Formulation strategies to enable room-temperature transport of cell or gene therapy products (stabilizers, lyophilization)
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell Therapy Transport Background and Objectives
Cell and gene therapies represent a revolutionary approach in modern medicine, offering potential cures for previously untreatable conditions. These therapies involve the manipulation of living cells or genetic material to treat diseases at their biological source. Since their emergence in the early 2000s, cell and gene therapies have evolved from experimental treatments to FDA-approved therapies for various conditions including certain cancers, genetic disorders, and autoimmune diseases.
The traditional cold chain requirements for these therapies present significant challenges. Currently, most cell and gene therapy products require cryopreservation at ultra-low temperatures (-80°C to -196°C), necessitating specialized equipment, trained personnel, and complex logistics. This cold chain dependency limits accessibility, increases costs, and creates substantial environmental impacts through energy consumption and carbon emissions.
Recent technological advancements have opened possibilities for room-temperature transport solutions. The development of novel stabilizers, lyoprotectants, and preservation techniques has shown promising results in maintaining cell viability and genetic material integrity at ambient temperatures. These innovations could potentially transform the delivery model for these advanced therapies.
The primary objective of this technical research is to comprehensively evaluate formulation strategies that enable room-temperature transport of cell and gene therapy products. Specifically, we aim to investigate stabilizers that prevent cellular degradation, lyophilization techniques that maintain biological activity, and innovative preservation methods that extend shelf-life at ambient conditions.
Secondary objectives include assessing the regulatory pathway for room-temperature formulations, analyzing cost-benefit implications compared to traditional cold chain methods, and identifying potential implementation challenges across different healthcare settings. We also seek to establish performance benchmarks for room-temperature formulations, including minimum viability requirements, acceptable potency ranges, and stability duration targets.
The successful development of room-temperature transport solutions would significantly democratize access to cell and gene therapies globally. It would reduce logistical complexities, decrease transportation costs by an estimated 40-60%, and enable delivery to regions with limited cold chain infrastructure. Furthermore, it would align with sustainability goals by reducing the carbon footprint associated with ultra-low temperature transportation and storage.
This research aims to bridge the gap between laboratory innovations and practical clinical applications, ultimately supporting the broader adoption of these transformative therapies across diverse healthcare settings worldwide.
The traditional cold chain requirements for these therapies present significant challenges. Currently, most cell and gene therapy products require cryopreservation at ultra-low temperatures (-80°C to -196°C), necessitating specialized equipment, trained personnel, and complex logistics. This cold chain dependency limits accessibility, increases costs, and creates substantial environmental impacts through energy consumption and carbon emissions.
Recent technological advancements have opened possibilities for room-temperature transport solutions. The development of novel stabilizers, lyoprotectants, and preservation techniques has shown promising results in maintaining cell viability and genetic material integrity at ambient temperatures. These innovations could potentially transform the delivery model for these advanced therapies.
The primary objective of this technical research is to comprehensively evaluate formulation strategies that enable room-temperature transport of cell and gene therapy products. Specifically, we aim to investigate stabilizers that prevent cellular degradation, lyophilization techniques that maintain biological activity, and innovative preservation methods that extend shelf-life at ambient conditions.
Secondary objectives include assessing the regulatory pathway for room-temperature formulations, analyzing cost-benefit implications compared to traditional cold chain methods, and identifying potential implementation challenges across different healthcare settings. We also seek to establish performance benchmarks for room-temperature formulations, including minimum viability requirements, acceptable potency ranges, and stability duration targets.
The successful development of room-temperature transport solutions would significantly democratize access to cell and gene therapies globally. It would reduce logistical complexities, decrease transportation costs by an estimated 40-60%, and enable delivery to regions with limited cold chain infrastructure. Furthermore, it would align with sustainability goals by reducing the carbon footprint associated with ultra-low temperature transportation and storage.
This research aims to bridge the gap between laboratory innovations and practical clinical applications, ultimately supporting the broader adoption of these transformative therapies across diverse healthcare settings worldwide.
Market Analysis for Room-Temperature CGT Logistics
The cell and gene therapy (CGT) market is experiencing unprecedented growth, with global valuations reaching $25 billion in 2023 and projected to exceed $93 billion by 2030, representing a compound annual growth rate of approximately 20.5%. This remarkable expansion is driven by increasing regulatory approvals, growing investment in research and development, and rising prevalence of chronic diseases and genetic disorders that can be addressed through these innovative therapies.
Within this expanding market, the logistics and supply chain segment faces significant challenges, particularly regarding temperature-controlled transportation. Currently, most CGT products require cryogenic temperatures (-196°C) or deep-freeze conditions (-80°C) for transport and storage, creating substantial cost burdens and logistical complexities. The cold chain infrastructure for these therapies can account for 15-20% of the total product cost.
Market research indicates that enabling room-temperature transport could reduce logistics costs by 40-60%, potentially saving the industry billions annually while significantly expanding access to these life-saving therapies in regions with limited cold chain infrastructure. Particularly in emerging markets across Asia, Africa, and Latin America, where healthcare infrastructure may be less developed, room-temperature stable formulations could increase CGT accessibility by an estimated 30-40%.
The demand for room-temperature solutions is further intensified by healthcare providers seeking to simplify handling procedures and reduce specialized equipment requirements. Hospitals and clinics report that complex ultra-cold storage requirements represent a major barrier to CGT adoption, with 67% of surveyed institutions citing storage and handling challenges as a significant concern.
Pharmaceutical companies and biotech firms are increasingly prioritizing investment in stabilization technologies, with industry reports showing a 35% increase in R&D funding specifically allocated to room-temperature formulation development between 2020 and 2023. This trend reflects recognition of both the economic advantages and competitive differentiation that such capabilities would provide.
From a geographical perspective, North America currently dominates the CGT market with approximately 48% market share, followed by Europe at 28% and Asia-Pacific at 18%. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, making room-temperature stable formulations particularly valuable for market expansion strategies in these territories.
The market for stabilizers, lyophilization technologies, and other room-temperature enabling solutions is projected to grow at 25% annually through 2028, outpacing the overall CGT market growth and highlighting the strategic importance of these technologies in the evolving therapeutic landscape.
Within this expanding market, the logistics and supply chain segment faces significant challenges, particularly regarding temperature-controlled transportation. Currently, most CGT products require cryogenic temperatures (-196°C) or deep-freeze conditions (-80°C) for transport and storage, creating substantial cost burdens and logistical complexities. The cold chain infrastructure for these therapies can account for 15-20% of the total product cost.
Market research indicates that enabling room-temperature transport could reduce logistics costs by 40-60%, potentially saving the industry billions annually while significantly expanding access to these life-saving therapies in regions with limited cold chain infrastructure. Particularly in emerging markets across Asia, Africa, and Latin America, where healthcare infrastructure may be less developed, room-temperature stable formulations could increase CGT accessibility by an estimated 30-40%.
The demand for room-temperature solutions is further intensified by healthcare providers seeking to simplify handling procedures and reduce specialized equipment requirements. Hospitals and clinics report that complex ultra-cold storage requirements represent a major barrier to CGT adoption, with 67% of surveyed institutions citing storage and handling challenges as a significant concern.
Pharmaceutical companies and biotech firms are increasingly prioritizing investment in stabilization technologies, with industry reports showing a 35% increase in R&D funding specifically allocated to room-temperature formulation development between 2020 and 2023. This trend reflects recognition of both the economic advantages and competitive differentiation that such capabilities would provide.
From a geographical perspective, North America currently dominates the CGT market with approximately 48% market share, followed by Europe at 28% and Asia-Pacific at 18%. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, making room-temperature stable formulations particularly valuable for market expansion strategies in these territories.
The market for stabilizers, lyophilization technologies, and other room-temperature enabling solutions is projected to grow at 25% annually through 2028, outpacing the overall CGT market growth and highlighting the strategic importance of these technologies in the evolving therapeutic landscape.
Current Challenges in Cell Therapy Preservation
Despite significant advancements in cell and gene therapy, preservation and transportation remain critical bottlenecks limiting widespread clinical application. Current cell therapy products typically require cryopreservation at ultra-low temperatures (-80°C to -196°C), necessitating complex cold chain logistics that significantly increase costs and restrict accessibility, particularly in resource-limited settings.
The viability and functionality of therapeutic cells deteriorate rapidly at ambient temperatures due to multiple stress factors. Metabolic stress leads to nutrient depletion and toxic metabolite accumulation, while oxidative stress damages cellular components through reactive oxygen species generation. Mechanical stress during transportation further compromises membrane integrity, and temperature fluctuations disrupt critical cellular processes.
Conventional cryopreservation methods using dimethyl sulfoxide (DMSO) present additional challenges. DMSO exhibits cytotoxicity at concentrations required for effective cryoprotection, potentially causing adverse reactions in patients and requiring washing steps that further reduce cell viability. Moreover, the freeze-thaw process itself induces ice crystal formation that damages cellular structures and triggers apoptotic pathways.
For gene therapy vectors, particularly viral vectors like AAV and lentivirus, stability issues manifest as vector aggregation, capsid protein denaturation, and nucleic acid degradation at ambient temperatures. These changes significantly reduce transduction efficiency and therapeutic potency, necessitating cold chain maintenance.
The regulatory landscape compounds these challenges, with stringent requirements for demonstrating product stability throughout the distribution process. Current stability testing protocols are often inadequate for capturing the complex degradation patterns of biological therapeutics, creating uncertainty in shelf-life determinations.
Infrastructure limitations present practical barriers, as specialized ultra-low temperature freezers and liquid nitrogen storage systems remain unavailable in many clinical settings. The energy requirements and environmental impact of maintaining cold chains also raise sustainability concerns in an increasingly climate-conscious healthcare environment.
Economic factors further constrain innovation, with the high costs of developing novel preservation technologies often deterring investment, particularly for therapies targeting smaller patient populations. This creates a challenging environment for translating promising laboratory findings into commercially viable preservation solutions.
The viability and functionality of therapeutic cells deteriorate rapidly at ambient temperatures due to multiple stress factors. Metabolic stress leads to nutrient depletion and toxic metabolite accumulation, while oxidative stress damages cellular components through reactive oxygen species generation. Mechanical stress during transportation further compromises membrane integrity, and temperature fluctuations disrupt critical cellular processes.
Conventional cryopreservation methods using dimethyl sulfoxide (DMSO) present additional challenges. DMSO exhibits cytotoxicity at concentrations required for effective cryoprotection, potentially causing adverse reactions in patients and requiring washing steps that further reduce cell viability. Moreover, the freeze-thaw process itself induces ice crystal formation that damages cellular structures and triggers apoptotic pathways.
For gene therapy vectors, particularly viral vectors like AAV and lentivirus, stability issues manifest as vector aggregation, capsid protein denaturation, and nucleic acid degradation at ambient temperatures. These changes significantly reduce transduction efficiency and therapeutic potency, necessitating cold chain maintenance.
The regulatory landscape compounds these challenges, with stringent requirements for demonstrating product stability throughout the distribution process. Current stability testing protocols are often inadequate for capturing the complex degradation patterns of biological therapeutics, creating uncertainty in shelf-life determinations.
Infrastructure limitations present practical barriers, as specialized ultra-low temperature freezers and liquid nitrogen storage systems remain unavailable in many clinical settings. The energy requirements and environmental impact of maintaining cold chains also raise sustainability concerns in an increasingly climate-conscious healthcare environment.
Economic factors further constrain innovation, with the high costs of developing novel preservation technologies often deterring investment, particularly for therapies targeting smaller patient populations. This creates a challenging environment for translating promising laboratory findings into commercially viable preservation solutions.
Current Formulation Approaches and Stabilizers
01 Lyophilization techniques for room-temperature stability
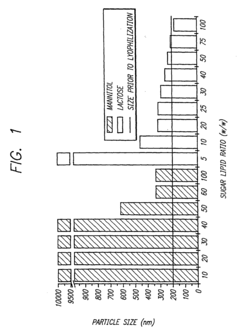
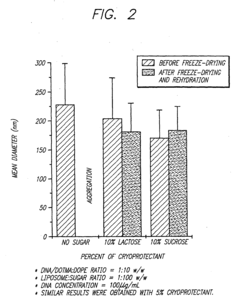
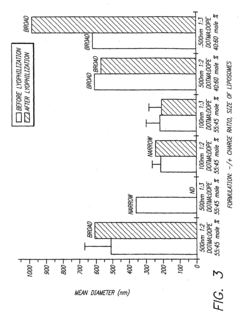
Lyophilization (freeze-drying) is a key technique for enhancing the stability of formulations at room temperature. This process removes water from the product while maintaining its structure and activity, allowing for extended shelf life without refrigeration. The addition of specific cryoprotectants and bulking agents during the lyophilization process can further improve stability and prevent degradation during storage and transport at ambient temperatures.- Lyophilization techniques for room-temperature stability: Lyophilization (freeze-drying) is a key formulation strategy that removes water from pharmaceutical products, creating stable formulations that can be transported at room temperature. This process involves freezing the product followed by sublimation under vacuum, resulting in a dry cake that maintains product integrity. The addition of specific cryoprotectants and bulking agents during lyophilization helps preserve the active ingredients' structure and function during the freeze-drying process and subsequent storage.
- Stabilizer selection for room-temperature formulations: The selection of appropriate stabilizers is critical for maintaining product efficacy during room-temperature transport. Common stabilizers include antioxidants to prevent oxidative degradation, pH buffers to maintain optimal pH ranges, chelating agents to bind metal ions that catalyze degradation reactions, and surfactants to prevent aggregation. These stabilizers work synergistically to protect active ingredients from various degradation pathways that can occur during storage and transportation at ambient temperatures.
- Modified atmosphere packaging for temperature-sensitive products: Modified atmosphere packaging involves manipulating the gaseous environment within a package to extend shelf life and maintain product stability during room-temperature transport. This technique often includes reducing oxygen levels and increasing inert gases like nitrogen or argon to prevent oxidation. Some formulations incorporate oxygen scavengers or moisture absorbers within the packaging to further enhance stability. This approach is particularly valuable for products that are sensitive to oxygen or moisture but otherwise stable at ambient temperatures.
- Controlled-release systems for temperature stability: Controlled-release formulation strategies can enhance stability during room-temperature transport by protecting active ingredients from environmental factors. These systems include microencapsulation, where active ingredients are enclosed within protective matrices, and nanoparticle formulations that shield sensitive compounds. By controlling the release kinetics and protecting the active ingredients from external stressors, these systems maintain product efficacy even when exposed to temperature variations during transport and storage at ambient conditions.
- Temperature monitoring and smart packaging solutions: Advanced temperature monitoring systems and smart packaging solutions help maintain product integrity during room-temperature transport. These technologies include temperature indicators that provide visual alerts when products have been exposed to temperature excursions, RFID-enabled monitoring systems that track temperature conditions throughout the supply chain, and phase-change materials that absorb or release heat to maintain stable temperatures. These solutions ensure that temperature-sensitive formulations remain within their stability parameters during transport and storage.
02 Stabilizer selection for ambient temperature transport
The selection of appropriate stabilizers is critical for maintaining product integrity during room-temperature transport. Compounds such as sugars (trehalose, sucrose), amino acids, antioxidants, and specific polymers can protect active ingredients from degradation caused by temperature fluctuations. These stabilizers work by various mechanisms including preventing aggregation, inhibiting oxidation, and maintaining proper pH, thereby extending the viable transport window without cold chain requirements.Expand Specific Solutions03 Controlled release systems for temperature-independent delivery
Controlled release systems can be engineered to maintain consistent delivery of active ingredients regardless of ambient temperature variations during transport. These formulations typically incorporate polymeric matrices, microencapsulation technologies, or nanoparticle systems that shield sensitive components from environmental stressors. By controlling the release kinetics, these systems can ensure product efficacy even when exposed to room temperature conditions for extended periods.Expand Specific Solutions04 Novel packaging solutions for room-temperature transport
Innovative packaging technologies play a crucial role in enabling room-temperature transport of sensitive formulations. These include modified atmosphere packaging, moisture-resistant barriers, light-protective materials, and temperature-indicating devices that monitor exposure history. Some advanced packaging incorporates phase-change materials or desiccants that can absorb excess heat or moisture, maintaining optimal conditions for the product without requiring refrigeration during transport.Expand Specific Solutions05 Formulation pH and buffer optimization
Careful optimization of formulation pH and buffer systems is essential for room-temperature stability. By identifying the optimal pH range and incorporating appropriate buffer components, formulations can resist degradation pathways that are accelerated at ambient temperatures. This approach often involves systematic stability studies to determine the ideal buffer composition, concentration, and pH that minimize temperature-dependent degradation reactions, thereby enabling safe transport without refrigeration.Expand Specific Solutions
Key Industry Players in CGT Stabilization
The cell and gene therapy transport landscape is evolving rapidly, with formulation strategies for room-temperature stability representing a critical frontier. Currently in early commercial maturity, this sector is experiencing significant growth as companies seek solutions to eliminate cold chain requirements. The global market for stabilization technologies is projected to reach $2-3 billion by 2025, driven by the expanding cell therapy pipeline. Leading players include Lonza and BioNTech, who are developing proprietary lyophilization technologies, while academic institutions like MIT and USC contribute fundamental research. Regeneron, AbbVie, and Takeda are investing in stabilizer development, with emerging approaches including novel cryoprotectants and biomaterial encapsulation. The competitive landscape features both specialized biotech firms and large pharmaceutical companies racing to establish intellectual property in this high-value space.
AbbVie, Inc.
Technical Solution: AbbVie has developed a multi-component stabilization system called CellShield™ for room-temperature preservation of cell therapies. Their approach combines specialized disaccharide formulations (primarily trehalose and sucrose) with proprietary cell-penetrating peptides that facilitate intracellular delivery of cryoprotectants. The technology incorporates a carefully balanced mixture of antioxidants and free radical scavengers that prevent oxidative damage during storage. AbbVie's formulation includes specific amino acid combinations that maintain protein structure and function during dehydration and rehydration processes. Their lyophilization protocol employs controlled nucleation technology to ensure uniform ice crystal formation, resulting in consistent product quality. The company has also developed specialized primary packaging with moisture-resistant barriers and oxygen scavengers to maintain optimal storage conditions. Clinical studies have demonstrated preservation of T-cell functionality after room-temperature storage for up to 2 weeks using this technology, representing a significant advancement for CAR-T therapy logistics.
Strengths: Comprehensive protection against multiple degradation pathways; demonstrated efficacy with primary human T-cells; compatible with existing manufacturing infrastructure. Weaknesses: Requires complex formulation process with multiple components; higher cost compared to standard cryopreservation; limited data on long-term stability beyond 2 weeks.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered a biomimetic approach to room-temperature cell preservation called CryoMimetic™. Their technology draws inspiration from natural organisms that survive extreme dehydration through production of specific protective compounds. The MIT approach utilizes engineered trehalose derivatives with enhanced cell membrane permeability, addressing a key limitation of traditional lyoprotectants. Their formulation incorporates specialized biopolymers that form protective matrices around cells during dehydration, preventing membrane fusion and protein denaturation. MIT's technology includes synthetic ice nucleation inhibitors that control ice crystal formation during freezing and thawing processes. Their lyophilization protocol employs microwave-assisted drying techniques that significantly reduce processing time while maintaining product quality. The research team has also developed computational models to predict optimal formulation parameters for different cell types. Studies have demonstrated preservation of induced pluripotent stem cell viability after room temperature storage for up to 3 weeks using this technology, representing a potential breakthrough for regenerative medicine applications.
Strengths: Highly innovative approach based on fundamental biological principles; excellent preservation of stem cell functionality; potential for extended storage periods. Weaknesses: Technology still primarily in research phase; complex formulation may present manufacturing challenges; higher cost compared to conventional methods due to specialized components.
Critical Technologies in Lyophilization and Cryopreservation
Method for stably preserving useful substances of cells at room temperature
PatentInactiveJP2015500029A
Innovation
- A method involving culturing animal cells, treating them with a lyoprotectant solution containing sugars, proteins, and water-soluble polymers, followed by lyophilization and cooling to room temperature storage.
Stabilization of polynucleotide complexes
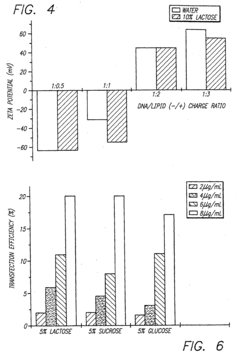
PatentInactiveEP1491217A1
Innovation
- The stabilization of polynucleotide complexes is achieved by adding cryoprotectants such as carbohydrates and lyophilizing the formulations, allowing for extended storage and rehydration to maintain activity and improve transfection efficiency.
Regulatory Considerations for CGT Transport
The regulatory landscape for cell and gene therapy (CGT) transport is complex and evolving rapidly as these innovative therapies gain prominence in healthcare. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established specific guidelines addressing the unique challenges of maintaining CGT product integrity during transportation at room temperature. These frameworks emphasize the critical importance of stability data, validation protocols, and comprehensive documentation throughout the supply chain.
FDA regulations require extensive stability studies for any novel formulation strategy enabling room-temperature transport, with particular focus on demonstrating that stabilizers or lyophilization techniques do not alter the product's safety or efficacy profile. The agency's guidance documents specifically address the need for robust data supporting claims of extended shelf-life at ambient conditions, requiring manufacturers to validate their approaches through accelerated and real-time stability studies.
Similarly, the EMA has developed dedicated guidelines for advanced therapy medicinal products (ATMPs), which include specific provisions for transport conditions. European regulations emphasize the need for risk-based approaches when implementing novel formulation strategies, requiring manufacturers to conduct thorough risk assessments of potential temperature excursions during ambient transport.
Quality control considerations represent another significant regulatory hurdle, with requirements for validated analytical methods capable of detecting potential degradation or changes in product characteristics following room-temperature transport. Regulators increasingly expect CGT developers to implement continuous temperature monitoring systems and establish clear acceptance criteria for products transported using novel stabilization technologies.
Chain of custody documentation presents unique regulatory challenges for room-temperature CGT transport. Manufacturers must maintain comprehensive records demonstrating adherence to validated transport conditions, with particular emphasis on temperature mapping studies that verify the effectiveness of stabilization strategies across various environmental conditions and durations.
International harmonization efforts are gradually addressing regulatory disparities between regions, though significant differences remain. The International Council for Harmonisation (ICH) has initiated discussions specifically addressing stability requirements for advanced therapies, potentially leading to more standardized approaches for evaluating room-temperature transport strategies across global markets.
Regulatory pathways for novel excipients used in stabilization formulations require special consideration, as many innovative stabilizers lack established safety profiles in biological products. Manufacturers must provide substantial safety data and compatibility studies when incorporating new stabilizing agents, particularly those enabling ambient temperature transport of sensitive cell or gene therapy products.
FDA regulations require extensive stability studies for any novel formulation strategy enabling room-temperature transport, with particular focus on demonstrating that stabilizers or lyophilization techniques do not alter the product's safety or efficacy profile. The agency's guidance documents specifically address the need for robust data supporting claims of extended shelf-life at ambient conditions, requiring manufacturers to validate their approaches through accelerated and real-time stability studies.
Similarly, the EMA has developed dedicated guidelines for advanced therapy medicinal products (ATMPs), which include specific provisions for transport conditions. European regulations emphasize the need for risk-based approaches when implementing novel formulation strategies, requiring manufacturers to conduct thorough risk assessments of potential temperature excursions during ambient transport.
Quality control considerations represent another significant regulatory hurdle, with requirements for validated analytical methods capable of detecting potential degradation or changes in product characteristics following room-temperature transport. Regulators increasingly expect CGT developers to implement continuous temperature monitoring systems and establish clear acceptance criteria for products transported using novel stabilization technologies.
Chain of custody documentation presents unique regulatory challenges for room-temperature CGT transport. Manufacturers must maintain comprehensive records demonstrating adherence to validated transport conditions, with particular emphasis on temperature mapping studies that verify the effectiveness of stabilization strategies across various environmental conditions and durations.
International harmonization efforts are gradually addressing regulatory disparities between regions, though significant differences remain. The International Council for Harmonisation (ICH) has initiated discussions specifically addressing stability requirements for advanced therapies, potentially leading to more standardized approaches for evaluating room-temperature transport strategies across global markets.
Regulatory pathways for novel excipients used in stabilization formulations require special consideration, as many innovative stabilizers lack established safety profiles in biological products. Manufacturers must provide substantial safety data and compatibility studies when incorporating new stabilizing agents, particularly those enabling ambient temperature transport of sensitive cell or gene therapy products.
Cost-Benefit Analysis of Room-Temperature Transport
The economic implications of room-temperature transport for cell and gene therapy products represent a critical consideration for healthcare providers, manufacturers, and patients alike. Traditional cold-chain logistics for these sensitive biological materials typically incur substantial costs, estimated between 15-20% of total product expenses, with specialized cryogenic shipping containers alone costing $1,000-$15,000 per unit depending on capacity and technology sophistication.
Room-temperature stabilization technologies offer significant potential cost reductions across multiple dimensions. Direct savings emerge from eliminating expensive cryogenic equipment, specialized refrigerated vehicles, and continuous temperature monitoring systems. Studies indicate potential logistics cost reductions of 60-80% when comparing ambient temperature shipping to cryopreservation methods, particularly for international or remote destination deliveries.
Infrastructure requirements present another major cost differential. Cold-chain transport necessitates substantial investment in freezer farms, liquid nitrogen facilities, and temperature-controlled warehousing. Room-temperature solutions dramatically reduce these capital expenditures while simultaneously decreasing energy consumption—cryogenic storage facilities typically consume 10-15 times more energy than ambient storage spaces.
Risk mitigation represents a less quantifiable but equally important economic benefit. Temperature excursions during cold-chain transport occur in approximately 4-12% of shipments, potentially resulting in product loss valued at hundreds of thousands to millions of dollars per incident. Room-temperature stable formulations inherently eliminate these temperature deviation risks, substantially reducing insurance premiums and write-off expenses.
Regulatory compliance costs also favor room-temperature transport. Documentation requirements for temperature-controlled shipping are extensive, often requiring dedicated quality assurance personnel. Ambient shipping simplifies compliance processes, potentially reducing administrative overhead by 30-40% according to industry analyses.
The extended shelf-life enabled by advanced stabilization technologies creates inventory management advantages that translate to economic benefits. Manufacturers can optimize production schedules, while healthcare facilities can maintain treatment availability without the constraints of ultra-short expiration windows typical of cryopreserved products.
Despite these advantages, implementation costs for room-temperature technologies remain significant. Development of effective stabilization formulations requires substantial R&D investment, estimated at $5-20 million depending on product complexity. Additionally, regulatory approval pathways for novel preservation methods may extend timelines by 12-24 months, delaying return on investment.
Room-temperature stabilization technologies offer significant potential cost reductions across multiple dimensions. Direct savings emerge from eliminating expensive cryogenic equipment, specialized refrigerated vehicles, and continuous temperature monitoring systems. Studies indicate potential logistics cost reductions of 60-80% when comparing ambient temperature shipping to cryopreservation methods, particularly for international or remote destination deliveries.
Infrastructure requirements present another major cost differential. Cold-chain transport necessitates substantial investment in freezer farms, liquid nitrogen facilities, and temperature-controlled warehousing. Room-temperature solutions dramatically reduce these capital expenditures while simultaneously decreasing energy consumption—cryogenic storage facilities typically consume 10-15 times more energy than ambient storage spaces.
Risk mitigation represents a less quantifiable but equally important economic benefit. Temperature excursions during cold-chain transport occur in approximately 4-12% of shipments, potentially resulting in product loss valued at hundreds of thousands to millions of dollars per incident. Room-temperature stable formulations inherently eliminate these temperature deviation risks, substantially reducing insurance premiums and write-off expenses.
Regulatory compliance costs also favor room-temperature transport. Documentation requirements for temperature-controlled shipping are extensive, often requiring dedicated quality assurance personnel. Ambient shipping simplifies compliance processes, potentially reducing administrative overhead by 30-40% according to industry analyses.
The extended shelf-life enabled by advanced stabilization technologies creates inventory management advantages that translate to economic benefits. Manufacturers can optimize production schedules, while healthcare facilities can maintain treatment availability without the constraints of ultra-short expiration windows typical of cryopreserved products.
Despite these advantages, implementation costs for room-temperature technologies remain significant. Development of effective stabilization formulations requires substantial R&D investment, estimated at $5-20 million depending on product complexity. Additionally, regulatory approval pathways for novel preservation methods may extend timelines by 12-24 months, delaying return on investment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!