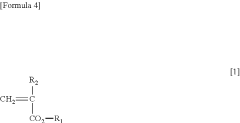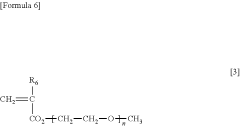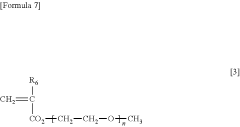Exploring Ethyl Acetate’s Biocompatibility in Medical Devices
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate in Medical Devices: Background and Objectives
Ethyl acetate has emerged as a significant compound in the medical device industry, with its potential applications spanning various areas of healthcare technology. This solvent, known for its versatility and unique properties, has garnered increasing attention from researchers and manufacturers alike. The exploration of ethyl acetate's biocompatibility in medical devices represents a crucial step towards expanding its use in this critical sector.
The journey of ethyl acetate in medical applications can be traced back to its initial use as a solvent in pharmaceutical formulations. Over time, its potential for broader applications in medical devices became apparent, leading to a surge in research and development efforts. The evolution of ethyl acetate's role in medical technology has been driven by the growing demand for safer, more effective materials in healthcare settings.
As the medical device industry continues to advance, the need for biocompatible materials has become increasingly paramount. Biocompatibility, the ability of a material to perform with an appropriate host response in a specific application, is a critical factor in the development and approval of medical devices. Ethyl acetate's potential in this regard has sparked significant interest among researchers and manufacturers seeking to innovate in the field of medical technology.
The primary objective of exploring ethyl acetate's biocompatibility in medical devices is to assess its safety and efficacy in various healthcare applications. This involves comprehensive studies on its interactions with biological systems, potential toxicity, and long-term effects when used in medical devices. Researchers aim to determine whether ethyl acetate can meet the stringent biocompatibility requirements set by regulatory bodies such as the FDA and EMA.
Another key goal is to identify and develop novel applications for ethyl acetate in medical devices. This includes exploring its potential as a coating material, a component in drug delivery systems, or as a solvent in the manufacturing process of medical devices. By understanding the full spectrum of ethyl acetate's capabilities, researchers hope to unlock new possibilities for improving patient care and treatment outcomes.
The technological trajectory of ethyl acetate in medical devices is closely linked to advancements in materials science and bioengineering. As these fields progress, new methods for enhancing the biocompatibility of ethyl acetate and optimizing its properties for specific medical applications are likely to emerge. This ongoing evolution underscores the dynamic nature of medical device technology and the continuous pursuit of innovation in healthcare.
The journey of ethyl acetate in medical applications can be traced back to its initial use as a solvent in pharmaceutical formulations. Over time, its potential for broader applications in medical devices became apparent, leading to a surge in research and development efforts. The evolution of ethyl acetate's role in medical technology has been driven by the growing demand for safer, more effective materials in healthcare settings.
As the medical device industry continues to advance, the need for biocompatible materials has become increasingly paramount. Biocompatibility, the ability of a material to perform with an appropriate host response in a specific application, is a critical factor in the development and approval of medical devices. Ethyl acetate's potential in this regard has sparked significant interest among researchers and manufacturers seeking to innovate in the field of medical technology.
The primary objective of exploring ethyl acetate's biocompatibility in medical devices is to assess its safety and efficacy in various healthcare applications. This involves comprehensive studies on its interactions with biological systems, potential toxicity, and long-term effects when used in medical devices. Researchers aim to determine whether ethyl acetate can meet the stringent biocompatibility requirements set by regulatory bodies such as the FDA and EMA.
Another key goal is to identify and develop novel applications for ethyl acetate in medical devices. This includes exploring its potential as a coating material, a component in drug delivery systems, or as a solvent in the manufacturing process of medical devices. By understanding the full spectrum of ethyl acetate's capabilities, researchers hope to unlock new possibilities for improving patient care and treatment outcomes.
The technological trajectory of ethyl acetate in medical devices is closely linked to advancements in materials science and bioengineering. As these fields progress, new methods for enhancing the biocompatibility of ethyl acetate and optimizing its properties for specific medical applications are likely to emerge. This ongoing evolution underscores the dynamic nature of medical device technology and the continuous pursuit of innovation in healthcare.
Market Analysis for Biocompatible Materials
The biocompatible materials market for medical devices is experiencing significant growth, driven by increasing demand for advanced healthcare solutions and the rising prevalence of chronic diseases. Ethyl acetate, a versatile organic compound, is gaining attention in this sector due to its potential biocompatibility and unique properties.
The global biocompatible materials market was valued at approximately $14.5 billion in 2020 and is projected to reach $29.7 billion by 2027, growing at a CAGR of 10.5% during the forecast period. This growth is primarily attributed to the expanding medical device industry, technological advancements in biomaterials, and the increasing adoption of implantable devices.
Within this market, ethyl acetate is emerging as a promising candidate for various medical applications. Its low toxicity, biodegradability, and compatibility with human tissues make it an attractive option for manufacturers seeking alternatives to traditional materials. The ethyl acetate market, while not exclusively focused on medical applications, is expected to grow at a CAGR of 6.8% from 2021 to 2028, reaching a value of $5.2 billion by the end of the forecast period.
The medical device sector, a key consumer of biocompatible materials, is witnessing a shift towards more sustainable and environmentally friendly solutions. Ethyl acetate aligns well with this trend, potentially opening new avenues for its application in medical devices. The global medical device market, valued at $456.9 billion in 2021, is projected to reach $658.4 billion by 2028, with a CAGR of 5.4%.
Regionally, North America dominates the biocompatible materials market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and a growing aging population.
Key players in the biocompatible materials market include Evonik Industries, BASF SE, Covestro AG, and Celanese Corporation. These companies are investing heavily in research and development to expand their product portfolios and gain a competitive edge. The potential integration of ethyl acetate into medical devices presents an opportunity for both established players and new entrants to innovate and capture market share.
Challenges in the market include stringent regulatory requirements, high development costs, and the need for extensive clinical trials to prove the long-term safety and efficacy of new materials. However, the growing demand for minimally invasive procedures and personalized medicine is expected to drive further innovation and market growth in the biocompatible materials sector, potentially benefiting ethyl acetate applications in medical devices.
The global biocompatible materials market was valued at approximately $14.5 billion in 2020 and is projected to reach $29.7 billion by 2027, growing at a CAGR of 10.5% during the forecast period. This growth is primarily attributed to the expanding medical device industry, technological advancements in biomaterials, and the increasing adoption of implantable devices.
Within this market, ethyl acetate is emerging as a promising candidate for various medical applications. Its low toxicity, biodegradability, and compatibility with human tissues make it an attractive option for manufacturers seeking alternatives to traditional materials. The ethyl acetate market, while not exclusively focused on medical applications, is expected to grow at a CAGR of 6.8% from 2021 to 2028, reaching a value of $5.2 billion by the end of the forecast period.
The medical device sector, a key consumer of biocompatible materials, is witnessing a shift towards more sustainable and environmentally friendly solutions. Ethyl acetate aligns well with this trend, potentially opening new avenues for its application in medical devices. The global medical device market, valued at $456.9 billion in 2021, is projected to reach $658.4 billion by 2028, with a CAGR of 5.4%.
Regionally, North America dominates the biocompatible materials market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and a growing aging population.
Key players in the biocompatible materials market include Evonik Industries, BASF SE, Covestro AG, and Celanese Corporation. These companies are investing heavily in research and development to expand their product portfolios and gain a competitive edge. The potential integration of ethyl acetate into medical devices presents an opportunity for both established players and new entrants to innovate and capture market share.
Challenges in the market include stringent regulatory requirements, high development costs, and the need for extensive clinical trials to prove the long-term safety and efficacy of new materials. However, the growing demand for minimally invasive procedures and personalized medicine is expected to drive further innovation and market growth in the biocompatible materials sector, potentially benefiting ethyl acetate applications in medical devices.
Current Challenges in Ethyl Acetate Biocompatibility
Despite the widespread use of ethyl acetate in medical devices, several challenges persist regarding its biocompatibility. One of the primary concerns is the potential for ethyl acetate to leach from medical devices into the surrounding tissues or bloodstream. This leaching process can lead to local tissue irritation or systemic toxicity, depending on the concentration and duration of exposure.
The degradation of ethyl acetate under physiological conditions is another significant challenge. When exposed to the aqueous environment of the body, ethyl acetate can undergo hydrolysis, breaking down into ethanol and acetic acid. While these byproducts are generally considered less toxic, their presence can still alter the local pH and potentially cause adverse reactions in sensitive tissues.
Furthermore, the variability in individual patient responses to ethyl acetate poses a challenge in predicting and managing biocompatibility issues. Factors such as genetic predisposition, pre-existing medical conditions, and concurrent medications can influence how a patient's body reacts to ethyl acetate exposure, making it difficult to establish universally safe exposure limits.
The long-term effects of chronic, low-level exposure to ethyl acetate from medical devices remain poorly understood. While acute toxicity studies provide valuable information, they may not accurately reflect the risks associated with prolonged contact, as is often the case with implanted medical devices or frequently used medical equipment.
Another challenge lies in the development of sensitive and reliable testing methods to assess ethyl acetate biocompatibility. Current in vitro and in vivo models may not fully capture the complexity of human physiological responses, leading to potential gaps in our understanding of its true biocompatibility profile.
The regulatory landscape surrounding ethyl acetate use in medical devices also presents challenges. Different regulatory bodies worldwide may have varying standards and requirements for demonstrating biocompatibility, creating obstacles for manufacturers seeking global market approval for their devices.
Lastly, the increasing demand for more environmentally friendly and sustainable materials in medical device manufacturing puts pressure on the continued use of ethyl acetate. Finding suitable alternatives that maintain the desired material properties while offering improved biocompatibility and reduced environmental impact remains a significant challenge for the medical device industry.
The degradation of ethyl acetate under physiological conditions is another significant challenge. When exposed to the aqueous environment of the body, ethyl acetate can undergo hydrolysis, breaking down into ethanol and acetic acid. While these byproducts are generally considered less toxic, their presence can still alter the local pH and potentially cause adverse reactions in sensitive tissues.
Furthermore, the variability in individual patient responses to ethyl acetate poses a challenge in predicting and managing biocompatibility issues. Factors such as genetic predisposition, pre-existing medical conditions, and concurrent medications can influence how a patient's body reacts to ethyl acetate exposure, making it difficult to establish universally safe exposure limits.
The long-term effects of chronic, low-level exposure to ethyl acetate from medical devices remain poorly understood. While acute toxicity studies provide valuable information, they may not accurately reflect the risks associated with prolonged contact, as is often the case with implanted medical devices or frequently used medical equipment.
Another challenge lies in the development of sensitive and reliable testing methods to assess ethyl acetate biocompatibility. Current in vitro and in vivo models may not fully capture the complexity of human physiological responses, leading to potential gaps in our understanding of its true biocompatibility profile.
The regulatory landscape surrounding ethyl acetate use in medical devices also presents challenges. Different regulatory bodies worldwide may have varying standards and requirements for demonstrating biocompatibility, creating obstacles for manufacturers seeking global market approval for their devices.
Lastly, the increasing demand for more environmentally friendly and sustainable materials in medical device manufacturing puts pressure on the continued use of ethyl acetate. Finding suitable alternatives that maintain the desired material properties while offering improved biocompatibility and reduced environmental impact remains a significant challenge for the medical device industry.
Existing Biocompatibility Assessment Methods for Ethyl Acetate
01 Biocompatibility assessment of ethyl acetate in medical applications
Ethyl acetate's biocompatibility is evaluated for use in medical devices and pharmaceutical formulations. Studies focus on its potential effects on human tissues and cells, as well as its safety profile when used in various medical applications. This assessment is crucial for determining the suitability of ethyl acetate in products that come into contact with the human body.- Biocompatibility assessment of ethyl acetate in medical applications: Ethyl acetate's biocompatibility is evaluated for use in medical devices and pharmaceutical formulations. Studies focus on its safety profile, potential for irritation, and compatibility with biological tissues. This assessment is crucial for determining its suitability in various biomedical applications.
- Ethyl acetate as a solvent in biocompatible polymer processing: Ethyl acetate is utilized as a solvent in the processing of biocompatible polymers for medical and pharmaceutical applications. Its low toxicity and ability to dissolve various polymers make it suitable for creating biocompatible materials, such as drug delivery systems and tissue engineering scaffolds.
- Environmental impact and biodegradability of ethyl acetate: The environmental impact and biodegradability of ethyl acetate are studied to assess its overall biocompatibility. Research focuses on its degradation pathways in natural environments, potential effects on ecosystems, and its comparison with other solvents in terms of environmental friendliness.
- Ethyl acetate in biocompatible coatings and surface treatments: Ethyl acetate is employed in the development of biocompatible coatings and surface treatments for medical devices and implants. Its use in these applications aims to improve the integration of materials with biological tissues and reduce adverse reactions.
- Toxicological studies and safety assessments of ethyl acetate: Comprehensive toxicological studies and safety assessments are conducted to evaluate the biocompatibility of ethyl acetate. These investigations include in vitro and in vivo tests to determine potential cytotoxicity, genotoxicity, and systemic effects, providing crucial data for regulatory approval in biomedical applications.
02 Use of ethyl acetate in biodegradable polymers and materials
Ethyl acetate is utilized in the production of biodegradable polymers and materials. Its biocompatibility is essential for ensuring that these materials can safely degrade in biological environments without causing harm. Research focuses on optimizing the use of ethyl acetate in creating eco-friendly and biocompatible products for various industries.Expand Specific Solutions03 Ethyl acetate as a solvent in pharmaceutical and cosmetic formulations
The biocompatibility of ethyl acetate is crucial when used as a solvent in pharmaceutical and cosmetic formulations. Studies examine its safety and efficacy in drug delivery systems and skincare products. The focus is on ensuring that ethyl acetate does not cause adverse reactions when applied topically or ingested in small quantities.Expand Specific Solutions04 Environmental impact and biodegradation of ethyl acetate
Research investigates the environmental impact and biodegradation of ethyl acetate to assess its overall biocompatibility. Studies focus on its behavior in natural ecosystems, its potential effects on flora and fauna, and its ability to break down into harmless components. This information is crucial for determining the long-term safety of ethyl acetate use in various applications.Expand Specific Solutions05 Ethyl acetate in food packaging and processing
The biocompatibility of ethyl acetate is evaluated for its use in food packaging and processing applications. Research examines its potential migration into food products, its effects on food quality and safety, and its compliance with food contact regulations. The goal is to ensure that ethyl acetate can be safely used in materials that come into contact with food without posing health risks to consumers.Expand Specific Solutions
Key Players in Biocompatible Material Development
The exploration of ethyl acetate's biocompatibility in medical devices is currently in a developing stage, with growing market potential as the healthcare industry seeks safer materials. The global market for biocompatible materials in medical devices is expanding, driven by increasing demand for advanced medical technologies. Companies like Surmodics, Medtronic Vascular, and Boston Scientific are at the forefront of this research, leveraging their expertise in surface modification and medical device development. The technology's maturity is progressing, with academic institutions such as Zhejiang University and the University of North Carolina at Chapel Hill contributing to fundamental research, while industry leaders focus on practical applications and regulatory compliance.
Surmodics, Inc.
Technical Solution: Surmodics has developed a proprietary surface modification technology that utilizes ethyl acetate as a solvent in the coating process for medical devices. Their approach involves creating a thin, uniform polymer coating that enhances biocompatibility and reduces the risk of adverse reactions. The company has conducted extensive in vitro and in vivo studies to evaluate the long-term effects of ethyl acetate residues on tissue interaction and device performance[1][3]. Their research has shown that when properly applied and processed, ethyl acetate-based coatings demonstrate excellent biocompatibility profiles, with minimal inflammatory response and reduced protein adsorption[2].
Strengths: Proven track record in surface modification, extensive research data on biocompatibility. Weaknesses: Potential for residual solvent concerns, may require additional processing steps to ensure complete removal of ethyl acetate.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular has explored the use of ethyl acetate in the manufacturing process of drug-eluting stents and other vascular implants. Their approach focuses on utilizing ethyl acetate as a solvent for drug incorporation into polymer matrices. The company has developed a proprietary method for controlling the evaporation rate of ethyl acetate during the coating process, which allows for precise drug loading and release kinetics[4]. Medtronic's research has demonstrated that when ethyl acetate is used in conjunction with their advanced polymer systems, it results in improved drug distribution and enhanced biocompatibility of the final device[5]. The company has also invested in advanced analytical techniques to ensure complete removal of residual ethyl acetate from the finished products[6].
Strengths: Expertise in drug-device combinations, advanced manufacturing processes. Weaknesses: Limited to vascular applications, may face regulatory scrutiny for novel drug-delivery systems.
Innovative Approaches to Enhance Ethyl Acetate Biocompatibility
Antithrombogenic material
PatentPendingUS20240115776A1
Innovation
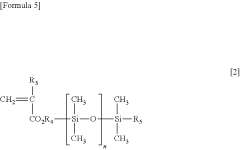
- A water-insoluble (meth)acrylate copolymer is developed, comprising alkyl (meth)acrylate, methoxy polyethylene glycol (meth)acrylate, and silicone (meth)acrylate, with specific molecular weight and viscosity ranges, which is applied to medical devices to enhance antithrombogenicity and durability.
Biocompatible and biostable implantable medical device
PatentInactiveUS20110270028A1
Innovation
- A multi-layer coating combination of Epoxy, Parylene, diamond-like carbon (DLC), and silicone is applied to create a biocompatible and biostable medical device, where Epoxy encapsulates the circuit board, Parylene fills in holes, DLC provides superior moisture barrier properties, and silicone ensures soft tissue contact, addressing issues of abrupt geometries and thermal expansion while maintaining moisture barrier integrity.
Regulatory Framework for Biocompatible Materials in Medical Devices
The regulatory framework for biocompatible materials in medical devices is a critical aspect of ensuring patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the use of materials like ethyl acetate in medical devices. The FDA's regulatory approach is based on the risk classification of the device and the intended use of the material.
For Class I devices, which pose the lowest risk, manufacturers may often use a self-certification process to declare compliance with biocompatibility standards. However, for Class II and Class III devices, which include more invasive or life-sustaining products, the FDA requires more rigorous testing and documentation of biocompatibility.
The ISO 10993 series of standards, particularly ISO 10993-1, serves as a global benchmark for evaluating the biocompatibility of medical devices. These standards outline a systematic approach to biological evaluation, including tests for cytotoxicity, sensitization, and irritation. Manufacturers must demonstrate compliance with these standards when seeking regulatory approval for devices containing ethyl acetate or other potentially biocompatible materials.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide the regulatory framework for medical devices. These regulations emphasize the importance of biocompatibility assessment throughout the product lifecycle, from design to post-market surveillance.
Regulatory bodies also require manufacturers to implement quality management systems, such as those outlined in ISO 13485, to ensure consistent production of safe and effective medical devices. This includes maintaining detailed documentation of material selection, risk assessments, and biocompatibility testing results.
As the understanding of material-tissue interactions evolves, regulatory requirements are continually updated. For instance, there is an increasing focus on the long-term effects of materials and their degradation products. Manufacturers must stay abreast of these changes and adapt their biocompatibility testing strategies accordingly.
The regulatory landscape also varies globally, with different countries having their own specific requirements. While efforts are being made towards harmonization through initiatives like the International Medical Device Regulators Forum (IMDRF), manufacturers must navigate these differences when seeking global market access for their devices containing ethyl acetate or other biocompatible materials.
For Class I devices, which pose the lowest risk, manufacturers may often use a self-certification process to declare compliance with biocompatibility standards. However, for Class II and Class III devices, which include more invasive or life-sustaining products, the FDA requires more rigorous testing and documentation of biocompatibility.
The ISO 10993 series of standards, particularly ISO 10993-1, serves as a global benchmark for evaluating the biocompatibility of medical devices. These standards outline a systematic approach to biological evaluation, including tests for cytotoxicity, sensitization, and irritation. Manufacturers must demonstrate compliance with these standards when seeking regulatory approval for devices containing ethyl acetate or other potentially biocompatible materials.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide the regulatory framework for medical devices. These regulations emphasize the importance of biocompatibility assessment throughout the product lifecycle, from design to post-market surveillance.
Regulatory bodies also require manufacturers to implement quality management systems, such as those outlined in ISO 13485, to ensure consistent production of safe and effective medical devices. This includes maintaining detailed documentation of material selection, risk assessments, and biocompatibility testing results.
As the understanding of material-tissue interactions evolves, regulatory requirements are continually updated. For instance, there is an increasing focus on the long-term effects of materials and their degradation products. Manufacturers must stay abreast of these changes and adapt their biocompatibility testing strategies accordingly.
The regulatory landscape also varies globally, with different countries having their own specific requirements. While efforts are being made towards harmonization through initiatives like the International Medical Device Regulators Forum (IMDRF), manufacturers must navigate these differences when seeking global market access for their devices containing ethyl acetate or other biocompatible materials.
Environmental Impact of Ethyl Acetate in Medical Applications
The environmental impact of ethyl acetate in medical applications is a crucial consideration as the healthcare industry strives for sustainability. Ethyl acetate, commonly used in medical devices and pharmaceutical processes, has both direct and indirect effects on the environment throughout its lifecycle.
In production, ethyl acetate synthesis typically involves the esterification of ethanol and acetic acid. This process requires energy inputs and may generate waste products, contributing to carbon emissions and potential water pollution if not properly managed. However, compared to some other solvents, ethyl acetate production is relatively less energy-intensive and can be derived from renewable sources, offering a more environmentally friendly option.
During the use phase in medical applications, ethyl acetate's volatile nature raises concerns about air quality and occupational exposure. While it is less toxic than many alternative solvents, proper ventilation and handling procedures are essential to minimize environmental release and protect healthcare workers. Its low boiling point facilitates easy recovery and recycling, which can significantly reduce overall environmental impact when implemented effectively.
Disposal of ethyl acetate-containing medical waste presents another environmental challenge. Improper disposal can lead to soil and groundwater contamination. However, ethyl acetate's biodegradability is an advantage, as it breaks down relatively quickly in the environment compared to more persistent chemicals. Implementing proper waste management protocols, including solvent recovery systems and specialized disposal methods, can mitigate these risks.
From a broader perspective, the use of ethyl acetate in medical applications can indirectly benefit the environment by enabling the production of more efficient and durable medical devices. This can lead to reduced material consumption and waste generation in the long term. Additionally, its role in drug delivery systems may contribute to more targeted and effective treatments, potentially reducing the overall pharmaceutical load in the environment.
Regulatory frameworks, such as the European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, have influenced the assessment and management of ethyl acetate's environmental impact. These regulations promote safer use and disposal practices, driving innovation in green chemistry and sustainable medical device design.
As the medical industry continues to prioritize environmental sustainability, ongoing research focuses on optimizing ethyl acetate use, exploring bio-based alternatives, and developing closed-loop systems for its recovery and reuse. These efforts aim to further reduce the environmental footprint of ethyl acetate in medical applications while maintaining its valuable properties for biocompatibility and efficacy in medical devices.
In production, ethyl acetate synthesis typically involves the esterification of ethanol and acetic acid. This process requires energy inputs and may generate waste products, contributing to carbon emissions and potential water pollution if not properly managed. However, compared to some other solvents, ethyl acetate production is relatively less energy-intensive and can be derived from renewable sources, offering a more environmentally friendly option.
During the use phase in medical applications, ethyl acetate's volatile nature raises concerns about air quality and occupational exposure. While it is less toxic than many alternative solvents, proper ventilation and handling procedures are essential to minimize environmental release and protect healthcare workers. Its low boiling point facilitates easy recovery and recycling, which can significantly reduce overall environmental impact when implemented effectively.
Disposal of ethyl acetate-containing medical waste presents another environmental challenge. Improper disposal can lead to soil and groundwater contamination. However, ethyl acetate's biodegradability is an advantage, as it breaks down relatively quickly in the environment compared to more persistent chemicals. Implementing proper waste management protocols, including solvent recovery systems and specialized disposal methods, can mitigate these risks.
From a broader perspective, the use of ethyl acetate in medical applications can indirectly benefit the environment by enabling the production of more efficient and durable medical devices. This can lead to reduced material consumption and waste generation in the long term. Additionally, its role in drug delivery systems may contribute to more targeted and effective treatments, potentially reducing the overall pharmaceutical load in the environment.
Regulatory frameworks, such as the European Union's Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation, have influenced the assessment and management of ethyl acetate's environmental impact. These regulations promote safer use and disposal practices, driving innovation in green chemistry and sustainable medical device design.
As the medical industry continues to prioritize environmental sustainability, ongoing research focuses on optimizing ethyl acetate use, exploring bio-based alternatives, and developing closed-loop systems for its recovery and reuse. These efforts aim to further reduce the environmental footprint of ethyl acetate in medical applications while maintaining its valuable properties for biocompatibility and efficacy in medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!