How Ethyl Acetate Drives Solvent Recovery Advances?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Recovery Background and Objectives
Ethyl acetate recovery has emerged as a critical focus in the chemical industry, driven by the increasing demand for sustainable practices and cost-effective solvent management. The evolution of ethyl acetate recovery techniques can be traced back to the early 20th century, with significant advancements occurring in recent decades. As environmental regulations have become more stringent and resource efficiency has gained prominence, the importance of effective solvent recovery has grown exponentially.
The primary objective of ethyl acetate recovery is to minimize waste, reduce environmental impact, and improve the economic viability of industrial processes that utilize this versatile solvent. Ethyl acetate, widely used in industries such as pharmaceuticals, paints, and coatings, presents both challenges and opportunities in terms of recovery and reuse. The technical goals for recovery processes include achieving high purity levels, maximizing recovery rates, and minimizing energy consumption.
Recent technological trends in ethyl acetate recovery have focused on developing more efficient separation methods, such as advanced distillation techniques, membrane technologies, and hybrid systems. These innovations aim to overcome traditional limitations in recovery processes, such as azeotrope formation and energy-intensive operations. The industry is moving towards more integrated and automated recovery systems that can adapt to varying feed compositions and process conditions.
Another key objective in ethyl acetate recovery is the development of green technologies that align with circular economy principles. This includes exploring bio-based alternatives for ethyl acetate production and recovery, as well as integrating recovery processes with other waste management and energy recovery systems. The ultimate aim is to create closed-loop systems that maximize resource utilization and minimize environmental footprint.
The global push towards sustainability has also spurred research into novel applications of recovered ethyl acetate, expanding its potential uses beyond traditional industries. This not only enhances the economic value of recovery processes but also contributes to the overall reduction of virgin solvent production. As such, ethyl acetate recovery is increasingly viewed not just as a waste management strategy, but as a key component in sustainable chemical manufacturing and circular economy models.
Looking ahead, the field of ethyl acetate recovery is poised for further innovation, with emerging technologies such as artificial intelligence and advanced analytics expected to play a significant role in optimizing recovery processes. The ongoing research and development in this area underscore its importance in driving solvent recovery advances and shaping the future of sustainable industrial practices.
The primary objective of ethyl acetate recovery is to minimize waste, reduce environmental impact, and improve the economic viability of industrial processes that utilize this versatile solvent. Ethyl acetate, widely used in industries such as pharmaceuticals, paints, and coatings, presents both challenges and opportunities in terms of recovery and reuse. The technical goals for recovery processes include achieving high purity levels, maximizing recovery rates, and minimizing energy consumption.
Recent technological trends in ethyl acetate recovery have focused on developing more efficient separation methods, such as advanced distillation techniques, membrane technologies, and hybrid systems. These innovations aim to overcome traditional limitations in recovery processes, such as azeotrope formation and energy-intensive operations. The industry is moving towards more integrated and automated recovery systems that can adapt to varying feed compositions and process conditions.
Another key objective in ethyl acetate recovery is the development of green technologies that align with circular economy principles. This includes exploring bio-based alternatives for ethyl acetate production and recovery, as well as integrating recovery processes with other waste management and energy recovery systems. The ultimate aim is to create closed-loop systems that maximize resource utilization and minimize environmental footprint.
The global push towards sustainability has also spurred research into novel applications of recovered ethyl acetate, expanding its potential uses beyond traditional industries. This not only enhances the economic value of recovery processes but also contributes to the overall reduction of virgin solvent production. As such, ethyl acetate recovery is increasingly viewed not just as a waste management strategy, but as a key component in sustainable chemical manufacturing and circular economy models.
Looking ahead, the field of ethyl acetate recovery is poised for further innovation, with emerging technologies such as artificial intelligence and advanced analytics expected to play a significant role in optimizing recovery processes. The ongoing research and development in this area underscore its importance in driving solvent recovery advances and shaping the future of sustainable industrial practices.
Market Analysis for Ethyl Acetate Recycling
The ethyl acetate recycling market has witnessed significant growth in recent years, driven by increasing environmental concerns and the rising demand for sustainable practices in various industries. This market is primarily fueled by the chemical, pharmaceutical, and paint industries, where ethyl acetate is extensively used as a solvent. The global ethyl acetate market size was valued at approximately $3.3 billion in 2020 and is projected to reach $4.9 billion by 2028, growing at a CAGR of 5.1% during the forecast period.
The pharmaceutical industry represents a major consumer of ethyl acetate, utilizing it in the production of various drugs and medicines. The growing pharmaceutical sector, particularly in emerging economies, is expected to drive the demand for ethyl acetate recycling. Additionally, the paint and coatings industry, which uses ethyl acetate as a solvent in formulations, is experiencing steady growth, further boosting the recycling market.
Geographically, Asia-Pacific dominates the ethyl acetate recycling market, accounting for over 40% of the global market share. This can be attributed to the rapid industrialization in countries like China and India, coupled with stringent environmental regulations. North America and Europe follow closely, with increasing adoption of sustainable practices and circular economy principles driving market growth in these regions.
The market is characterized by the presence of both large multinational corporations and small to medium-sized enterprises. Key players in the ethyl acetate recycling market include Celanese Corporation, Eastman Chemical Company, and INEOS. These companies are investing heavily in research and development to improve recycling technologies and expand their market presence.
One of the key trends shaping the market is the adoption of advanced recycling technologies. Distillation and membrane separation techniques are gaining traction due to their efficiency in recovering high-purity ethyl acetate. Moreover, the integration of IoT and AI in recycling processes is enhancing operational efficiency and reducing energy consumption, making recycling more cost-effective.
The market also faces certain challenges, such as high initial investment costs for recycling equipment and the availability of cheaper alternatives. However, the increasing focus on sustainability and circular economy practices is expected to overcome these barriers in the long run. Government initiatives and regulations promoting solvent recycling are further propelling market growth, creating a favorable environment for ethyl acetate recycling technologies.
The pharmaceutical industry represents a major consumer of ethyl acetate, utilizing it in the production of various drugs and medicines. The growing pharmaceutical sector, particularly in emerging economies, is expected to drive the demand for ethyl acetate recycling. Additionally, the paint and coatings industry, which uses ethyl acetate as a solvent in formulations, is experiencing steady growth, further boosting the recycling market.
Geographically, Asia-Pacific dominates the ethyl acetate recycling market, accounting for over 40% of the global market share. This can be attributed to the rapid industrialization in countries like China and India, coupled with stringent environmental regulations. North America and Europe follow closely, with increasing adoption of sustainable practices and circular economy principles driving market growth in these regions.
The market is characterized by the presence of both large multinational corporations and small to medium-sized enterprises. Key players in the ethyl acetate recycling market include Celanese Corporation, Eastman Chemical Company, and INEOS. These companies are investing heavily in research and development to improve recycling technologies and expand their market presence.
One of the key trends shaping the market is the adoption of advanced recycling technologies. Distillation and membrane separation techniques are gaining traction due to their efficiency in recovering high-purity ethyl acetate. Moreover, the integration of IoT and AI in recycling processes is enhancing operational efficiency and reducing energy consumption, making recycling more cost-effective.
The market also faces certain challenges, such as high initial investment costs for recycling equipment and the availability of cheaper alternatives. However, the increasing focus on sustainability and circular economy practices is expected to overcome these barriers in the long run. Government initiatives and regulations promoting solvent recycling are further propelling market growth, creating a favorable environment for ethyl acetate recycling technologies.
Current Challenges in Solvent Recovery
Solvent recovery, particularly in the context of ethyl acetate, faces several significant challenges in the current industrial landscape. One of the primary issues is the energy-intensive nature of traditional recovery methods. Distillation, the most commonly used technique, requires substantial thermal energy input, leading to high operational costs and environmental concerns due to increased carbon emissions.
Another challenge lies in the efficiency of separation processes. Ethyl acetate often forms azeotropic mixtures with water and other solvents, making complete separation difficult through conventional means. This results in reduced purity of recovered solvents and potential loss of valuable materials, impacting both economic and environmental aspects of industrial operations.
The presence of impurities and contaminants in recovered solvents poses a significant hurdle. In many industrial applications, especially in pharmaceuticals and electronics, even trace amounts of impurities can compromise product quality. Developing effective purification techniques that can handle diverse contaminants while maintaining the integrity of ethyl acetate remains a complex task.
Scale-up issues present another set of challenges. While laboratory-scale solvent recovery systems may demonstrate promising results, translating these successes to industrial-scale operations often encounters unforeseen complications. These can include reduced efficiency, increased energy consumption, and difficulties in maintaining consistent quality across large volumes.
Regulatory compliance adds another layer of complexity to solvent recovery processes. Stringent environmental regulations and safety standards necessitate careful handling and disposal of solvents, as well as meticulous documentation of recovery processes. Meeting these requirements while maintaining cost-effectiveness and operational efficiency is an ongoing challenge for many industries.
The volatility of ethyl acetate itself presents unique challenges in recovery processes. Its low boiling point and high vapor pressure make it prone to losses during handling and storage, requiring specialized equipment and procedures to minimize emissions and ensure worker safety.
Lastly, the integration of solvent recovery systems into existing industrial processes without disrupting ongoing operations remains a significant challenge. Retrofitting existing plants with advanced recovery technologies often requires substantial capital investment and may necessitate temporary production halts, creating resistance to adoption despite long-term benefits.
Addressing these challenges requires innovative approaches that combine advanced separation technologies, energy-efficient processes, and intelligent system integration. As industries strive for greater sustainability and cost-effectiveness, overcoming these hurdles in ethyl acetate recovery becomes increasingly crucial for maintaining competitive edge and environmental responsibility.
Another challenge lies in the efficiency of separation processes. Ethyl acetate often forms azeotropic mixtures with water and other solvents, making complete separation difficult through conventional means. This results in reduced purity of recovered solvents and potential loss of valuable materials, impacting both economic and environmental aspects of industrial operations.
The presence of impurities and contaminants in recovered solvents poses a significant hurdle. In many industrial applications, especially in pharmaceuticals and electronics, even trace amounts of impurities can compromise product quality. Developing effective purification techniques that can handle diverse contaminants while maintaining the integrity of ethyl acetate remains a complex task.
Scale-up issues present another set of challenges. While laboratory-scale solvent recovery systems may demonstrate promising results, translating these successes to industrial-scale operations often encounters unforeseen complications. These can include reduced efficiency, increased energy consumption, and difficulties in maintaining consistent quality across large volumes.
Regulatory compliance adds another layer of complexity to solvent recovery processes. Stringent environmental regulations and safety standards necessitate careful handling and disposal of solvents, as well as meticulous documentation of recovery processes. Meeting these requirements while maintaining cost-effectiveness and operational efficiency is an ongoing challenge for many industries.
The volatility of ethyl acetate itself presents unique challenges in recovery processes. Its low boiling point and high vapor pressure make it prone to losses during handling and storage, requiring specialized equipment and procedures to minimize emissions and ensure worker safety.
Lastly, the integration of solvent recovery systems into existing industrial processes without disrupting ongoing operations remains a significant challenge. Retrofitting existing plants with advanced recovery technologies often requires substantial capital investment and may necessitate temporary production halts, creating resistance to adoption despite long-term benefits.
Addressing these challenges requires innovative approaches that combine advanced separation technologies, energy-efficient processes, and intelligent system integration. As industries strive for greater sustainability and cost-effectiveness, overcoming these hurdles in ethyl acetate recovery becomes increasingly crucial for maintaining competitive edge and environmental responsibility.
Existing Ethyl Acetate Recovery Methods
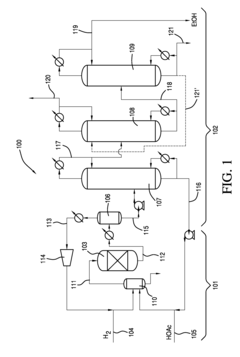
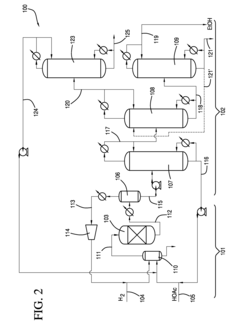
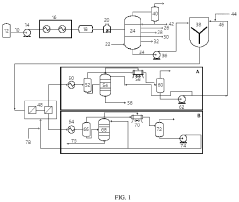
01 Distillation-based recovery methods
Distillation is a common method for ethyl acetate solvent recovery. This process involves heating the solvent mixture to separate components based on their boiling points. Various distillation techniques, such as fractional distillation or vacuum distillation, can be employed to improve efficiency and purity of recovered ethyl acetate.- Distillation and condensation techniques: Ethyl acetate solvent recovery often employs distillation and condensation methods. The process involves heating the solvent mixture to separate ethyl acetate based on its boiling point, followed by cooling and condensing the vapor back into liquid form. This technique allows for efficient separation and recovery of ethyl acetate from other solvents or impurities.
- Membrane separation technology: Membrane separation is an emerging technology for ethyl acetate recovery. This method uses selective membranes to separate ethyl acetate from other components based on molecular size or chemical properties. Membrane separation can be more energy-efficient than traditional distillation methods and can handle mixtures that are difficult to separate by other means.
- Adsorption and desorption processes: Adsorption-based recovery systems use materials like activated carbon or zeolites to selectively adsorb ethyl acetate from a mixture. The adsorbed ethyl acetate is then recovered through desorption processes, often using heat or pressure swing techniques. This method can be particularly effective for dilute solutions or when high purity recovery is required.
- Integrated solvent recovery systems: Integrated systems combine multiple recovery techniques to maximize efficiency and purity. These systems may incorporate distillation, membrane separation, and adsorption processes in a single setup. By leveraging the strengths of each method, integrated systems can achieve higher recovery rates and handle a wider range of solvent mixtures.
- Energy-efficient and sustainable recovery methods: Recent innovations focus on improving the energy efficiency and sustainability of ethyl acetate recovery processes. This includes the use of heat integration, low-temperature separation techniques, and renewable energy sources to power recovery systems. These methods aim to reduce the environmental impact and operational costs associated with solvent recovery.
02 Adsorption and membrane separation techniques
Adsorption and membrane separation are alternative methods for ethyl acetate recovery. These techniques utilize materials with selective affinity for ethyl acetate or membranes with specific pore sizes to separate the solvent from other components. These methods can be particularly useful for mixtures that are difficult to separate by distillation alone.Expand Specific Solutions03 Integrated solvent recovery systems
Integrated systems combine multiple recovery techniques to maximize efficiency and purity of recovered ethyl acetate. These systems may incorporate distillation, adsorption, membrane separation, and other methods in a single process. Such integrated approaches can be tailored to specific industrial applications and solvent mixtures.Expand Specific Solutions04 Energy-efficient and sustainable recovery processes
Recent innovations focus on developing energy-efficient and sustainable ethyl acetate recovery processes. These may include the use of heat integration, renewable energy sources, or novel materials to reduce energy consumption and environmental impact. Some approaches also aim to minimize waste generation and improve overall process sustainability.Expand Specific Solutions05 Solvent recovery in specific industrial applications
Ethyl acetate recovery methods are often tailored to specific industrial applications, such as pharmaceutical manufacturing, paint production, or electronics industry. These specialized recovery processes consider factors like solvent purity requirements, presence of contaminants, and integration with existing production processes to optimize recovery efficiency and product quality.Expand Specific Solutions
Key Players in Solvent Recovery Industry
The ethyl acetate solvent recovery market is in a growth phase, driven by increasing environmental regulations and cost-saving initiatives across industries. The global market size is projected to expand significantly in the coming years, with key players like Celanese International Corp., SK Chemicals Co. Ltd., and China Petroleum & Chemical Corp. leading the way. These companies are investing in advanced technologies to improve recovery efficiency and purity levels. The technology is reaching maturity, with established processes, but ongoing research by companies like Wacker Chemie AG and universities such as South China University of Technology is focused on developing more sustainable and cost-effective recovery methods. This competitive landscape is characterized by a mix of large chemical corporations and specialized solvent recovery firms, all striving to innovate and capture market share in this growing sector.
Celanese International Corp.
Technical Solution: Celanese International Corp. has developed a state-of-the-art ethyl acetate recovery system based on their proprietary VAntage® technology. This process combines advanced distillation techniques with innovative mass transfer internals to achieve high-efficiency separation. Celanese's system utilizes structured packing with enhanced surface area and optimized liquid distribution, resulting in improved mass transfer and reduced pressure drop. The company has also implemented a heat pump system to recover and reuse waste heat, significantly reducing energy consumption. Celanese reports recovery rates of over 99% for ethyl acetate, with purities exceeding 99.9%[13]. Their technology is scalable and has been successfully implemented in large-scale industrial applications, with capacities ranging from 50,000 to 200,000 tons per year[15]. Additionally, Celanese has integrated advanced process control systems to optimize operation and maintain consistent product quality[17].
Strengths: High recovery rates and purity, energy efficiency, and scalability for large industrial applications. Weaknesses: High initial capital investment and complexity of the advanced distillation system.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced ethyl acetate solvent recovery system utilizing a multi-stage distillation process. Their technology employs a series of distillation columns with optimized operating conditions to achieve high purity ethyl acetate recovery. The system incorporates energy-efficient heat integration and vapor recompression to minimize energy consumption. Sinopec's process achieves recovery rates of over 99% for ethyl acetate from industrial waste streams[1]. The company has also implemented membrane separation technology in conjunction with distillation to further enhance separation efficiency and reduce energy requirements[3]. Their solvent recovery units are designed to handle large-scale industrial applications, with capacities ranging from 10,000 to 100,000 tons per year[5].
Strengths: High recovery rates, energy efficiency, and large-scale applicability. Weaknesses: High initial capital investment and complexity of the multi-stage system.
Innovative Technologies for Ethyl Acetate Reclamation
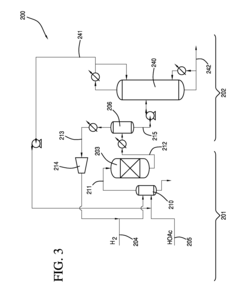
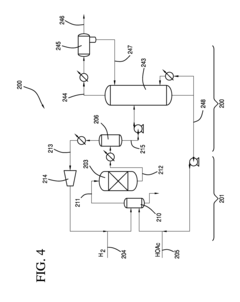
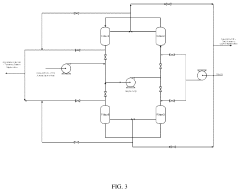
Process for producing an ethyl acetate solvent and co-production of ethanol
PatentInactiveUS20110190531A1
Innovation
- A process involving the hydrogenation of acetic acid in the presence of a catalyst, followed by a series of distillation columns to separate and recover ethanol and ethyl acetate solvent, with specific catalyst compositions and conditions to optimize ethanol and ethyl acetate production, including the use of platinum-based catalysts and modified silica supports.
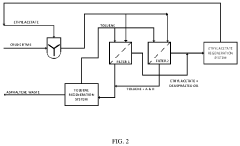
Method for removing asphaltenes, resins and heavy metals from crude oil
PatentPendingUS20230407188A1
Innovation
- The use of ethyl acetate as a solvent at ambient temperatures and pressures to precipitate asphaltenes, resins, and heavy metals in a single step, with a solvent-to-feed ratio that is less than conventional methods, allowing for efficient recovery and recycling of the solvent.
Environmental Impact and Sustainability
The use of ethyl acetate in solvent recovery processes has significant implications for environmental impact and sustainability. As industries strive to reduce their ecological footprint, the adoption of ethyl acetate-based recovery systems presents both opportunities and challenges.
Ethyl acetate's relatively low toxicity and biodegradability make it an attractive alternative to more harmful solvents. Its use in recovery processes can lead to reduced emissions of volatile organic compounds (VOCs), which are known contributors to air pollution and smog formation. This aligns with increasingly stringent environmental regulations and corporate sustainability goals.
The recovery of ethyl acetate itself is a key factor in its environmental profile. Advanced recovery techniques, such as membrane separation and adsorption processes, have improved the efficiency of ethyl acetate reclamation. These methods not only minimize waste but also reduce the need for fresh solvent production, thereby conserving resources and energy.
However, the environmental benefits of ethyl acetate-driven solvent recovery must be weighed against the energy requirements of the recovery process. While modern systems are more energy-efficient than their predecessors, there is still room for improvement. Ongoing research focuses on developing low-energy recovery methods to further enhance the sustainability of these systems.
Life cycle assessments of ethyl acetate-based recovery processes have shown promising results in terms of reduced carbon footprint compared to traditional solvent systems. This is particularly evident in industries such as pharmaceuticals and coatings, where large volumes of solvents are used and recovered.
The sustainability aspect extends beyond environmental considerations to include economic factors. Efficient solvent recovery using ethyl acetate can lead to significant cost savings for industries, making sustainable practices more economically viable. This alignment of environmental and economic benefits is crucial for widespread adoption of greener technologies.
As industries move towards circular economy models, the role of ethyl acetate in solvent recovery becomes increasingly important. Its ability to be recycled multiple times without significant loss of quality supports closed-loop manufacturing processes, reducing the overall environmental impact of industrial operations.
Looking forward, the integration of ethyl acetate-based recovery systems with renewable energy sources and smart manufacturing technologies holds promise for further improving the sustainability profile of industrial processes. This holistic approach to sustainability in solvent recovery represents a significant step towards more environmentally responsible industrial practices.
Ethyl acetate's relatively low toxicity and biodegradability make it an attractive alternative to more harmful solvents. Its use in recovery processes can lead to reduced emissions of volatile organic compounds (VOCs), which are known contributors to air pollution and smog formation. This aligns with increasingly stringent environmental regulations and corporate sustainability goals.
The recovery of ethyl acetate itself is a key factor in its environmental profile. Advanced recovery techniques, such as membrane separation and adsorption processes, have improved the efficiency of ethyl acetate reclamation. These methods not only minimize waste but also reduce the need for fresh solvent production, thereby conserving resources and energy.
However, the environmental benefits of ethyl acetate-driven solvent recovery must be weighed against the energy requirements of the recovery process. While modern systems are more energy-efficient than their predecessors, there is still room for improvement. Ongoing research focuses on developing low-energy recovery methods to further enhance the sustainability of these systems.
Life cycle assessments of ethyl acetate-based recovery processes have shown promising results in terms of reduced carbon footprint compared to traditional solvent systems. This is particularly evident in industries such as pharmaceuticals and coatings, where large volumes of solvents are used and recovered.
The sustainability aspect extends beyond environmental considerations to include economic factors. Efficient solvent recovery using ethyl acetate can lead to significant cost savings for industries, making sustainable practices more economically viable. This alignment of environmental and economic benefits is crucial for widespread adoption of greener technologies.
As industries move towards circular economy models, the role of ethyl acetate in solvent recovery becomes increasingly important. Its ability to be recycled multiple times without significant loss of quality supports closed-loop manufacturing processes, reducing the overall environmental impact of industrial operations.
Looking forward, the integration of ethyl acetate-based recovery systems with renewable energy sources and smart manufacturing technologies holds promise for further improving the sustainability profile of industrial processes. This holistic approach to sustainability in solvent recovery represents a significant step towards more environmentally responsible industrial practices.
Economic Feasibility of Recovery Systems
The economic feasibility of ethyl acetate recovery systems is a critical factor in determining the viability of solvent recovery advances. These systems offer significant potential for cost savings and environmental benefits, but their implementation requires careful consideration of various economic factors.
Initial investment costs for ethyl acetate recovery systems can be substantial, including equipment purchases, installation, and facility modifications. However, these upfront expenses are often offset by long-term savings in solvent procurement and waste disposal costs. The payback period for such investments typically ranges from 1 to 3 years, depending on the scale of operations and the efficiency of the recovery system.
Operational costs are another crucial aspect of economic feasibility. Energy consumption is a primary concern, as distillation and other separation processes require significant heat input. However, modern recovery systems often incorporate heat integration techniques, reducing overall energy requirements and associated costs. Maintenance expenses should also be factored in, including regular equipment servicing and occasional component replacements.
The recovery rate of ethyl acetate is a key determinant of economic viability. Advanced systems can achieve recovery rates of up to 99%, significantly reducing the need for fresh solvent purchases. This high recovery rate not only cuts raw material costs but also minimizes waste disposal expenses, which can be substantial due to environmental regulations.
Market conditions play a vital role in the economic feasibility of recovery systems. Fluctuations in ethyl acetate prices can impact the cost-benefit analysis. When solvent prices are high, recovery systems become more attractive. Conversely, during periods of low solvent costs, the economic incentive may be reduced, although environmental considerations often still justify their use.
Regulatory factors also influence economic feasibility. Stringent environmental regulations regarding solvent emissions and waste disposal can make recovery systems more economically attractive by reducing compliance costs and potential fines. In some regions, government incentives or tax benefits for implementing environmentally friendly technologies can further enhance the economic appeal of these systems.
The scale of operations is another critical factor. Larger facilities with higher solvent usage volumes generally see greater economic benefits from recovery systems due to economies of scale. Smaller operations may find the initial investment more challenging to justify, although modular and scalable recovery solutions are becoming increasingly available to address this market segment.
In conclusion, while the economic feasibility of ethyl acetate recovery systems varies depending on specific operational contexts, they generally offer a compelling value proposition. The combination of reduced solvent procurement costs, minimized waste disposal expenses, and potential regulatory benefits often results in a favorable return on investment, particularly for medium to large-scale operations in regions with strict environmental regulations.
Initial investment costs for ethyl acetate recovery systems can be substantial, including equipment purchases, installation, and facility modifications. However, these upfront expenses are often offset by long-term savings in solvent procurement and waste disposal costs. The payback period for such investments typically ranges from 1 to 3 years, depending on the scale of operations and the efficiency of the recovery system.
Operational costs are another crucial aspect of economic feasibility. Energy consumption is a primary concern, as distillation and other separation processes require significant heat input. However, modern recovery systems often incorporate heat integration techniques, reducing overall energy requirements and associated costs. Maintenance expenses should also be factored in, including regular equipment servicing and occasional component replacements.
The recovery rate of ethyl acetate is a key determinant of economic viability. Advanced systems can achieve recovery rates of up to 99%, significantly reducing the need for fresh solvent purchases. This high recovery rate not only cuts raw material costs but also minimizes waste disposal expenses, which can be substantial due to environmental regulations.
Market conditions play a vital role in the economic feasibility of recovery systems. Fluctuations in ethyl acetate prices can impact the cost-benefit analysis. When solvent prices are high, recovery systems become more attractive. Conversely, during periods of low solvent costs, the economic incentive may be reduced, although environmental considerations often still justify their use.
Regulatory factors also influence economic feasibility. Stringent environmental regulations regarding solvent emissions and waste disposal can make recovery systems more economically attractive by reducing compliance costs and potential fines. In some regions, government incentives or tax benefits for implementing environmentally friendly technologies can further enhance the economic appeal of these systems.
The scale of operations is another critical factor. Larger facilities with higher solvent usage volumes generally see greater economic benefits from recovery systems due to economies of scale. Smaller operations may find the initial investment more challenging to justify, although modular and scalable recovery solutions are becoming increasingly available to address this market segment.
In conclusion, while the economic feasibility of ethyl acetate recovery systems varies depending on specific operational contexts, they generally offer a compelling value proposition. The combination of reduced solvent procurement costs, minimized waste disposal expenses, and potential regulatory benefits often results in a favorable return on investment, particularly for medium to large-scale operations in regions with strict environmental regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!