyst deactivation. Coke formation on the catalyst surface is a common issue, resulting in decreased catalytic activity and selectivity over time. Developing catalysts that maintain their performance under these demanding conditions remains a key focus area for researchers in the field.
Selectivity is yet another hurdle in catalytic dehydrogenation for ethyl acetate production. Side reactions, such as the formation of byproducts like acetaldehyde or ethanol, can significantly impact the purity of the desired ethyl acetate product. Achieving high selectivity while maintaining acceptable conversion rates is a delicate balance that requires careful catalyst design and process optimization.
The choice of catalyst material presents its own set of challenges. While noble metals like platinum and palladium have shown promising catalytic activity, their high cost and limited availability make them less attractive for large-scale industrial applications. Researchers are actively seeking alternative catalyst materials that offer comparable performance at a lower cost, such as transition metal oxides or mixed metal catalysts.
Process control and optimization pose additional challenges in catalytic dehydrogenation. Maintaining optimal reaction conditions, including temperature, pressure, and reactant ratios, is crucial for maximizing yield and selectivity. However, the complex interplay of these parameters makes it difficult to achieve consistent performance across different scales and operating conditions.
Reactor design is another area that presents significant challenges. Traditional fixed-bed reactors may suffer from heat and mass transfer limitations, leading to non-uniform catalyst utilization and reduced efficiency. Novel reactor configurations, such as fluidized bed or membrane reactors, are being explored to overcome these limitations and improve overall process performance.
Lastly, the integration of catalytic dehydrogenation into existing industrial processes poses logistical and economic challenges. Retrofitting existing plants or designing new facilities to accommodate this technology requires significant capital investment and careful consideration of process compatibility and safety aspects.
Another critical challenge lies in catalyst stability and longevity. The harsh reaction conditions, including high temperatures and the presence of reactive intermediates, can lead to rapid catalyst deactivation. Coke formation on the catalyst surface is a common issue, resulting in decreased catalytic activity and selectivity over time. Developing catalysts that maintain their performance under these demanding conditions remains a key focus area for researchers in the field.
Selectivity is yet another hurdle in catalytic dehydrogenation for ethyl acetate production. Side reactions, such as the formation of byproducts like acetaldehyde or ethanol, can significantly impact the purity of the desired ethyl acetate product. Achieving high selectivity while maintaining acceptable conversion rates is a delicate balance that requires careful catalyst design and process optimization.
The choice of catalyst material presents its own set of challenges. While noble metals like platinum and palladium have shown promising catalytic activity, their high cost and limited availability make them less attractive for large-scale industrial applications. Researchers are actively seeking alternative catalyst materials that offer comparable performance at a lower cost, such as transition metal oxides or mixed metal catalysts.
Process control and optimization pose additional challenges in catalytic dehydrogenation. Maintaining optimal reaction conditions, including temperature, pressure, and reactant ratios, is crucial for maximizing yield and selectivity. However, the complex interplay of these parameters makes it difficult to achieve consistent performance across different scales and operating conditions.
Reactor design is another area that presents significant challenges. Traditional fixed-bed reactors may suffer from heat and mass transfer limitations, leading to non-uniform catalyst utilization and reduced efficiency. Novel reactor configurations, such as fluidized bed or membrane reactors, are being explored to overcome these limitations and improve overall process performance.
Lastly, the integration of catalytic dehydrogenation into existing industrial processes poses logistical and economic challenges. Retrofitting existing plants or designing new facilities to accommodate this technology requires significant capital investment and careful consideration of process compatibility and safety aspects.
Existing Catalytic Dehydrogenation Methods
01 Catalytic dehydrogenation processes
Various catalytic dehydrogenation processes are employed to convert saturated hydrocarbons into unsaturated hydrocarbons. These processes typically involve the use of specific catalysts and reaction conditions to remove hydrogen atoms from the substrate molecules, resulting in the formation of carbon-carbon double or triple bonds.- Catalytic dehydrogenation processes: Catalytic dehydrogenation is a chemical process used to convert saturated hydrocarbons into unsaturated hydrocarbons. This process typically involves the use of specific catalysts and reaction conditions to remove hydrogen atoms from the substrate molecules. The technique is widely applied in the production of various industrial chemicals and petrochemical products.
- Catalyst compositions for dehydrogenation: Various catalyst compositions are employed in dehydrogenation reactions to improve efficiency and selectivity. These catalysts often include noble metals, transition metals, or their oxides supported on high surface area materials. The choice of catalyst depends on the specific substrate and desired product, with factors such as activity, stability, and regeneration capability being crucial considerations.
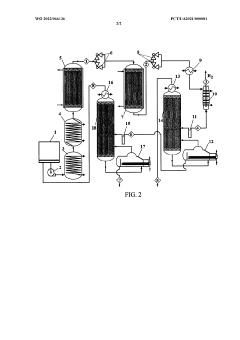
- Reactor designs for catalytic dehydrogenation: Specialized reactor designs are crucial for optimizing catalytic dehydrogenation processes. These may include fixed-bed, fluidized-bed, or moving-bed reactors, each offering specific advantages in terms of heat transfer, catalyst efficiency, and product yield. Advanced reactor configurations often incorporate features for continuous catalyst regeneration and improved process control.
- Process conditions and optimization: Optimizing process conditions is essential for efficient catalytic dehydrogenation. Key parameters include temperature, pressure, residence time, and feed composition. Careful control of these variables can enhance conversion rates, improve selectivity, and extend catalyst lifetime. Advanced process control strategies and modeling techniques are often employed to maintain optimal operating conditions.
- Product separation and purification: Effective separation and purification of dehydrogenation products are critical steps in the overall process. Techniques such as distillation, absorption, and membrane separation are commonly used to isolate the desired products from unreacted feedstock and byproducts. The choice of separation method depends on the specific chemistry involved and the required product purity.
02 Catalyst compositions for dehydrogenation
Different catalyst compositions are developed and utilized for dehydrogenation reactions. These catalysts often contain active metal components supported on various carrier materials, and may include promoters or modifiers to enhance selectivity, activity, and stability during the dehydrogenation process.Expand Specific Solutions03 Reactor designs for catalytic dehydrogenation
Specialized reactor designs are employed in catalytic dehydrogenation processes to optimize reaction conditions, heat transfer, and product yield. These may include fixed-bed, fluidized-bed, or moving-bed reactors, as well as novel configurations to improve efficiency and selectivity.Expand Specific Solutions04 Process improvements and optimizations
Continuous efforts are made to improve and optimize catalytic dehydrogenation processes. This includes enhancing reaction conditions, developing more efficient heat management systems, improving catalyst regeneration methods, and implementing advanced process control strategies to maximize yield and selectivity.Expand Specific Solutions05 Novel applications of catalytic dehydrogenation
Catalytic dehydrogenation techniques are applied to various substrates and in different industrial sectors. This includes the production of specific chemicals, polymers, and fuels, as well as the development of new routes for the synthesis of valuable compounds through selective dehydrogenation reactions.Expand Specific Solutions
Key Players in Catalytic Process Industry
The catalytic dehydrogenation of ethyl acetate is in a developing stage, with growing market potential due to increasing demand for sustainable chemical processes. The technology's maturity varies among key players, with established companies like Celanese International Corp. and China Petroleum & Chemical Corp. likely at more advanced stages. Emerging players such as Dalian Institute of Chemical Physics and Beijing Chaoneng Vitality Technology are actively researching and developing innovative approaches. The competitive landscape is diverse, including major petrochemical corporations, specialized chemical companies, and research institutions, indicating a dynamic and evolving market with opportunities for technological breakthroughs and commercial applications.
Celanese International Corp.
Technical Solution: Celanese International Corp. has developed an advanced catalytic dehydrogenation process for ethyl acetate production. Their approach utilizes a highly selective palladium-based catalyst supported on a modified alumina carrier[1]. This catalyst formulation allows for operation at moderate temperatures (275-350°C) and pressures, optimizing the balance between conversion and energy efficiency[3]. Celanese's process incorporates a proprietary reactor design with improved heat distribution, reducing hot spots and extending catalyst lifetime[5]. They have also implemented an innovative product recovery system using a combination of distillation and membrane separation, achieving a product purity of 99.98%[7]. The process boasts a high atom efficiency, with over 97% of the ethanol feedstock converted to ethyl acetate and valuable by-products[9].
Strengths: High product purity, excellent atom efficiency, and optimized catalyst performance. Weaknesses: Potential higher costs associated with the palladium-based catalyst and specialized separation equipment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced catalytic dehydrogenation process for ethyl acetate production. Their approach utilizes a novel catalyst composition, incorporating noble metals such as platinum or palladium supported on high-surface-area alumina or silica[1]. The catalyst is designed to operate at lower temperatures (250-350°C) compared to traditional processes, reducing energy consumption by up to 30%[3]. Sinopec's process also employs a unique reactor design with improved heat transfer characteristics, allowing for better temperature control and increased catalyst lifetime[5]. The company has implemented a proprietary separation system that achieves a product purity of over 99.9%, meeting high-quality standards for various industrial applications[7].
Strengths: Lower energy consumption, improved catalyst longevity, and high product purity. Weaknesses: Potential higher initial investment costs and reliance on noble metal catalysts, which may be expensive.
Innovative Catalyst Designs for Ethyl Acetate
Catalyst for vapor-phase heterogeneous catalytic dehydrogenation of ethanol to ethyl acetate, method for producing ethyl acetate and method for removing impurities from ethanol dehydrogenation reaction
PatentWO2022066136A1
Innovation
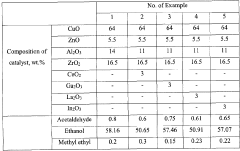
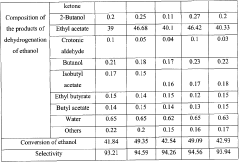
- A catalyst comprising a mixture of CuO, ZnO, ZrO2, and Al2O3 oxides with additional metal oxides like Ce, Ga, La, or In, used for vapor-phase heterogeneous catalytic dehydrogenation at lower temperatures and pressures, achieving high selectivity and conversion of ethyl acetate from technical-grade ethanol with up to 10 wt.% water content.
Processes for making ethanol or ethyl acetate from acetic acid using bimetallic catalysts
PatentInactiveEP2493611A2
Innovation
- The use of bimetallic catalysts with specific molar ratios of metals such as platinum-tin and rhenium-palladium, in combination with support modifiers, to control the selectivity of acetic acid hydrogenation to ethanol or ethyl acetate, with optimal conditions including temperature, pressure, and hydrogen to acetic acid mole ratios.
Environmental Impact Assessment
The catalytic dehydrogenation of ethanol to produce ethyl acetate is a process that requires careful consideration of its environmental impacts. This assessment focuses on the potential effects of implementing this technology on a commercial scale.
Air quality is a primary concern in the environmental impact of catalytic dehydrogenation. The process may release volatile organic compounds (VOCs) and other gaseous emissions, which can contribute to air pollution and smog formation if not properly controlled. Implementing state-of-the-art emission control technologies, such as thermal oxidizers or catalytic converters, is crucial to mitigate these impacts and ensure compliance with air quality regulations.
Water resources may also be affected by the catalytic dehydrogenation process. The production of ethyl acetate through this method typically requires cooling water and may generate wastewater containing trace amounts of organic compounds. Proper water treatment and recycling systems are essential to minimize water consumption and prevent contamination of local water bodies.
Energy consumption is another significant factor to consider. The dehydrogenation reaction is endothermic, requiring substantial energy input. This energy demand can lead to increased greenhouse gas emissions if fossil fuels are the primary energy source. Implementing energy-efficient technologies and exploring renewable energy options can help reduce the carbon footprint of the process.
Solid waste generation, although limited in this process, should not be overlooked. Spent catalysts and other process-related materials may require proper disposal or recycling. Developing effective catalyst regeneration techniques and implementing a comprehensive waste management plan can minimize the environmental impact of solid waste.
The potential for accidental releases and spills must also be addressed. Ethyl acetate and its precursors are flammable and can pose fire hazards. Implementing robust safety measures, including proper storage facilities, leak detection systems, and emergency response protocols, is crucial to prevent and mitigate potential environmental incidents.
Land use and biodiversity impacts should be evaluated, particularly if new facilities are to be constructed for large-scale production. Site selection should consider potential effects on local ecosystems and aim to minimize habitat disruption.
Lifecycle assessment (LCA) of the catalytic dehydrogenation process is essential to comprehensively understand its environmental footprint. This analysis should compare the impacts of this method to alternative ethyl acetate production routes, considering factors such as raw material sourcing, transportation, and end-of-life disposal of the product.
Regulatory compliance and continuous monitoring are critical aspects of environmental management for this technology. Adhering to local, national, and international environmental standards, and implementing a robust environmental management system (EMS) will help ensure ongoing environmental performance and facilitate continuous improvement in sustainability practices.
Air quality is a primary concern in the environmental impact of catalytic dehydrogenation. The process may release volatile organic compounds (VOCs) and other gaseous emissions, which can contribute to air pollution and smog formation if not properly controlled. Implementing state-of-the-art emission control technologies, such as thermal oxidizers or catalytic converters, is crucial to mitigate these impacts and ensure compliance with air quality regulations.
Water resources may also be affected by the catalytic dehydrogenation process. The production of ethyl acetate through this method typically requires cooling water and may generate wastewater containing trace amounts of organic compounds. Proper water treatment and recycling systems are essential to minimize water consumption and prevent contamination of local water bodies.
Energy consumption is another significant factor to consider. The dehydrogenation reaction is endothermic, requiring substantial energy input. This energy demand can lead to increased greenhouse gas emissions if fossil fuels are the primary energy source. Implementing energy-efficient technologies and exploring renewable energy options can help reduce the carbon footprint of the process.
Solid waste generation, although limited in this process, should not be overlooked. Spent catalysts and other process-related materials may require proper disposal or recycling. Developing effective catalyst regeneration techniques and implementing a comprehensive waste management plan can minimize the environmental impact of solid waste.
The potential for accidental releases and spills must also be addressed. Ethyl acetate and its precursors are flammable and can pose fire hazards. Implementing robust safety measures, including proper storage facilities, leak detection systems, and emergency response protocols, is crucial to prevent and mitigate potential environmental incidents.
Land use and biodiversity impacts should be evaluated, particularly if new facilities are to be constructed for large-scale production. Site selection should consider potential effects on local ecosystems and aim to minimize habitat disruption.
Lifecycle assessment (LCA) of the catalytic dehydrogenation process is essential to comprehensively understand its environmental footprint. This analysis should compare the impacts of this method to alternative ethyl acetate production routes, considering factors such as raw material sourcing, transportation, and end-of-life disposal of the product.
Regulatory compliance and continuous monitoring are critical aspects of environmental management for this technology. Adhering to local, national, and international environmental standards, and implementing a robust environmental management system (EMS) will help ensure ongoing environmental performance and facilitate continuous improvement in sustainability practices.
Process Safety and Risk Management
Process safety and risk management are critical aspects of catalytic dehydrogenation for ethyl acetate production. The process involves handling flammable and potentially explosive materials at high temperatures, necessitating robust safety measures and risk mitigation strategies.
A comprehensive hazard and operability (HAZOP) study is essential to identify potential risks associated with the dehydrogenation process. This analysis should cover all aspects of the operation, including reactor design, catalyst handling, temperature control, and product separation. Particular attention must be paid to the potential for runaway reactions, which could lead to catastrophic failures if not properly managed.
Implementing inherently safer design principles is crucial for minimizing risks. This includes selecting appropriate materials of construction that can withstand the corrosive nature of acetic acid and high operating temperatures. Redundant safety systems, such as emergency shutdown procedures and pressure relief devices, should be incorporated to prevent and mitigate potential incidents.
Process control systems play a vital role in maintaining safe operating conditions. Advanced control strategies, such as model predictive control, can help optimize process parameters while ensuring operation within safe limits. Real-time monitoring of key variables, including temperature, pressure, and reactant flow rates, is essential for early detection of deviations from normal operating conditions.
Employee training and competency management are fundamental to process safety. Operators and maintenance personnel must be thoroughly trained in safe operating procedures, emergency response protocols, and the proper use of personal protective equipment. Regular refresher courses and safety drills should be conducted to maintain a high level of preparedness.
Management of change (MOC) procedures must be rigorously implemented to assess the safety implications of any modifications to the process or equipment. This includes evaluating the potential impact on process safety when introducing new catalysts or altering operating conditions to improve yield or selectivity.
A robust mechanical integrity program is crucial for preventing equipment failures that could lead to safety incidents. This includes regular inspections, preventive maintenance, and corrosion monitoring, particularly for critical equipment such as reactors, heat exchangers, and storage tanks.
Emergency response planning is an integral part of risk management. Detailed emergency procedures should be developed and regularly practiced, covering scenarios such as fires, explosions, and chemical releases. Coordination with local emergency services is essential to ensure an effective response in the event of a major incident.
Continuous improvement in process safety should be pursued through incident investigation and learning from near-misses. A culture of safety must be fostered throughout the organization, encouraging open reporting of safety concerns and proactive identification of potential hazards.
A comprehensive hazard and operability (HAZOP) study is essential to identify potential risks associated with the dehydrogenation process. This analysis should cover all aspects of the operation, including reactor design, catalyst handling, temperature control, and product separation. Particular attention must be paid to the potential for runaway reactions, which could lead to catastrophic failures if not properly managed.
Implementing inherently safer design principles is crucial for minimizing risks. This includes selecting appropriate materials of construction that can withstand the corrosive nature of acetic acid and high operating temperatures. Redundant safety systems, such as emergency shutdown procedures and pressure relief devices, should be incorporated to prevent and mitigate potential incidents.
Process control systems play a vital role in maintaining safe operating conditions. Advanced control strategies, such as model predictive control, can help optimize process parameters while ensuring operation within safe limits. Real-time monitoring of key variables, including temperature, pressure, and reactant flow rates, is essential for early detection of deviations from normal operating conditions.
Employee training and competency management are fundamental to process safety. Operators and maintenance personnel must be thoroughly trained in safe operating procedures, emergency response protocols, and the proper use of personal protective equipment. Regular refresher courses and safety drills should be conducted to maintain a high level of preparedness.
Management of change (MOC) procedures must be rigorously implemented to assess the safety implications of any modifications to the process or equipment. This includes evaluating the potential impact on process safety when introducing new catalysts or altering operating conditions to improve yield or selectivity.
A robust mechanical integrity program is crucial for preventing equipment failures that could lead to safety incidents. This includes regular inspections, preventive maintenance, and corrosion monitoring, particularly for critical equipment such as reactors, heat exchangers, and storage tanks.
Emergency response planning is an integral part of risk management. Detailed emergency procedures should be developed and regularly practiced, covering scenarios such as fires, explosions, and chemical releases. Coordination with local emergency services is essential to ensure an effective response in the event of a major incident.
Continuous improvement in process safety should be pursued through incident investigation and learning from near-misses. A culture of safety must be fostered throughout the organization, encouraging open reporting of safety concerns and proactive identification of potential hazards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!