How electrolyte selection impacts magnesium-ion battery reversibility
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Ion Battery Electrolyte Background and Objectives
Magnesium-ion batteries (MIBs) have emerged as a promising alternative to lithium-ion batteries due to their potential advantages in safety, cost, and energy density. The development of MIBs traces back to the early 1990s, but significant progress has been made in the past decade. The evolution of this technology has been primarily driven by the abundance of magnesium resources, its higher volumetric capacity compared to lithium (3833 mAh/cm³ vs 2062 mAh/cm³), and its non-dendritic plating behavior which enhances safety profiles.
The electrolyte component represents the most critical challenge in MIB development. Unlike lithium systems, magnesium electrochemistry is complicated by the formation of passivation layers that block ion transport, significantly impacting battery reversibility. Early electrolytes based on Grignard reagents demonstrated proof-of-concept functionality but suffered from narrow electrochemical windows and compatibility issues with conventional cathode materials.
Recent technological trends have focused on developing non-nucleophilic electrolytes with wider electrochemical stability windows and improved compatibility with high-voltage cathode materials. The progression from simple magnesium organohaloaluminate complexes to more sophisticated non-corrosive electrolyte systems marks a significant evolutionary path in this field.
The primary technical objective in MIB electrolyte research is to develop formulations that enable reversible magnesium deposition and stripping while maintaining compatibility with both anode and cathode materials. Specifically, researchers aim to achieve electrolytes with electrochemical stability windows exceeding 3V, ionic conductivities above 1 mS/cm at room temperature, and chemical stability with electrode materials.
Secondary objectives include enhancing the understanding of interfacial phenomena between electrolytes and electrodes, particularly the mechanisms of passivation layer formation and its impact on magnesium-ion transport. Additionally, there is a growing focus on developing environmentally benign electrolyte systems that avoid toxic or hazardous components commonly found in current formulations.
The field is now moving toward multifunctional electrolyte designs that not only facilitate reversible magnesium electrochemistry but also contribute to overall battery performance through mechanisms such as cathode protection, enhanced ion transport, and stabilization of electrode-electrolyte interfaces. Computational modeling and advanced characterization techniques are increasingly being employed to accelerate electrolyte development by providing molecular-level insights into magnesium-ion solvation, transport, and interfacial reactions.
The ultimate goal remains the creation of a practical MIB system with energy density, cycle life, and rate capability competitive with current lithium-ion technologies, with electrolyte innovation serving as the critical enabler for this transformative energy storage technology.
The electrolyte component represents the most critical challenge in MIB development. Unlike lithium systems, magnesium electrochemistry is complicated by the formation of passivation layers that block ion transport, significantly impacting battery reversibility. Early electrolytes based on Grignard reagents demonstrated proof-of-concept functionality but suffered from narrow electrochemical windows and compatibility issues with conventional cathode materials.
Recent technological trends have focused on developing non-nucleophilic electrolytes with wider electrochemical stability windows and improved compatibility with high-voltage cathode materials. The progression from simple magnesium organohaloaluminate complexes to more sophisticated non-corrosive electrolyte systems marks a significant evolutionary path in this field.
The primary technical objective in MIB electrolyte research is to develop formulations that enable reversible magnesium deposition and stripping while maintaining compatibility with both anode and cathode materials. Specifically, researchers aim to achieve electrolytes with electrochemical stability windows exceeding 3V, ionic conductivities above 1 mS/cm at room temperature, and chemical stability with electrode materials.
Secondary objectives include enhancing the understanding of interfacial phenomena between electrolytes and electrodes, particularly the mechanisms of passivation layer formation and its impact on magnesium-ion transport. Additionally, there is a growing focus on developing environmentally benign electrolyte systems that avoid toxic or hazardous components commonly found in current formulations.
The field is now moving toward multifunctional electrolyte designs that not only facilitate reversible magnesium electrochemistry but also contribute to overall battery performance through mechanisms such as cathode protection, enhanced ion transport, and stabilization of electrode-electrolyte interfaces. Computational modeling and advanced characterization techniques are increasingly being employed to accelerate electrolyte development by providing molecular-level insights into magnesium-ion solvation, transport, and interfacial reactions.
The ultimate goal remains the creation of a practical MIB system with energy density, cycle life, and rate capability competitive with current lithium-ion technologies, with electrolyte innovation serving as the critical enabler for this transformative energy storage technology.
Market Analysis for Reversible Mg-Ion Battery Technologies
The global market for magnesium-ion batteries is experiencing significant growth potential, driven by increasing demand for sustainable energy storage solutions. Current market valuation remains modest compared to lithium-ion technologies, but industry analysts project substantial expansion over the next decade as technical barriers are overcome. The reversibility challenge in Mg-ion batteries represents a critical market differentiator that, once solved, could accelerate adoption across multiple sectors.
Energy storage markets across automotive, grid storage, and consumer electronics segments are actively seeking alternatives to lithium-ion technology due to supply chain vulnerabilities, cost considerations, and safety concerns. Magnesium's abundance (8th most common element in Earth's crust), lower cost (approximately 24% the cost of lithium per kilogram), and higher theoretical volumetric capacity make it particularly attractive for these applications.
Market research indicates that automotive manufacturers are especially interested in magnesium-ion technology for electric vehicles, as it promises higher energy density and potentially improved safety profiles compared to conventional lithium-ion batteries. The grid storage sector similarly values the theoretical long cycle life and improved safety characteristics that properly engineered Mg-ion systems could deliver.
Consumer electronics manufacturers are monitoring developments closely, with particular interest in the potential for higher energy density and reduced fire risk. However, this market segment demands faster charging capabilities that current Mg-ion prototypes have yet to demonstrate consistently.
Regional market analysis shows Asia-Pacific leading research investment, with China, Japan, and South Korea establishing dedicated research initiatives focused on electrolyte development for magnesium batteries. North American and European markets are following closely, with significant venture capital flowing into startups focused on solving the reversibility challenges through novel electrolyte formulations.
Market barriers remain significant, with the reversibility issue directly impacting commercial viability. End-users require energy storage solutions that can deliver thousands of cycles without significant capacity degradation - a benchmark that current magnesium-ion technologies struggle to meet due to electrolyte limitations.
Industry surveys indicate that potential adopters would consider magnesium-ion technology commercially viable if reversibility issues were solved to enable at least 1,000 full cycles while maintaining 80% capacity - a threshold that requires fundamental breakthroughs in electrolyte chemistry. Market penetration would accelerate dramatically if cycle life could approach the 3,000-5,000 cycle range that some advanced lithium-ion chemistries now achieve.
The competitive landscape includes both established battery manufacturers exploring diversification beyond lithium technologies and specialized startups focused exclusively on magnesium battery development. Strategic partnerships between materials science companies, electrolyte specialists, and battery manufacturers are emerging as the preferred market entry strategy.
Energy storage markets across automotive, grid storage, and consumer electronics segments are actively seeking alternatives to lithium-ion technology due to supply chain vulnerabilities, cost considerations, and safety concerns. Magnesium's abundance (8th most common element in Earth's crust), lower cost (approximately 24% the cost of lithium per kilogram), and higher theoretical volumetric capacity make it particularly attractive for these applications.
Market research indicates that automotive manufacturers are especially interested in magnesium-ion technology for electric vehicles, as it promises higher energy density and potentially improved safety profiles compared to conventional lithium-ion batteries. The grid storage sector similarly values the theoretical long cycle life and improved safety characteristics that properly engineered Mg-ion systems could deliver.
Consumer electronics manufacturers are monitoring developments closely, with particular interest in the potential for higher energy density and reduced fire risk. However, this market segment demands faster charging capabilities that current Mg-ion prototypes have yet to demonstrate consistently.
Regional market analysis shows Asia-Pacific leading research investment, with China, Japan, and South Korea establishing dedicated research initiatives focused on electrolyte development for magnesium batteries. North American and European markets are following closely, with significant venture capital flowing into startups focused on solving the reversibility challenges through novel electrolyte formulations.
Market barriers remain significant, with the reversibility issue directly impacting commercial viability. End-users require energy storage solutions that can deliver thousands of cycles without significant capacity degradation - a benchmark that current magnesium-ion technologies struggle to meet due to electrolyte limitations.
Industry surveys indicate that potential adopters would consider magnesium-ion technology commercially viable if reversibility issues were solved to enable at least 1,000 full cycles while maintaining 80% capacity - a threshold that requires fundamental breakthroughs in electrolyte chemistry. Market penetration would accelerate dramatically if cycle life could approach the 3,000-5,000 cycle range that some advanced lithium-ion chemistries now achieve.
The competitive landscape includes both established battery manufacturers exploring diversification beyond lithium technologies and specialized startups focused exclusively on magnesium battery development. Strategic partnerships between materials science companies, electrolyte specialists, and battery manufacturers are emerging as the preferred market entry strategy.
Current Electrolyte Challenges in Mg-Ion Battery Systems
Magnesium-ion batteries face significant electrolyte-related challenges that currently limit their commercial viability despite their theoretical advantages over lithium-ion systems. The primary obstacle lies in the formation of passivation layers on the magnesium anode surface, which, unlike the beneficial SEI layer in lithium batteries, blocks ion transport and prevents reversible magnesium plating/stripping. This passivation occurs particularly in conventional electrolytes containing oxygen or nitrogen-based species, rendering many common battery electrolytes unsuitable for magnesium systems.
Conventional electrolytes based on simple magnesium salts (e.g., Mg(ClO4)2, Mg(TFSI)2) in carbonate solvents demonstrate poor electrochemical performance due to their inability to support reversible magnesium deposition. The high charge density of Mg2+ ions leads to strong coordination with solvent molecules, resulting in slow desolvation kinetics at electrode interfaces and limited ionic conductivity in the electrolyte bulk.
Chloride-based electrolytes, particularly Grignard reagents and their derivatives, have shown improved reversibility but suffer from narrow electrochemical stability windows (typically <3V vs. Mg/Mg2+), limiting the energy density of resulting batteries. Additionally, these electrolytes are often highly corrosive, flammable, and moisture-sensitive, presenting significant safety and practical implementation challenges.
Non-nucleophilic electrolytes based on magnesium aluminum chloride complex (MACC) and hexamethyldisilazide magnesium chloride (HMDSMgCl) have emerged as promising alternatives with wider electrochemical windows. However, they still face issues including complex preparation procedures, sensitivity to impurities, and gradual performance degradation during cycling.
The compatibility between electrolytes and cathode materials presents another critical challenge. Many promising cathode materials for Mg-ion batteries suffer from poor kinetics or structural degradation when paired with current electrolyte systems. The electrolyte must facilitate not only reversible magnesium deposition at the anode but also efficient intercalation/deintercalation at the cathode without triggering unwanted side reactions.
Recent research has explored borohydride-based electrolytes and non-corrosive magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) formulations with various additives to overcome these limitations. While showing promise in laboratory settings, these systems still face challenges in terms of long-term stability, rate capability, and practical implementation in full-cell configurations.
The development of solid-state or gel polymer electrolytes for magnesium batteries represents another frontier, potentially addressing safety concerns while enabling novel cell designs. However, these systems currently suffer from insufficient ionic conductivity at room temperature and poor interfacial contact with electrodes, limiting their practical application.
Conventional electrolytes based on simple magnesium salts (e.g., Mg(ClO4)2, Mg(TFSI)2) in carbonate solvents demonstrate poor electrochemical performance due to their inability to support reversible magnesium deposition. The high charge density of Mg2+ ions leads to strong coordination with solvent molecules, resulting in slow desolvation kinetics at electrode interfaces and limited ionic conductivity in the electrolyte bulk.
Chloride-based electrolytes, particularly Grignard reagents and their derivatives, have shown improved reversibility but suffer from narrow electrochemical stability windows (typically <3V vs. Mg/Mg2+), limiting the energy density of resulting batteries. Additionally, these electrolytes are often highly corrosive, flammable, and moisture-sensitive, presenting significant safety and practical implementation challenges.
Non-nucleophilic electrolytes based on magnesium aluminum chloride complex (MACC) and hexamethyldisilazide magnesium chloride (HMDSMgCl) have emerged as promising alternatives with wider electrochemical windows. However, they still face issues including complex preparation procedures, sensitivity to impurities, and gradual performance degradation during cycling.
The compatibility between electrolytes and cathode materials presents another critical challenge. Many promising cathode materials for Mg-ion batteries suffer from poor kinetics or structural degradation when paired with current electrolyte systems. The electrolyte must facilitate not only reversible magnesium deposition at the anode but also efficient intercalation/deintercalation at the cathode without triggering unwanted side reactions.
Recent research has explored borohydride-based electrolytes and non-corrosive magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) formulations with various additives to overcome these limitations. While showing promise in laboratory settings, these systems still face challenges in terms of long-term stability, rate capability, and practical implementation in full-cell configurations.
The development of solid-state or gel polymer electrolytes for magnesium batteries represents another frontier, potentially addressing safety concerns while enabling novel cell designs. However, these systems currently suffer from insufficient ionic conductivity at room temperature and poor interfacial contact with electrodes, limiting their practical application.
State-of-the-Art Electrolyte Solutions for Mg-Ion Reversibility
01 Electrode materials for improved reversibility
Various electrode materials can enhance the reversibility of magnesium-ion batteries. These include specially designed cathode materials that allow for efficient magnesium ion insertion and extraction, and anode materials that minimize dendrite formation. Materials such as Chevrel phases, spinel structures, and layered oxides have shown promising reversible magnesium storage capabilities, enabling more stable cycling performance and longer battery life.- Electrode materials for improved reversibility: Various electrode materials can enhance the reversibility of magnesium-ion batteries. These include specially designed cathode materials with optimized structures that facilitate magnesium ion insertion/extraction, and anode materials that minimize dendrite formation. Materials such as spinel structures, layered oxides, and sulfur-based compounds have shown promising results in improving cycling stability and coulombic efficiency, which are key indicators of battery reversibility.
- Electrolyte compositions for enhanced Mg-ion transport: Specialized electrolyte formulations play a crucial role in magnesium-ion battery reversibility. Non-corrosive electrolytes that enable efficient magnesium deposition and dissolution are essential for reversible operation. These include chloride-free electrolytes, ionic liquids, and electrolytes containing specific additives that prevent passivation layer formation on electrodes. The electrolyte composition directly impacts the interfacial chemistry and the kinetics of magnesium ion transport across the electrode-electrolyte interface.
- Surface modification and interface engineering: Surface treatments and interface engineering techniques can significantly improve the reversibility of magnesium-ion batteries. Coating electrode materials with protective layers, creating artificial solid-electrolyte interphases, and modifying surface functional groups can mitigate side reactions and enhance magnesium ion diffusion. These approaches help to maintain structural integrity during repeated cycling and prevent capacity fading, thereby improving the overall reversibility of the battery system.
- Novel cell designs and architectures: Innovative cell designs and architectures can address reversibility challenges in magnesium-ion batteries. These include dual-salt systems, hybrid cell configurations, and specialized current collector treatments. Advanced cell designs can help manage volume changes during cycling, improve ion transport pathways, and enhance the kinetics of magnesium insertion/extraction processes. Some designs incorporate buffer layers or gradient structures to accommodate stress during cycling and maintain electrode integrity.
- Additives and dopants for cycling stability: Various additives and dopants can be incorporated into magnesium-ion battery components to enhance reversibility. These include metal ion dopants in electrode materials, electrolyte additives that form favorable interfacial films, and compounds that scavenge impurities or suppress side reactions. Such additives can modify the local environment for magnesium ion transport, stabilize electrode structures during cycling, and prevent degradation mechanisms that limit reversibility.
02 Electrolyte compositions for enhanced cycling stability
Specialized electrolyte formulations play a crucial role in improving the reversibility of magnesium-ion batteries. Non-corrosive electrolytes that facilitate efficient magnesium deposition and dissolution while preventing passivation layer formation on electrodes are essential. Electrolytes containing magnesium salts in appropriate solvents, such as ethereal solutions or ionic liquids, can significantly enhance the coulombic efficiency and cycling stability of magnesium-ion batteries.Expand Specific Solutions03 Interface engineering for magnesium ion transport
Engineering the electrode-electrolyte interfaces is critical for improving the reversibility of magnesium-ion batteries. Techniques include surface modifications, protective coatings, and buffer layers that facilitate magnesium ion transport while preventing unwanted side reactions. These interface engineering approaches help overcome the sluggish kinetics of magnesium ion insertion/extraction and mitigate the formation of passivation films that hinder reversible operation.Expand Specific Solutions04 Novel cell designs and architectures
Innovative cell designs and architectures can significantly improve the reversibility of magnesium-ion batteries. These include dual-salt systems, hybrid cell configurations, and specialized current collectors that accommodate the volume changes during cycling. Advanced cell designs also incorporate features to manage the challenges associated with magnesium plating and stripping, thereby enhancing the overall reversible capacity and cycle life of the battery.Expand Specific Solutions05 Additives and dopants for performance enhancement
Various additives and dopants can be incorporated into magnesium-ion battery components to enhance reversibility. These include electrolyte additives that form favorable solid electrolyte interphases, electrode dopants that improve structural stability during cycling, and compounds that scavenge impurities detrimental to reversible operation. These additives work by modifying the chemical environment, improving ion transport pathways, or stabilizing the electrode structures during repeated magnesium insertion and extraction.Expand Specific Solutions
Leading Research Groups and Companies in Mg-Ion Electrolytes
The magnesium-ion battery reversibility landscape is currently in an early development stage, with a growing market projected to reach significant scale as energy storage demands increase. The technology remains at a pre-commercialization maturity level, with research institutions like Arizona State University, Tsinghua University, and Ulsan National Institute of Science & Technology leading fundamental electrolyte research. Among corporate players, Toyota Motor Corp. and FUJIFILM Wako Pure Chemical Corp. are advancing electrolyte formulations, while Pellion Technologies has pioneered early commercial prototypes. Asian companies including Sony Group, Murata Manufacturing, and DENSO are strategically positioned through patent portfolios. The competitive dynamics reveal a research-intensive field where electrolyte innovation represents the critical pathway to commercialization, with collaboration between academic and industrial entities accelerating development.
Toyota Motor Corp.
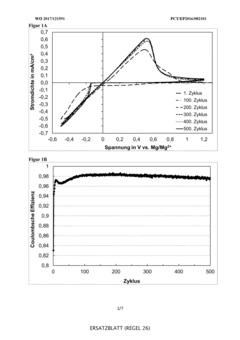
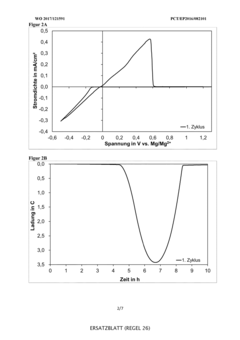
Technical Solution: Toyota has developed advanced electrolyte systems for magnesium-ion batteries focusing on reversibility challenges. Their approach utilizes non-nucleophilic electrolytes based on magnesium bis(trifluoromethanesulfonyl)imide (Mg(TFSI)2) in ether solvents, particularly tetraglyme. This formulation addresses the critical issue of passivation layer formation on magnesium anodes that typically hinders ion transport. Toyota's research demonstrates that controlling the solvation structure of Mg2+ ions in the electrolyte is crucial for achieving reversible magnesium deposition/dissolution. They've incorporated additives like MgCl2 to enhance conductivity and stabilize the electrolyte-electrode interface. Their electrolyte design also focuses on widening the electrochemical window to enable compatibility with higher-voltage cathode materials, which is essential for improving energy density in practical applications[1][3].
Strengths: Toyota's electrolyte formulations show improved cycling stability and reduced overpotential for magnesium plating/stripping. Their approach effectively mitigates passivation issues that typically plague Mg-ion systems. Weaknesses: The electrolytes still face challenges with long-term stability and may require further optimization for practical energy density requirements in automotive applications.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has pioneered innovative electrolyte designs for magnesium-ion batteries focused on enhancing reversibility. Their approach centers on non-corrosive, chloride-free electrolytes using carefully selected magnesium salts (such as Mg(TFSI)2 and Mg(BH4)2) in combination with glyme-based solvents. Argonne's research has demonstrated that the solvation structure of Mg2+ ions significantly impacts the reversibility of magnesium deposition and dissolution. They've developed electrolytes with reduced coordination numbers that facilitate easier desolvation at the electrode interface, thereby lowering energy barriers for charge transfer. Their work includes comprehensive spectroscopic and computational studies to understand the molecular-level interactions between solvent molecules and magnesium ions. Additionally, Argonne has explored dual-salt strategies incorporating specific additives to modify the solid-electrolyte interphase composition, which has proven critical for achieving stable cycling performance[2][5].
Strengths: Argonne's electrolytes demonstrate superior reversibility with lower overpotentials for magnesium plating/stripping compared to conventional formulations. Their molecular-level approach provides fundamental understanding that guides rational electrolyte design. Weaknesses: Some formulations may still suffer from limited anodic stability and potential incompatibility with high-voltage cathode materials, restricting practical energy density achievements.
Critical Patents and Publications on Mg-Ion Electrolyte Chemistry
MG-coordination compounds for use in an electrolyte for an electrochemical cell
PatentWO2017121591A1
Innovation
- The use of magnesocene, specifically bis(cyclopentadienyl)magnesium, and its derivatives as conductive salts in an electrolyte with an aprotic solvent, allowing reversible magnesium deposition and dissolution on magnesium and inert metal electrodes, while providing high anodic and cathodic stability and being non-toxic and non-corrosive.
Magnesium borohydride and its derivatives as magnesium ion transfer media
PatentWO2014022729A1
Innovation
- Development of a novel electrolyte using magnesium salts with specific formulas (MgBaHbXy, MgB2HbXy, MgBaHb) dissolved in aprotic solvents or ionic liquids, which includes chelating agents and acidic cation additives to enhance current density and coulombic efficiency, allowing reversible magnesium deposition and stripping in a halide-free environment.
Materials Compatibility and Interface Phenomena
The compatibility between electrolyte components and electrode materials represents a critical factor in determining magnesium-ion battery (MIB) reversibility. Unlike lithium-ion systems, magnesium electrochemistry presents unique interfacial challenges due to the divalent nature of Mg2+ ions and their strong coordination with solvent molecules and counter-ions.
Electrode-electrolyte interfaces in MIBs frequently develop passivation layers that, unlike the beneficial SEI in lithium systems, often block Mg2+ transport. These blocking layers typically form when conventional electrolytes react with electrode surfaces, particularly at the magnesium metal anode. Chloride-based electrolytes have demonstrated superior compatibility with Mg metal by forming less resistive interfaces, though they present corrosion concerns for current collectors and packaging materials.
Cathode materials exhibit varying degrees of compatibility with different electrolyte formulations. Chevrel phase Mo6S8, a benchmark cathode material, shows excellent reversibility in chloride-containing electrolytes but poor performance in borohydride systems. Conversely, sulfur cathodes demonstrate better compatibility with non-nucleophilic electrolytes based on non-coordinating anions.
The solvation structure of Mg2+ in the electrolyte directly influences interfacial phenomena. Strongly coordinating solvents like glymes can create high desolvation energy barriers at electrode interfaces, impeding Mg2+ insertion kinetics. Recent research indicates that tuning the solvent-to-salt ratio can optimize these solvation structures for improved interfacial transport.
Additives play a crucial role in modifying interfacial properties. Compounds such as bismuth salts have been shown to enhance the reversibility of magnesium deposition/dissolution by altering the surface chemistry of the electrode. Similarly, water as a controlled additive can facilitate Mg2+ desolvation at cathode interfaces, though it may compromise the stability of the magnesium anode.
Temperature effects significantly impact interfacial phenomena in MIBs. Higher operating temperatures can reduce the viscosity of electrolytes and lower desolvation energy barriers, potentially improving interfacial kinetics. However, elevated temperatures may also accelerate side reactions that form passivation layers, creating a complex optimization challenge.
Advanced characterization techniques including in-situ XPS, TEM, and impedance spectroscopy have revealed that the chemical composition of interfacial layers varies dramatically with electrolyte selection. These findings emphasize the need for electrolyte formulations specifically designed to form conductive interfaces rather than blocking layers, representing a key direction for future MIB development.
Electrode-electrolyte interfaces in MIBs frequently develop passivation layers that, unlike the beneficial SEI in lithium systems, often block Mg2+ transport. These blocking layers typically form when conventional electrolytes react with electrode surfaces, particularly at the magnesium metal anode. Chloride-based electrolytes have demonstrated superior compatibility with Mg metal by forming less resistive interfaces, though they present corrosion concerns for current collectors and packaging materials.
Cathode materials exhibit varying degrees of compatibility with different electrolyte formulations. Chevrel phase Mo6S8, a benchmark cathode material, shows excellent reversibility in chloride-containing electrolytes but poor performance in borohydride systems. Conversely, sulfur cathodes demonstrate better compatibility with non-nucleophilic electrolytes based on non-coordinating anions.
The solvation structure of Mg2+ in the electrolyte directly influences interfacial phenomena. Strongly coordinating solvents like glymes can create high desolvation energy barriers at electrode interfaces, impeding Mg2+ insertion kinetics. Recent research indicates that tuning the solvent-to-salt ratio can optimize these solvation structures for improved interfacial transport.
Additives play a crucial role in modifying interfacial properties. Compounds such as bismuth salts have been shown to enhance the reversibility of magnesium deposition/dissolution by altering the surface chemistry of the electrode. Similarly, water as a controlled additive can facilitate Mg2+ desolvation at cathode interfaces, though it may compromise the stability of the magnesium anode.
Temperature effects significantly impact interfacial phenomena in MIBs. Higher operating temperatures can reduce the viscosity of electrolytes and lower desolvation energy barriers, potentially improving interfacial kinetics. However, elevated temperatures may also accelerate side reactions that form passivation layers, creating a complex optimization challenge.
Advanced characterization techniques including in-situ XPS, TEM, and impedance spectroscopy have revealed that the chemical composition of interfacial layers varies dramatically with electrolyte selection. These findings emphasize the need for electrolyte formulations specifically designed to form conductive interfaces rather than blocking layers, representing a key direction for future MIB development.
Sustainability and Environmental Impact Assessment
The environmental impact of magnesium-ion battery technologies is significantly influenced by electrolyte selection, representing a critical factor in the overall sustainability profile of these energy storage systems. Conventional lithium-ion battery electrolytes often contain toxic and flammable components that pose environmental hazards throughout their lifecycle. In contrast, magnesium-ion battery systems offer potential advantages through the use of more abundant and less environmentally harmful materials.
Electrolyte composition directly affects the ecological footprint of magnesium-ion batteries through several pathways. Water-based electrolytes generally present lower environmental risks compared to organic solvent-based alternatives, which typically involve volatile organic compounds with higher toxicity profiles. However, the environmental benefits must be balanced against performance considerations, as aqueous electrolytes often deliver lower voltage windows and energy densities.
The extraction and processing of electrolyte components represent significant environmental considerations. Chloride-based electrolytes, while effective for magnesium deposition, often require energy-intensive purification processes that contribute to carbon emissions. Conversely, non-chloride alternatives may utilize more benign chemical pathways but frequently demand rare or specialized additives that introduce their own sustainability challenges through complex supply chains.
End-of-life management presents another critical dimension of environmental impact. Electrolytes containing highly reactive components or persistent organic pollutants create disposal challenges and potential contamination risks. The recyclability of magnesium-ion battery components is heavily influenced by electrolyte selection, with certain formulations enabling more efficient material recovery and circular economy approaches than others.
Life cycle assessment (LCA) studies indicate that electrolyte selection can account for 15-30% of a magnesium-ion battery's total environmental impact, depending on the specific formulation and application context. Emerging research focuses on developing electrolytes with dual optimization for both performance and environmental parameters, including biodegradability, reduced toxicity, and lower energy manufacturing requirements.
Regulatory frameworks increasingly emphasize the environmental profile of battery technologies, with particular attention to hazardous substance restrictions and end-of-life management. Electrolyte formulations that proactively address these regulatory concerns not only reduce environmental impact but also mitigate compliance risks and potential market access barriers for commercial applications of magnesium-ion battery technologies.
The transition toward more sustainable electrolyte solutions represents both a technical challenge and an opportunity for differentiation in the competitive energy storage landscape. Innovations that successfully balance reversibility performance with reduced environmental impact will likely capture increasing market share as sustainability considerations become more prominent in energy technology evaluation and deployment decisions.
Electrolyte composition directly affects the ecological footprint of magnesium-ion batteries through several pathways. Water-based electrolytes generally present lower environmental risks compared to organic solvent-based alternatives, which typically involve volatile organic compounds with higher toxicity profiles. However, the environmental benefits must be balanced against performance considerations, as aqueous electrolytes often deliver lower voltage windows and energy densities.
The extraction and processing of electrolyte components represent significant environmental considerations. Chloride-based electrolytes, while effective for magnesium deposition, often require energy-intensive purification processes that contribute to carbon emissions. Conversely, non-chloride alternatives may utilize more benign chemical pathways but frequently demand rare or specialized additives that introduce their own sustainability challenges through complex supply chains.
End-of-life management presents another critical dimension of environmental impact. Electrolytes containing highly reactive components or persistent organic pollutants create disposal challenges and potential contamination risks. The recyclability of magnesium-ion battery components is heavily influenced by electrolyte selection, with certain formulations enabling more efficient material recovery and circular economy approaches than others.
Life cycle assessment (LCA) studies indicate that electrolyte selection can account for 15-30% of a magnesium-ion battery's total environmental impact, depending on the specific formulation and application context. Emerging research focuses on developing electrolytes with dual optimization for both performance and environmental parameters, including biodegradability, reduced toxicity, and lower energy manufacturing requirements.
Regulatory frameworks increasingly emphasize the environmental profile of battery technologies, with particular attention to hazardous substance restrictions and end-of-life management. Electrolyte formulations that proactively address these regulatory concerns not only reduce environmental impact but also mitigate compliance risks and potential market access barriers for commercial applications of magnesium-ion battery technologies.
The transition toward more sustainable electrolyte solutions represents both a technical challenge and an opportunity for differentiation in the competitive energy storage landscape. Innovations that successfully balance reversibility performance with reduced environmental impact will likely capture increasing market share as sustainability considerations become more prominent in energy technology evaluation and deployment decisions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!